ACEI-Inhibitory Peptides Naturally Generated in Meat and Meat Products and Their Health Relevance
Abstract
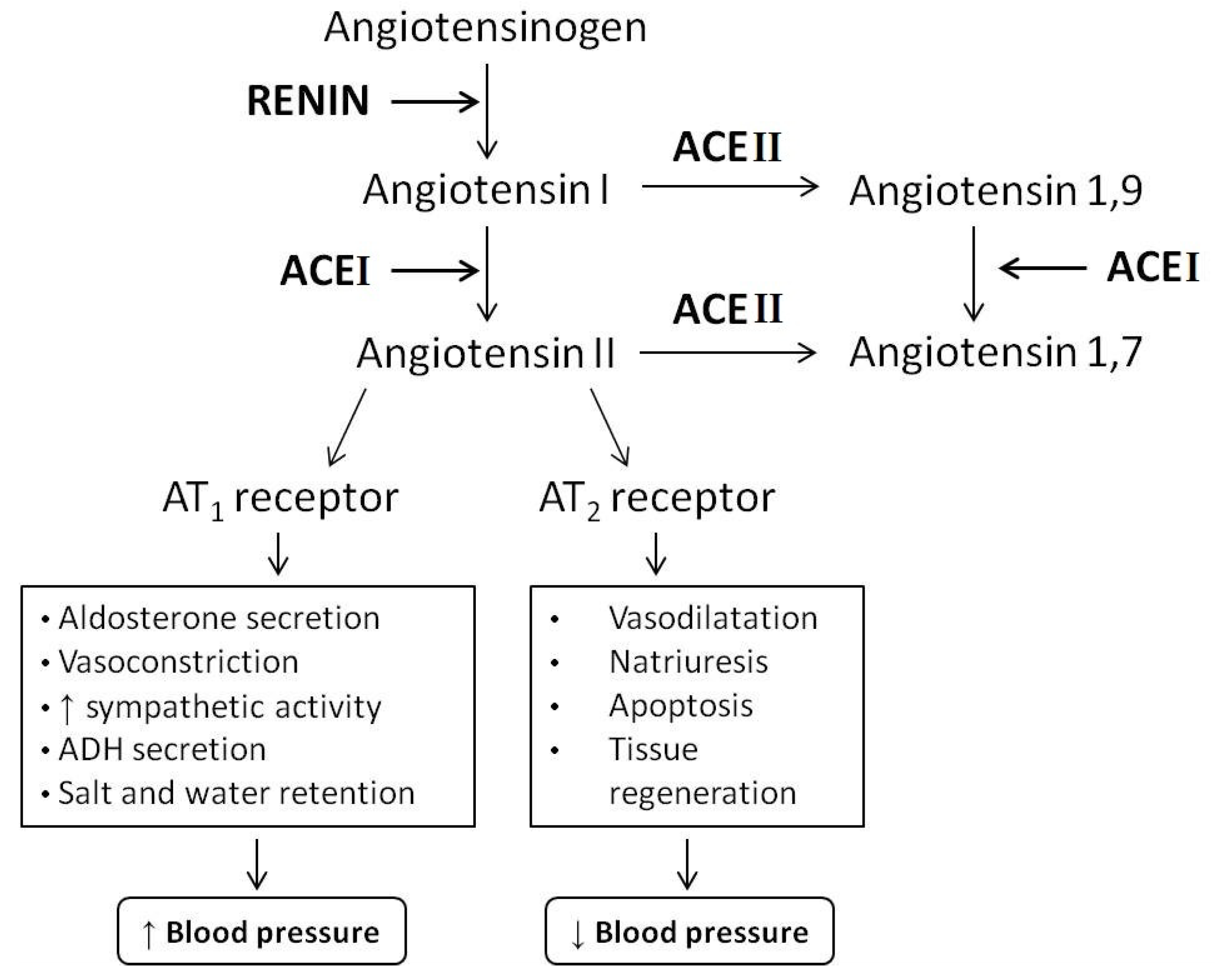
:1. Introduction
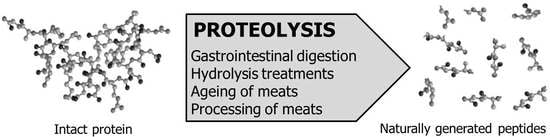
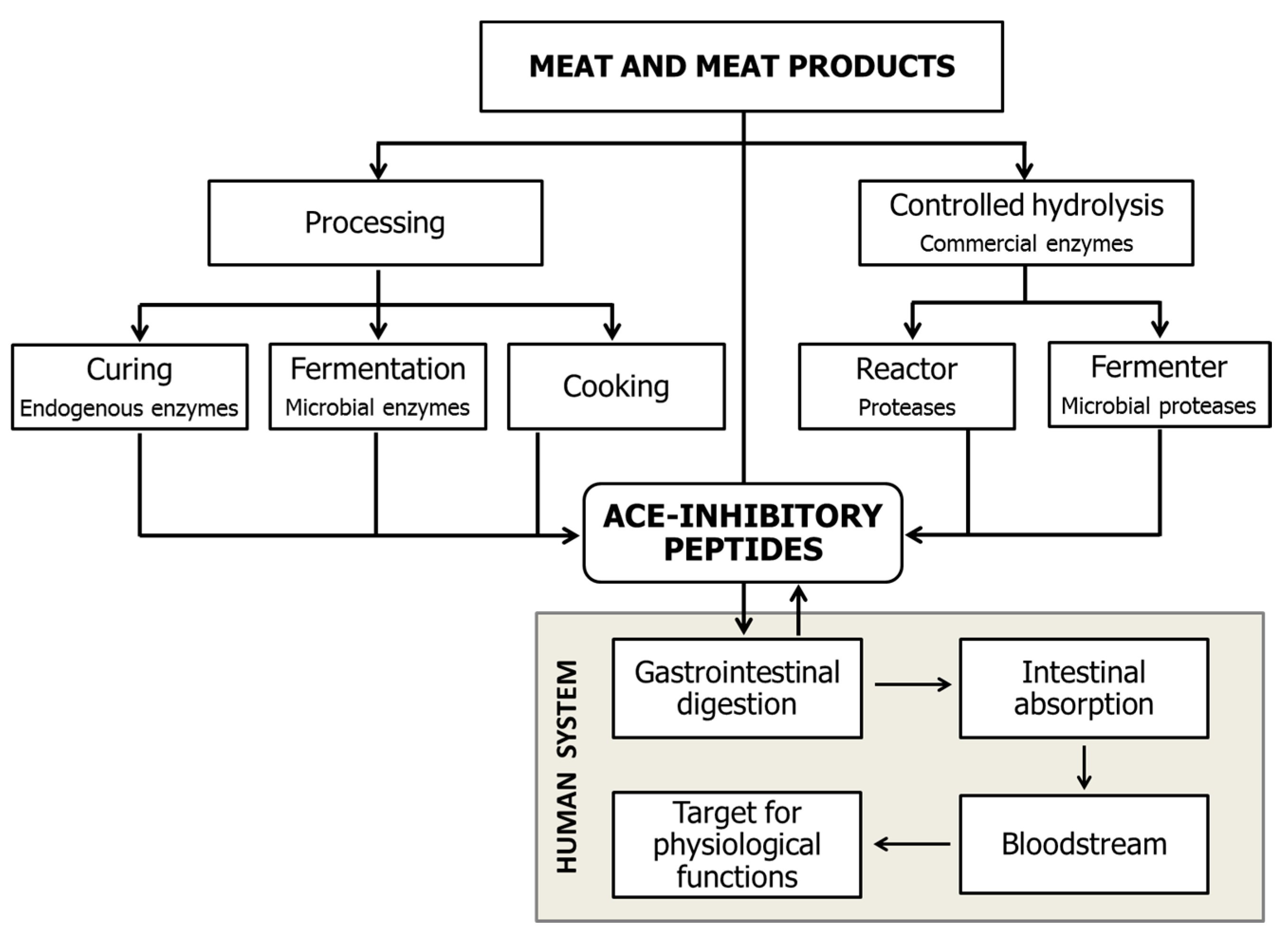
2. Generation of Meat-Derived ACEI-Inhibitory Peptides
2.1. Bioactive Peptides Generated during Gastrointestinal Digestion
2.2. Hydrolysis Treatments with Commercial Enzymes
2.3. Bioactive Peptide Generation during Ageing and the Processing of Meat
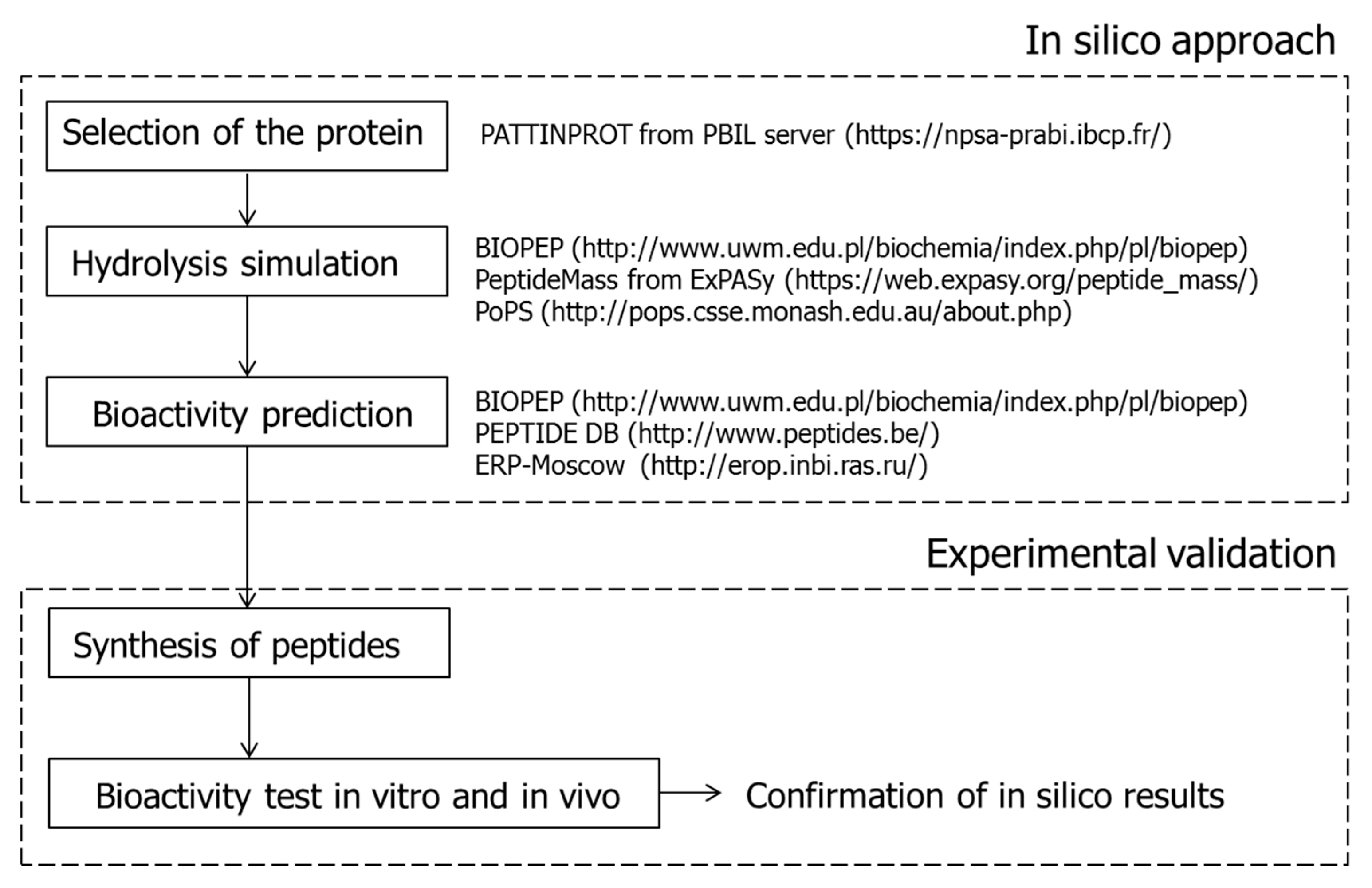
3. Identification of ACEI-Inhibitory Peptides
4. Bioavailability of ACEI-Inhibitory Peptides
5. Challenges and Limitations
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gallego, M.; Mora, L.; Toldrá, F. Health relevance of antihypertensive peptides in foods. Curr. Opin. Food Sci. 2018, 19, 8–14. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Munekata, P.E.S.; Gómez, B.; Barba, F.J.; Mora, L.; Pérez-Santaescolástica, C.; Toldrá, F. Bioactive peptides as natural antioxidants in food products—A review. Trends Food Sci. Technol. 2018, 79, 136–147. [Google Scholar] [CrossRef]
- Santos, J.C.P.; Sousa, R.C.S.; Otoni, C.G.; Moraes, A.R.F.; Souza, V.G.L.; Medeiros, E.A.A.; Espitia, P.J.P.; Pires, A.C.S.; Coimbra, J.S.R.; Soares, N.F.F. Nisin and other antimicrobial peptides: Production, mechanisms of action, and application in active food packaging. Innov. Food Sci. Emerg. Technol. 2018, 48, 179–194. [Google Scholar] [CrossRef]
- Moughan, P.J.; Rutherfurd-Markwick, K. Food bioactive proteins and peptides: antimicrobial, immunomodulatory and anti-inflammatory effects. In Diet, Immunity and Inflammation; Calder, P.C., Parveen, Y., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2013; pp. 313–340. ISBN 9780857090379. [Google Scholar]
- Chalamaiah, M.; Yu, W.; Wu, J. Immunomodulatory and anticancer protein hydrolysates (peptides) from food proteins: A review. Food Chem. 2018, 245, 205–222. [Google Scholar] [CrossRef] [PubMed]
- Arısoy, S.; Üstün-Aytekin, Ö. Hydrolysis of food-derived opioids by dipeptidyl peptidase IV from Lactococcus lactis spp. Lactis. Food Res. Int. 2018, 111, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Mora, L.; Aristoy, M.C.; Toldrá, F. Chapter Bioactive peptides. In Encyclopedia of Food Chemistry, 1st ed.; Varelis, P., Melton, L., Shahidi, F., Eds.; Elsevier: New York, NY, USA, 2018; ISBN 9780128140260. [Google Scholar]
- Toldrá, F.; Reig, M.; Aristoy, M.C.; Mora, L. Generation of bioactive peptides during processing. Food Chem. 2018, 267, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liao, W.; Udenigwe, C.C. Revisiting the mechanisms of ACE inhibitory peptides from food proteins. Trends Food Sci. Technol. 2017, 69, 214–219. [Google Scholar] [CrossRef]
- Girgih, A.T.; He, R.; Malomo, S.; Offengenden, M.; Wu, J.; Aluko, R.E. Structural and functional characterization of hemp seed (Cannabis sativa L.) protein derived antioxidant and antihypertensive peptides. J. Funct. Foods 2014, 6, 384–394. [Google Scholar] [CrossRef]
- Hernández-Ledesma, B.; del Mar Contreras, M.; Recio, I. Antihypertensive peptides: Production, bioavailability and incorporation into foods. Adv. Colloid Interface Sci. 2011, 165, 23–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lassoued, I.; Mora, L.; Nasri, R.; Jridi, M.; Toldrá, F.; Aristoy, M.-C.; Nasri, M. Characterization and comparative assessment of antioxidant and ACE inhibitory activities of thornback ray gelatin hydrolysates. J. Funct. Foods 2015, 13, 225–238. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Bioactive peptides. J. AOAC Int. 2008, 91, 914–931. [Google Scholar] [PubMed]
- Zheng, Y.; Li, Y.; Zhang, Y.; Ruan, X.; Zhang, R. Purification, characterization, synthesis, in vitro ACE inhibition and in vivo antihypertensive activity of bioactive peptides derived from oil palm kernel glutelin-2 hydrolysates. J. Funct. Foods 2017, 28, 48–58. [Google Scholar] [CrossRef]
- Jäkälä, P.; Vapaatalo, H. Antihypertensive peptides from milk proteins. Pharmaceuticals 2010, 3, 251–272. [Google Scholar] [CrossRef] [PubMed]
- Escudero, E.; Sentandreu, M.A.; Toldrá, F. Characterization of peptides released by in vitro digestion of pork meat. J. Agric. Food Chem. 2010, 58, 5160–5165. [Google Scholar] [CrossRef] [PubMed]
- Escudero, E.; Sentandreu, M.A.; Arihara, K.; Toldrá, F. Angiotensin I converting enzyme inhibitory peptides generated from in vitro gastrointestinal digestion of pork meat. J. Agric. Food Chem. 2010, 58, 2895–2901. [Google Scholar] [CrossRef] [PubMed]
- Sentandreu, M.A.; Toldrá, F. A rapid, simple and sensitive fluorescence method for the assay of angiotensin-I converting enzyme. Food Chem. 2006, 97, 546–554. [Google Scholar] [CrossRef]
- Escudero, E.; Toldrá, F.; Sentandreu, M.A.; Nishimura, H.; Arihara, K. Antihypertensive activity of peptides identified in the in vitro gastrointestinal digest of pork meat. Meat Sci. 2012, 91, 382–384. [Google Scholar] [CrossRef] [PubMed]
- Sayd, T.; Dufour, C.; Chambon, C.; Buffière, C.; Remond, D.; Santé-Lhoutellier, V. Combined in vivo and in silico approaches for predicting the release of bioactive peptides from meat digestion. Food Chem. 2018, 249, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Arihara, K.; Nakashima, Y.; Mukai, T.; Ishikawa, S.; Itoh, M. Peptide inhibitors for angiotensin I-converting enzyme from enzymatic hydrolysates of porcine skeletal muscle proteins. Meat Sci. 2001, 57, 319–324. [Google Scholar] [CrossRef]
- Katayama, K.; Tomatsu, M.; Fuchu, H.; Sugiyama, M.; Kawahara, S.; Yamauchi, K.; Kawamura, Y.; Muguruma, M. Purification and characterization of an angiotensin I-converting enzyme inhibitory peptide derived from porcine troponin C. Anim. Sci. J. 2003, 74, 53–58. [Google Scholar] [CrossRef]
- Fujita, H.; Yokoyama, K.; Yoshikawa, M. Classification and antihypertensive activity of angiotensin I-converting enzyme inhibitory peptides derived from food proteins. J. Food Sci. 2000, 65, 564–569. [Google Scholar]
- Terashima, M.; Baba, T.; Ikemoto, N.; Katayama, M.; Morimoto, T.; Matsumura, S. Novel angiotensin-converting enzyme (ACE) inhibitory peptides derived from boneless chicken leg meat. J. Agric. Food Chem. 2010, 58, 7432–7436. [Google Scholar] [CrossRef] [PubMed]
- Jang, A.; Lee, M. Purification and identification of angiotensin converting enzyme inhibitory peptides from beef hydrolysates. Meat Sci. 2005, 69, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Adje, E.Y.; Balti, R.; Guillochon, D.; Nedjar-Arroume, N. α 67–106 of bovine hemoglobin: A new family of antimicrobial and angiotensin I-converting enzyme inhibitory peptides. Eur. Food Res. Technol. 2011, 232, 637–646. [Google Scholar] [CrossRef]
- Banerjee, P.; Shanthi, C. Isolation of novel bioactive regions from bovine Achilles tendon collagen having angiotensin I-converting enzyme-inhibitory properties. Process Biochem. 2012, 47, 2335–2346. [Google Scholar] [CrossRef]
- Mora, L.; Gallego, M.; Escudero, E.; Reig, M.; Aristoy, M.C.; Toldrá, F. Small peptides hydrolysis in dry-cured meats. Int. J. Food Microbiol. 2015, 212, 9–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escudero, E.; Mora, L.; Fraser, P.D.; Aristoy, M.C.; Arihara, K.; Toldrá, F. Purification and identification of antihypertensive peptides in Spanish dry-cured ham. J. Proteomics 2013, 78, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Escudero, E.; Mora, L.; Toldrá, F. Stability of ACE inhibitory ham peptides against heat treatment and in vitro digestion. Food Chem. 2014, 161, 305–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mora, L.; Escudero, E.; Toldrá, F. Characterization of the peptide profile in Spanish Teruel, Italian Parma and Belgian dry-cured hams and its potential bioactivity. Food Res. Int. 2016, 89, 638–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mejri, L.; Vásquez-Villanueva, R.; Hassouna, M.; Marina, M.L.; García, M.C. Identification of peptides with antioxidant and antihypertensive capacities by RP-HPLC-Q-TOF-MS in dry fermented camel sausages inoculated with different starter cultures and ripening times. Food Res. Int. 2017, 100, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Gallego, M.; Mora, L.; Escudero, E.; Toldrá, F. Bioactive peptides and free amino acids profiles in different types of European dry-fermented sausages. Int. J. Food Microbiol. 2018, 276, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rivera, L.; Martínez-Maqueda, D.; Cruz-Huerta, E.; Miralles, B.; Recio, I. Peptidomics for discovery, bioavailability and monitoring of dairy bioactive peptides. Food Res. Int. 2014, 63, 170–181. [Google Scholar] [CrossRef] [Green Version]
- Nakashima, Y.; Arihara, K.; Sasaki, A.; Mio, H.; Ishikawa, S.; Itoh, M. Antihypertensive activities of peptides derived from porcine skeletal muscle myosin in spontaneously hypertensive rats. J. Food Sci. 2002, 67, 434–437. [Google Scholar] [CrossRef]
- Saiga, A.I.; Iwai, K.; Hayakawa, T.; Takahata, Y.; Kitamura, S.; Nishimura, T.; Morimatsu, F. Angiotensin I-converting enzyme-inhibitory peptides obtained from chicken collagen hydrolysate. J. Agric. Food Chem. 2008, 56, 9586–9591. [Google Scholar] [CrossRef] [PubMed]
- Mora, L.; Escudero, E.; Arihara, K.; Toldrá, F. Antihypertensive effect of peptides naturally generated during Iberian dry-cured ham processing. Food Res. Int. 2015, 78, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Iwai, K.; Saiga-Egusa, A.; Hayakawa, T.; Shimizu, M.; Takahata, Y.; Morimatsu, F. An angiotensin I-converting enzyme (ACE)-inhibitory peptide derived from chicken collagen hydrolysate lowers blood pressure in spontaneously hypertensive rats. J. Jpn. Soc. Food Sci. 2008, 55, 602–605. [Google Scholar] [CrossRef]
- Nakade, K.; Kamishima, R.; Inoue, Y.; Ahhmed, A.; Kawahara, S.; Nakayama, T.; Maruyama, M.; Numata, M.; Ohta, K.; Aoki, T.; et al. Identification of an antihypertensive peptide derived from chicken bone extract. Anim. Sci. J. 2008, 79, 710–715. [Google Scholar] [CrossRef]
- Katayama, K.; Mori, T.; Kawahara, S.; Miake, K.; Kodama, Y.; Sugiyama, M.; Kawamura, Y.; Nakayama, T.; Maruyama, M.; Muguruma, M. Angiotensin-I converting enzyme inhibitory peptide derived from porcine skeletal muscle myosin and its antihypertensive activity in spontaneously hypertensive rats. J. Food Sci. 2007, 72, S702–S706. [Google Scholar] [CrossRef] [PubMed]
- Katayama, K.; Anggraeni, H.E.; Mori, T.; Ahhmed, A.M.; Kawahara, S.; Sugiyama, M.; Nakayama, T.; Maruyama, M.; Muguruma, M. Porcine skeletal muscle troponin is a good source of peptides with angiotensin-I converting enzyme inhibitory activity and antihypertensive effects in spontaneously hypertensive rats. J. Agric. Food Chem. 2008, 56, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Muguruma, M.; Ahhmed, A.M.; Katayama, K.; Kawahara, S.; Maruyama, M.; Nakamura, T. Identification of pro-drug type ACE inhibitory peptide sourced from porcine myosin B: Evaluation of its antihypertensive effects in vivo. Food Chem. 2009, 114, 516–522. [Google Scholar] [CrossRef]
- Ichimura, T.; Yamanaka, A.; Otsuka, T.; Yamashita, E.; Maruyama, S. Antihypertensive effect of enzymatic hydrolysate of collagen and Gly-Pro in spontaneously hypertensive rats. Biosci. Biotechnol. Biochem. 2009, 73, 2317–2319. [Google Scholar] [CrossRef] [PubMed]
- Mirdhayati, I.; Hermanianto, J.; Wijaya, C.H.; Sajuthi, D.; Arihara, K. Angiotensin converting enzyme (ACE) inhibitory and antihypertensive activities of protein hydrolysate from meat of Kacang goat (Capra aegagrus hircus). J. Sci. Food Agric. 2016, 96, 3536–3542. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, J.M.; Burke, V.; Beilin, L.J.; Puddey, I.B. Partial substitution of carbohydrate intake with protein intake from lean red meat lowers blood pressure in hypertensive persons. Am. J. Clin. Nutr. 2006, 83, 780–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saiga-Egusa, A.; Iwai, K.; Hayakawa, T.; Takahata, Y.; Morimatsu, F. Antihypertensive effects and endothelial progenitor cell activation by intake of chicken collagen hydrolysate in pre- and mild-hypertension. Biosci. Biotechnol. Biochem. 2009, 73, 422–424. [Google Scholar] [CrossRef] [PubMed]
- López, M.R.C.; Bes-Rastrollo, M.; Zazpe, I.; Martínez, J.A.; Cuervo, M.; Martínez-González, M.A. Consumo de jamón curado e incidencia de episodios cardiovasculares, hipertensión arterial o ganancia de peso. Med. Clin. 2009, 133, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Montoro-García, S.; Zafrilla-Rentero, M.P.; Celdrán-de Haro, F.M.; Piñero-de Armas, J.J.; Toldrá, F.; Tejada-Portero, L.; Abellán-Alemán, J. Effects of dry-cured ham rich in bioactive peptides on cardiovascular health: A randomized controlled trial. J. Funct. Foods 2017, 38, 160–167. [Google Scholar] [CrossRef]
- Martínez-Sánchez, S.M.; Minguela, A.; Prieto-Merino, D.; Zafrilla-Rentero, M.P.; Abellán-Alemán, J.; Montoro-García, S. The effect of regular intake of dry-cured ham rich in bioactive peptides on inflammation, platelet and monocyte activation markers in humans. Nutrients 2017, 9, 321. [Google Scholar] [CrossRef] [PubMed]
- Agyei, D.; Ongkudon, C.M.; Wei, C.Y.; Chan, A.S.; Danquah, M.K. Bioprocess challenges to the isolation and purification of bioactive peptides. Food Bioprod. Process. 2016, 98, 244–256. [Google Scholar] [CrossRef]
- Carrasco-Castilla, J.; Hernández-Álvarez, A.J.; Jiménez-Martínez, C.; Gutiérrez-López, G.F.; Dávila-Ortiz, G. Use of proteomics and peptidomics methods in food bioactive peptide science and engineering. Food Eng. Rev. 2012, 4, 224–243. [Google Scholar] [CrossRef]
- Gu, Y.; Majumder, K.; Wu, J. QSAR-aided in silico approach in evaluation of food proteins as precursors of ACE inhibitory peptides. Food Res. Int. 2011, 44, 2465–2474. [Google Scholar] [CrossRef]
- Minkiewicz, P.; Dziuba, J.; Michalska, J. Bovine meat proteins as potential precursors of biologically active peptides-a computational study based on the BIOPEP database. Food Sci. Technol. Int. 2011, 17, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Lafarga, T.; O’Connor, P.; Hayes, M. Identification of novel dipeptidyl peptidase-IV and angiotensin-I-converting enzyme inhibitory peptides from meat proteins using in silico analysis. Peptides 2014, 59, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Pripp, A.H.; Isaksson, T.; Stepaniak, L.; Sørhaug, T. Quantitative structure-activity relationship modelling of ACE-inhibitory peptides derived from milk proteins. Eur. Food Res. Technol. 2004, 219, 579–583. [Google Scholar] [CrossRef]
- Vermeirssen, V.; Van Camp, J.; Verstraete, W. Bioavailability of angiotensin I converting enzyme inhibitory peptides. Br. J. Nutr. 2004, 92, 357–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mora, L.; Bolumar, T.; Heres, A.; Toldrá, F. Effect of cooking and simulated gastrointestinal digestion on the activity of generated bioactive peptides in aged beef meat. Food Funct. 2017, 8, 4347–4355. [Google Scholar] [CrossRef] [PubMed]
- Sangsawad, P.; Roytrakul, S.; Yongsawatdigul, J. Angiotensin converting enzyme (ACE) inhibitory peptides derived from the simulated in vitro gastrointestinal digestion of cooked chicken breast. J. Funct. Foods 2017, 29, 77–83. [Google Scholar] [CrossRef]
- Dellafiora, L.; Paolella, S.; Dall’Asta, C.; Dossena, A.; Cozzini, P.; Galaverna, G. Hybrid in silico/in vitro approach for the identification of angiotensin I converting enzyme inhibitory peptides from Parma dry-cured ham. J. Agric. Food Chem. 2015, 63, 6366–6375. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Young, J.F.; Rasmussen, M.K.; Dalsgaard, T.K.; Lametsch, R.; Aluko, R.E.; Therkildsen, M. Angiotensin I–converting enzyme–inhibitory peptides from bovine collagen: Insights into inhibitory mechanism and transepithelial transport. Food Res. Int. 2016, 89, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Gallego, M.; Grootaert, C.; Mora, L.; Aristoy, M.C.; Van Camp, J.; Toldrá, F. Transepithelial transport of dry-cured ham peptides with ACE inhibitory activity through a Caco-2 cell monolayer. J. Funct. Foods 2016, 21, 388–395. [Google Scholar] [CrossRef] [Green Version]
- Sangsawad, P.; Choowongkomon, K.; Kitts, D.D.; Chen, X.M.; Li-Chan, E.C.; Yongsawatdigul, J. Transepithelial transport and structural changes of chicken angiotensin I-converting enzyme (ACE) inhibitory peptides through Caco-2 cell monolayers. J. Funct. Foods 2018, 45, 401–408. [Google Scholar] [CrossRef]
- Segura-Campos, M.; Chel-Guerrero, L.; Betancur-Ancona, D.; Hernandez-Escalante, V.M. Bioavailability of bioactive peptides. Food Rev. Int. 2011, 27, 213–226. [Google Scholar] [CrossRef]
- Shen, W.; Matsui, T. Current knowledge of intestinal absorption of bioactive peptides. Food Funct. 2017, 8, 4306–4314. [Google Scholar] [CrossRef] [PubMed]
- Udenigwe, C.C.; Fogliano, V. Food matrix interaction and bioavailability of bioactive peptides: Two faces of the same coin? J. Funct. Foods 2017, 35, 9–12. [Google Scholar] [CrossRef]
- Jensen, I.J.; Dort, J.; Eilertsen, K.E. Proximate composition, antihypertensive and antioxidative properties of the semimembranosus muscle from pork and beef after cooking and in vitro digestion. Meat Sci. 2014, 96, 916–921. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Young, J.F.; Dalsgaard, T.K.; Therkildsen, M. Separation of angiotensin I-converting enzyme inhibitory peptides from bovine connective tissue and their stability towards temperature, pH and digestive enzymes. Int. J. Food Sci. Technol. 2015, 50, 1234–1243. [Google Scholar] [CrossRef]
- Mora, L.; Gallego, M.; Reig, M.; Toldrá, F. Challenges in the quantitation of naturally generated bioactive peptides in processed meats. Trends Food Sci. Technol. 2017, 69, 306–314. [Google Scholar] [CrossRef] [Green Version]
- Arroume, N.; Froidevaux, R.; Kapel, R.; Cudennec, B.; Ravallec, R.; Flahaut, C.; Bazinet, L.; Jacques, P.; Dhulster, P. Food peptides: Purification, identification and role in the metabolism. Curr. Opin. Food Sci. 2016, 7, 101–107. [Google Scholar] [CrossRef]

| Source | Peptide Sequence | Parent Protein | Hydrolysis Treatment | IC50 (µM) a | Dose (mg/kg BW) b | SBP (mmHg) c | Time (h) d | Reference |
|---|---|---|---|---|---|---|---|---|
| Chicken muscle | IKW | — | Thermolysin | 0.21 | 60 | −0.17 | 4 | [23] |
| Chicken muscle | LKP | Aldolase | Thermolysin | 0.32 | 60 | −0.18 | 4 | [23] |
| Chicken muscle | FKGRYYP | Creatine kinase | Thermolysin | 0.55 | 60 | 0 | — | [23] |
| Chicken muscle | GA(Hyp)GL(Hyp)GP | Collagen | Proteases | 29.4 | 4.5 | −0.18 | 6 | [39] |
| Chicken bone | YYRA | Inmunoglobin heavy chain | Pepsin | 57.2 | 10 | −0.20 | 6 | [40] |
| Porcine muscle | MNPPK | Myosin | Thermolysin | 945.5 | 1 | −0.23 | 6 | [35] |
| Porcine muscle | ITTNP | Myosin | Thermolysin | 549 | 1 | −0.21 | 6 | [35] |
| Porcine muscle | VKKVLGNP | Myosin light chain | Pepsin | 29 | 10 | −0.24 | 3 | [41] |
| Porcine muscle | KRQKYDI | Troponin | Pepsin | 26.2 | 10 | −0.9 | 6 | [42] |
| Porcine muscle | KRVITY | Myosin heavy chain | Pepsin | 6.1 | 10 | −0.23 | 6 | [43] |
| Porcine muscle | VKAGF | Actin | Pepsin | 20.3 | 10 | −0.17 | 6 | [43] |
| Porcine muscle | RPR | Nebulin | Pepsin + pancreatin | 382 | 1 | −0.33 | 6 | [19] |
| Porcine muscle | KAPVA | Titin | Pepsin + pancreatin | 46.56 | 1 | −0.33 | 6 | [19] |
| Porcine muscle | PTPVP | Titin | Pepsin + pancreatin | 256.41 | 1 | −0.25 | 6 | [19] |
| Porcine skin | GF(Hyp)GP | Collagen | Aspergillus protease | 91 | 10 | −0.20 | 8 | [44] |
| Goat muscle | FQPS | — | Protamex® + Flavourzyme® | 27.0 | 2.39 | −0.10 | 8 | [45] |
| Spanish dry-cured ham | AAATP | Allantoicase | No treatment | 100 | 1 | −0.26 | 8 | [19] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mora, L.; Gallego, M.; Toldrá, F. ACEI-Inhibitory Peptides Naturally Generated in Meat and Meat Products and Their Health Relevance. Nutrients 2018, 10, 1259. https://doi.org/10.3390/nu10091259
Mora L, Gallego M, Toldrá F. ACEI-Inhibitory Peptides Naturally Generated in Meat and Meat Products and Their Health Relevance. Nutrients. 2018; 10(9):1259. https://doi.org/10.3390/nu10091259
Chicago/Turabian StyleMora, Leticia, Marta Gallego, and Fidel Toldrá. 2018. "ACEI-Inhibitory Peptides Naturally Generated in Meat and Meat Products and Their Health Relevance" Nutrients 10, no. 9: 1259. https://doi.org/10.3390/nu10091259