Body Mass Index, Vitamin D, and Type 2 Diabetes: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis and Outcome Measures
3. Results
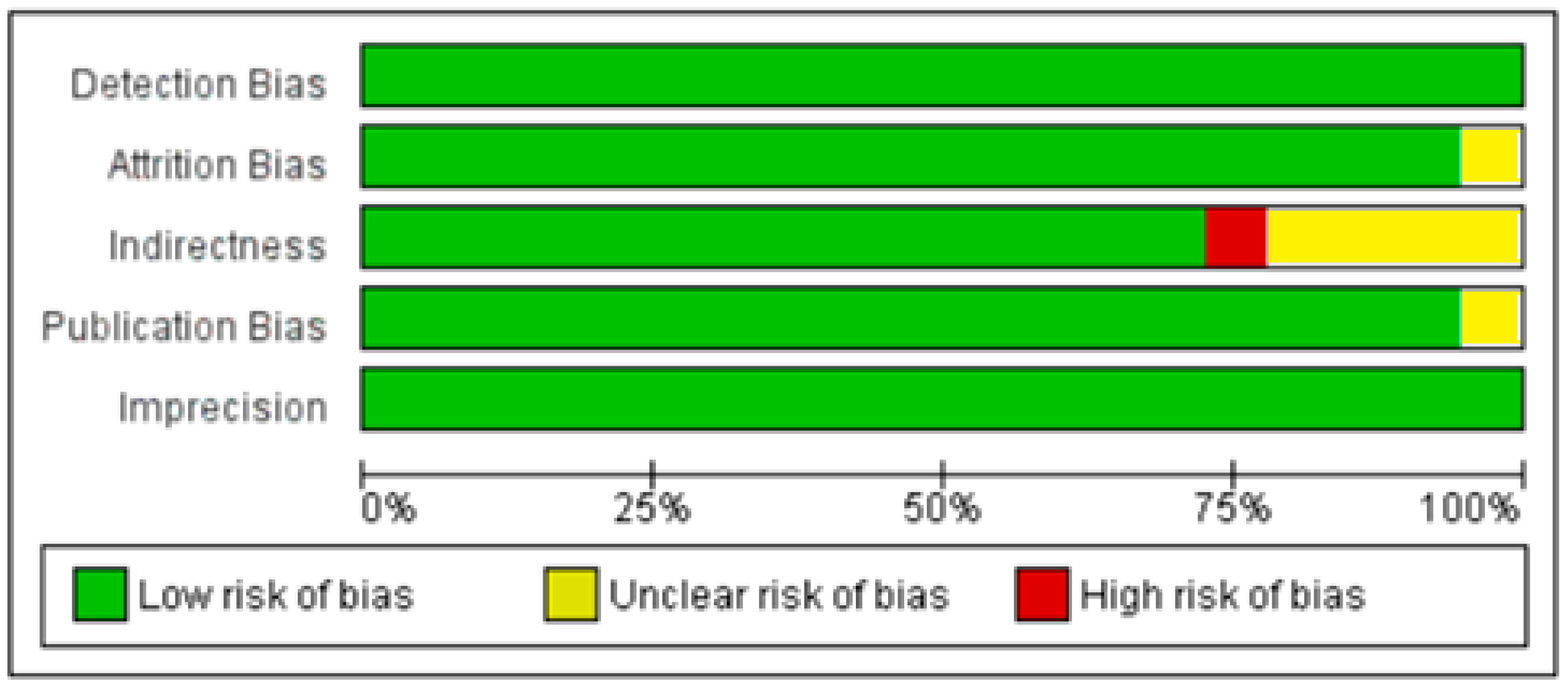
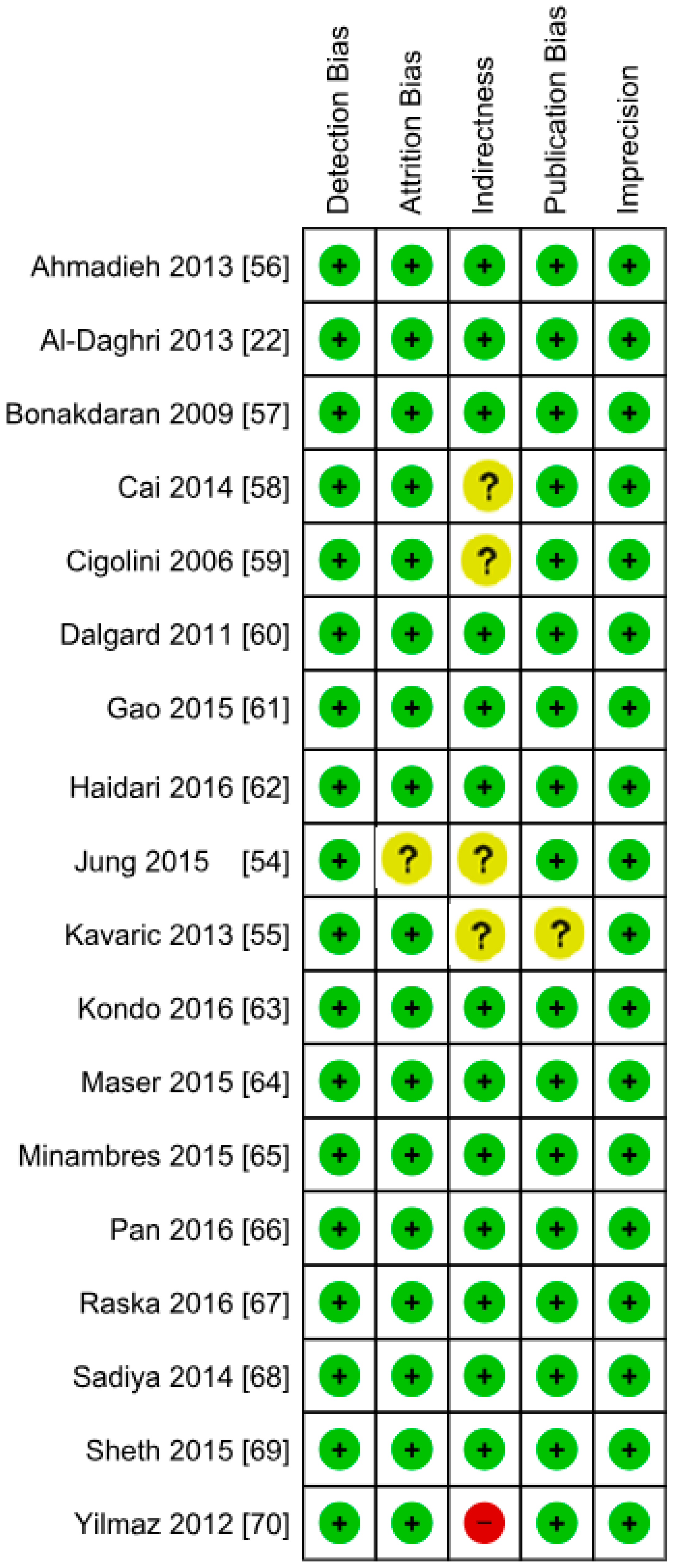
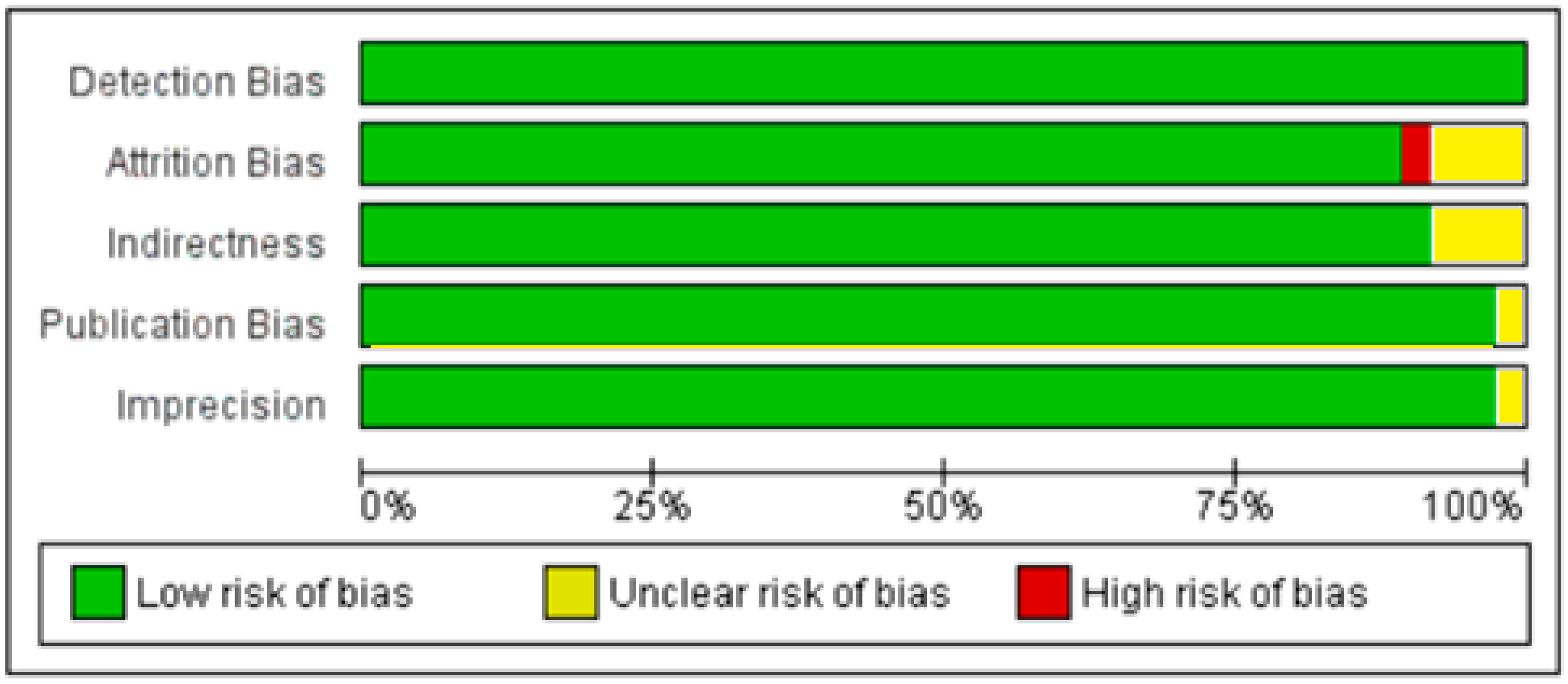
3.1. Included Studies
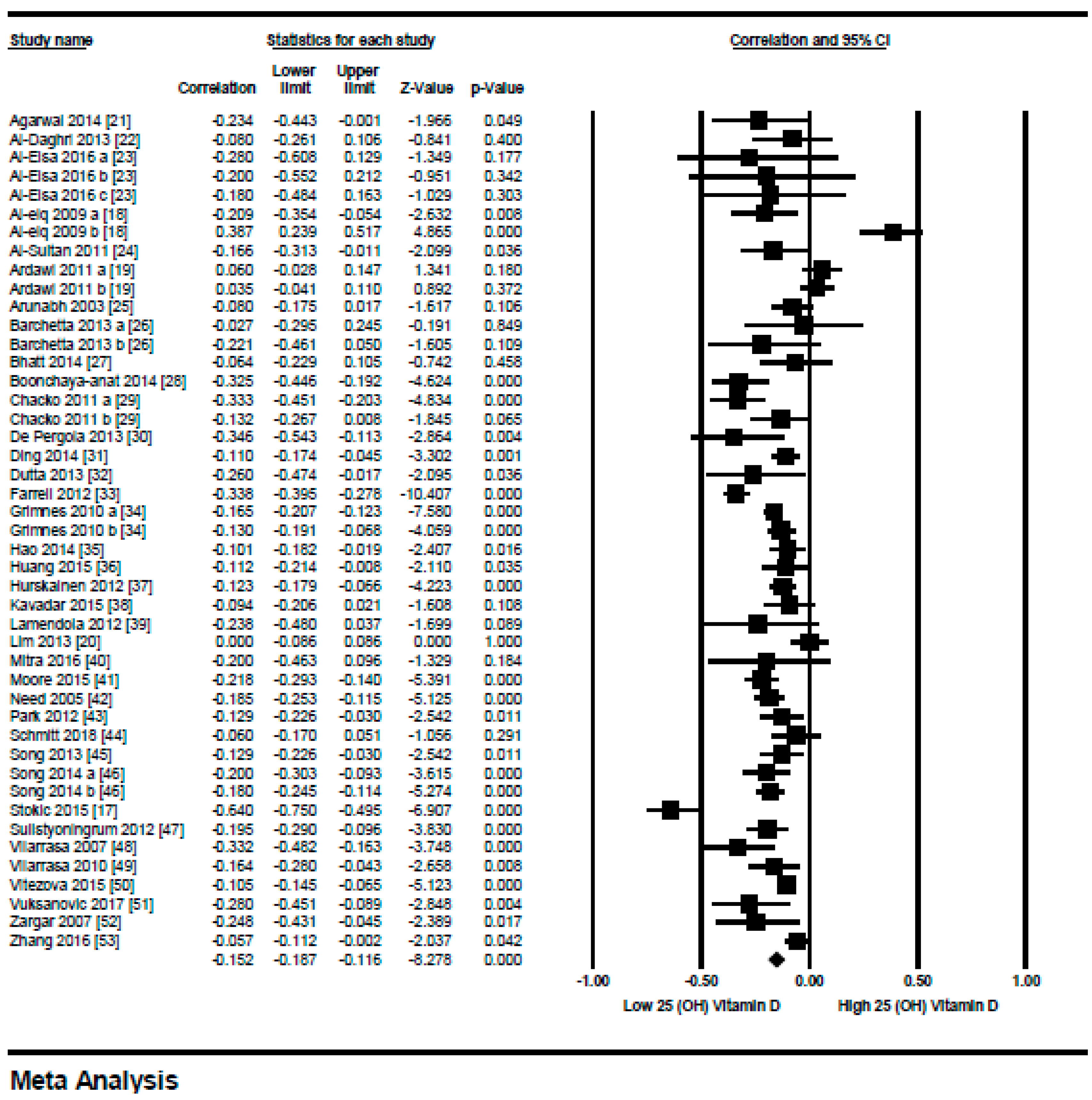
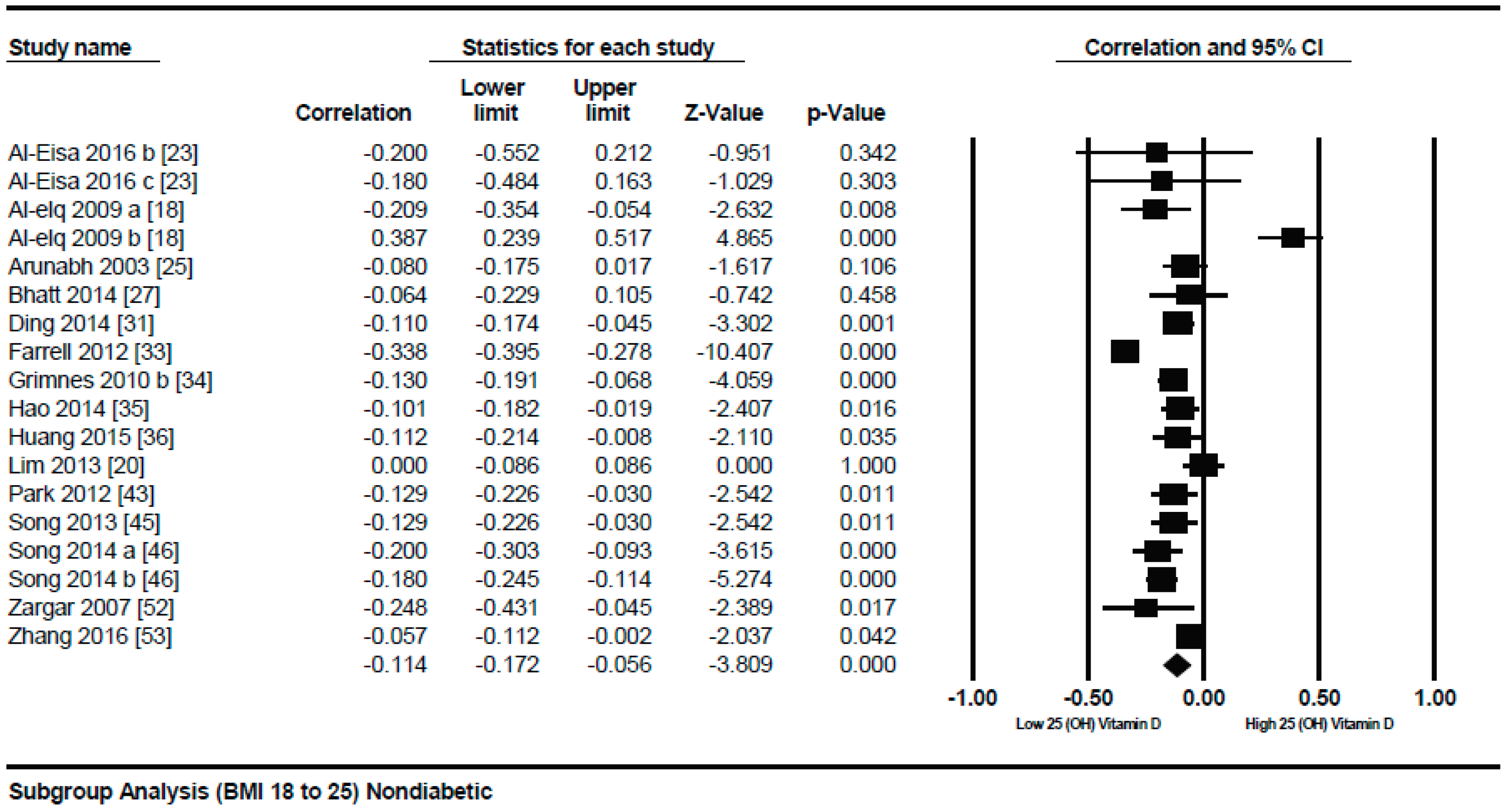
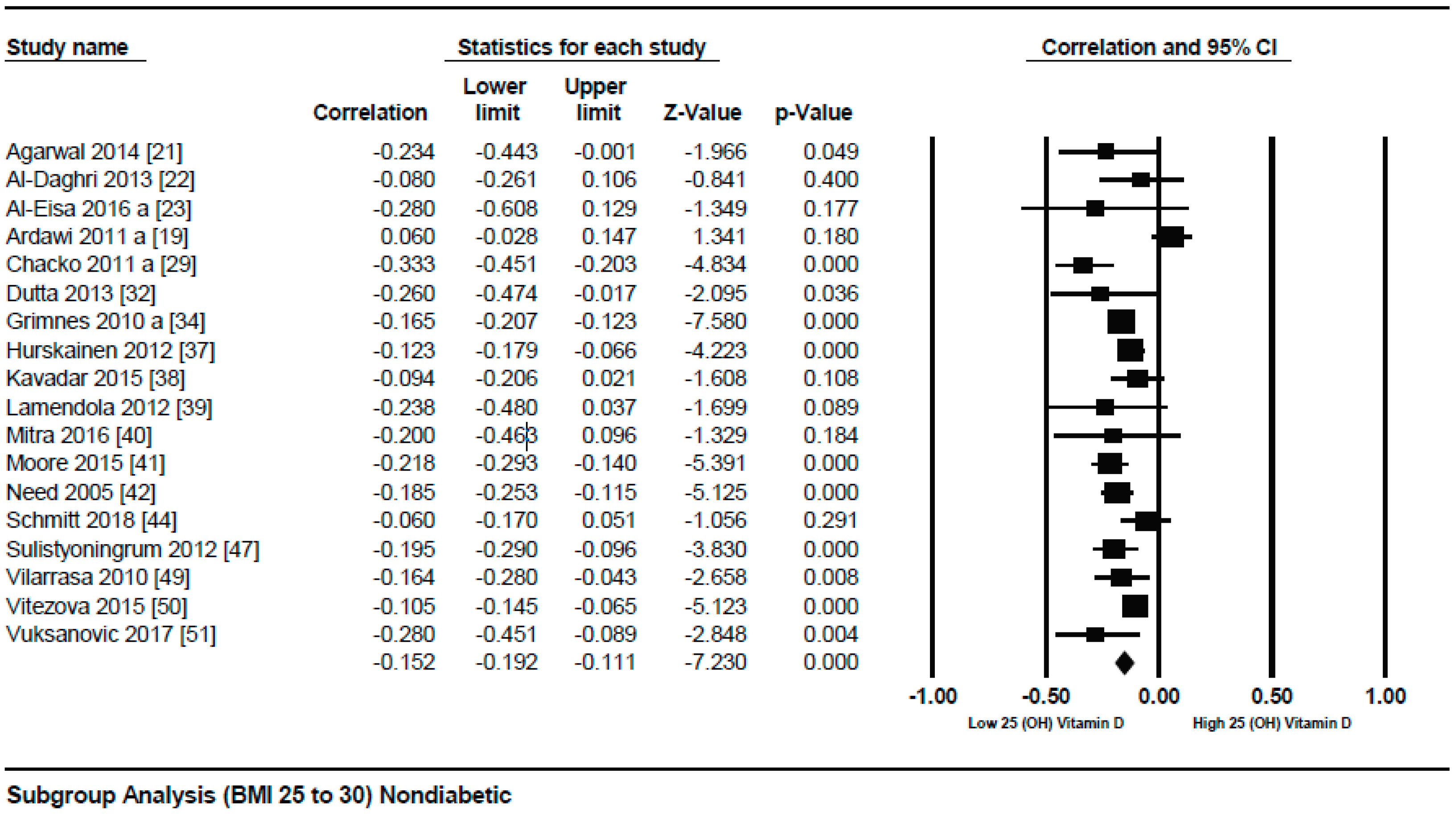
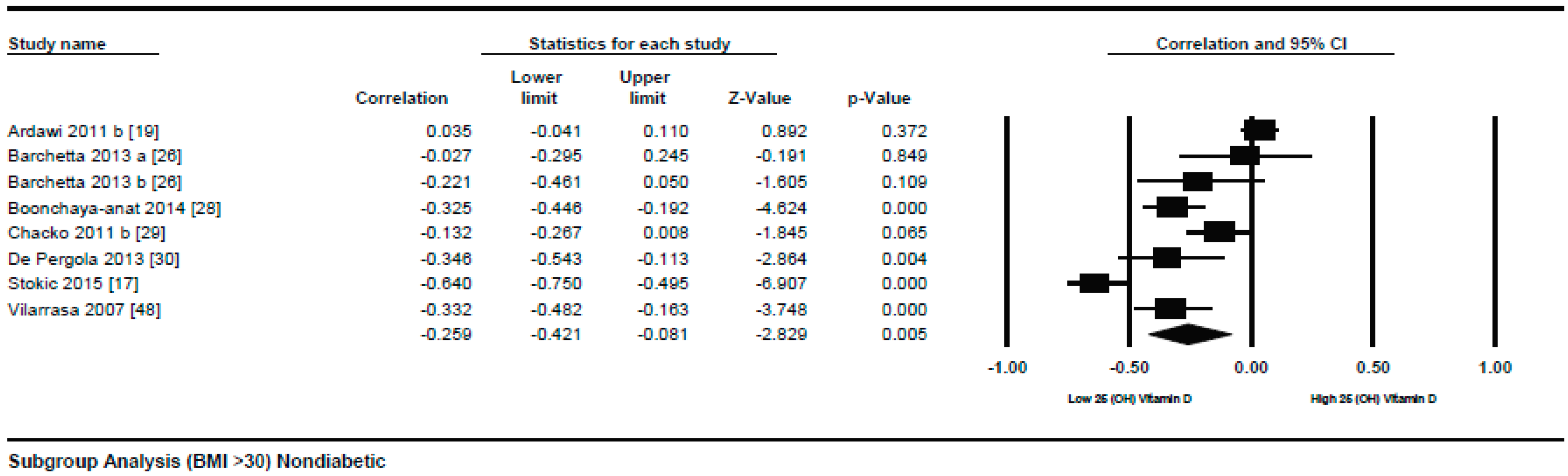
3.1.1. Meta-Analysis of the Association of Vitamin D and BMI in Non-Diabetics
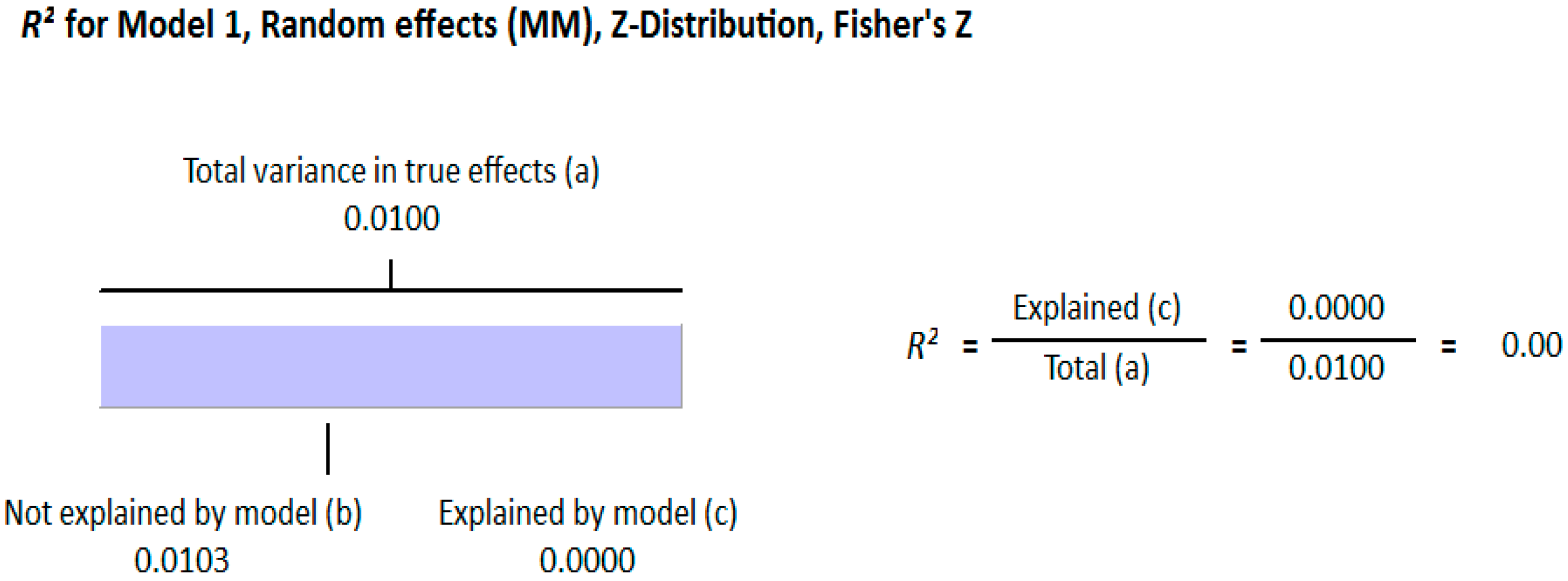
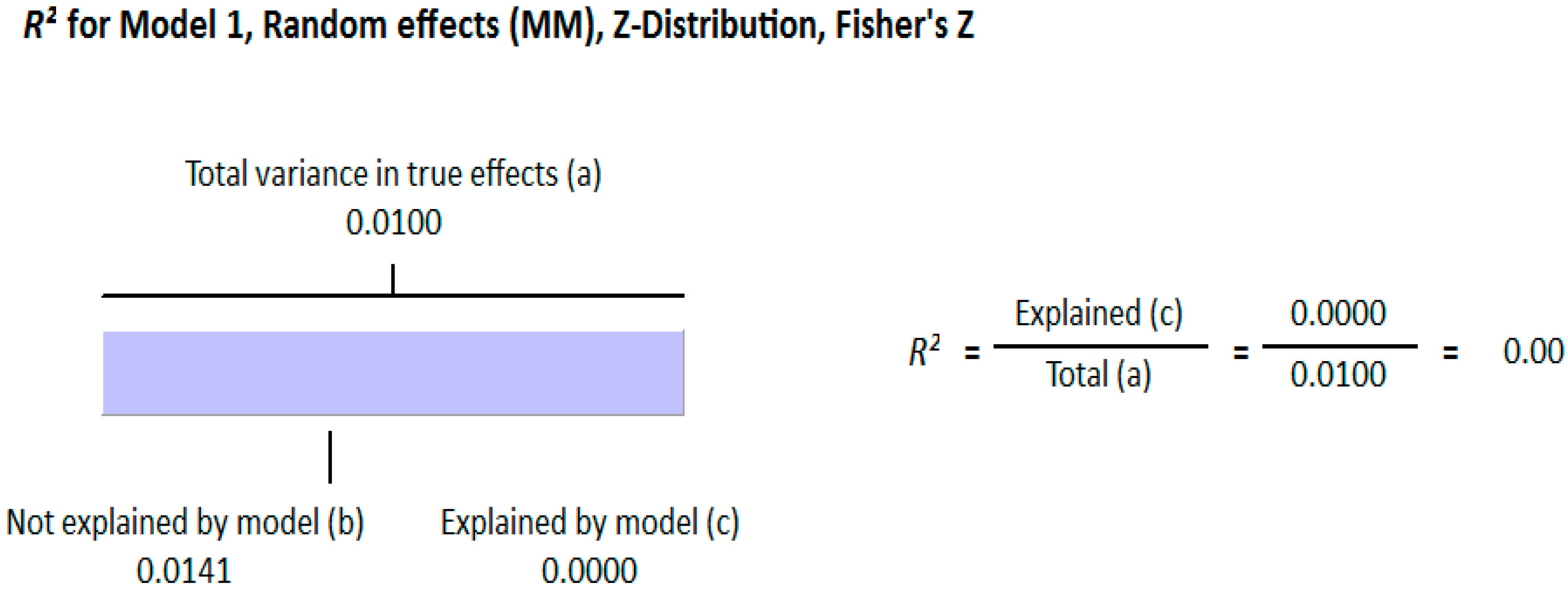
3.1.2. Meta Regression Analysis
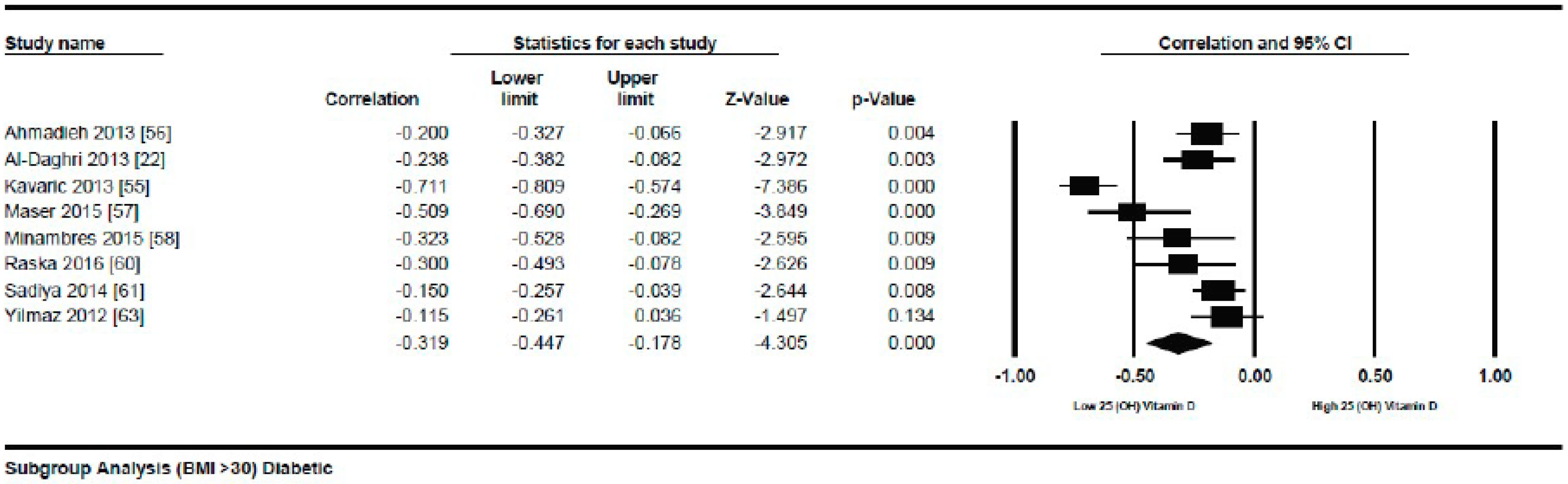
Meta-Analysis of the Association of Vitamin D and BMI in Diabetics
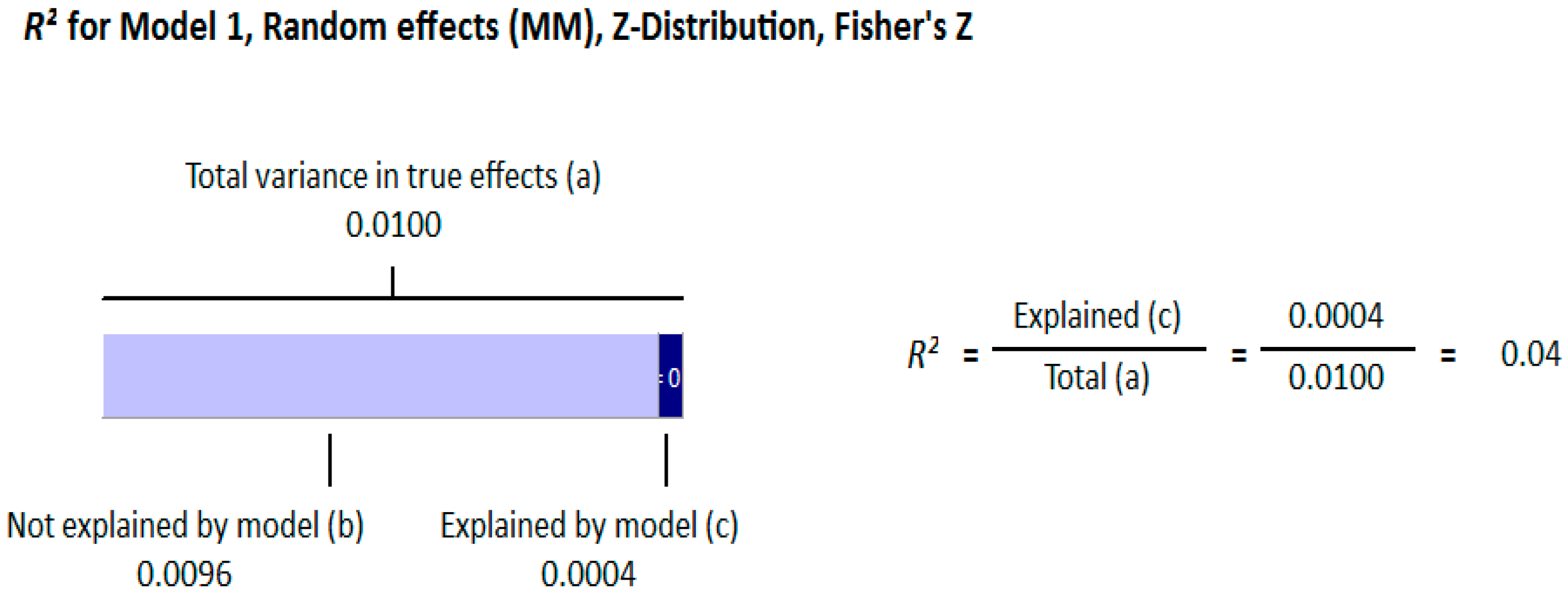
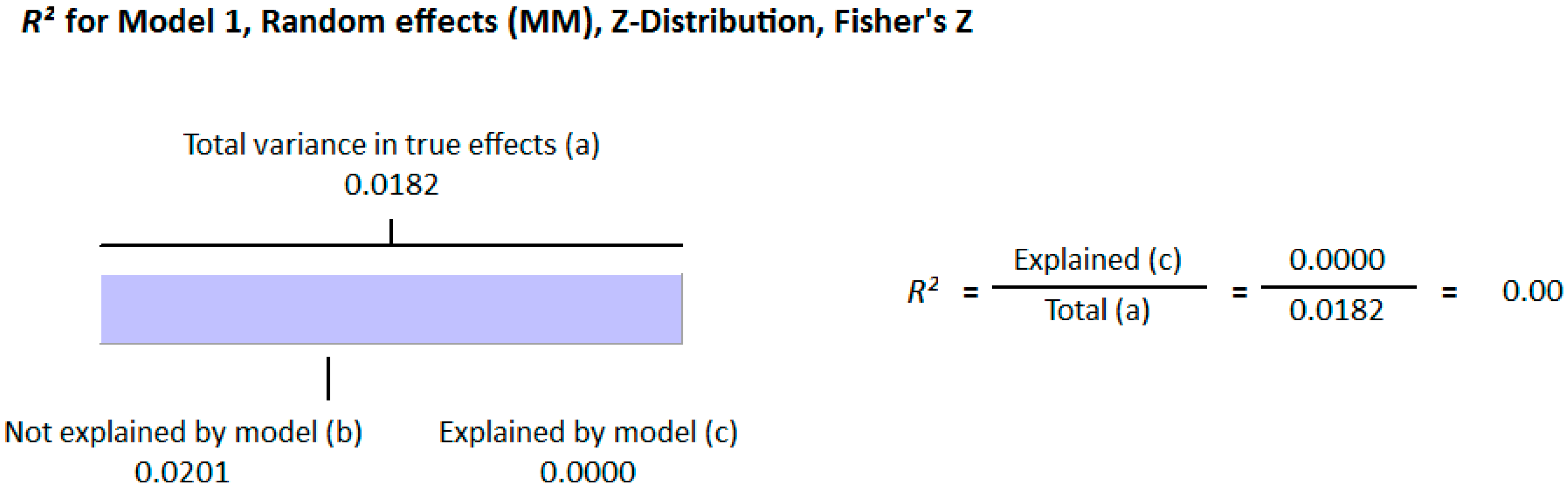
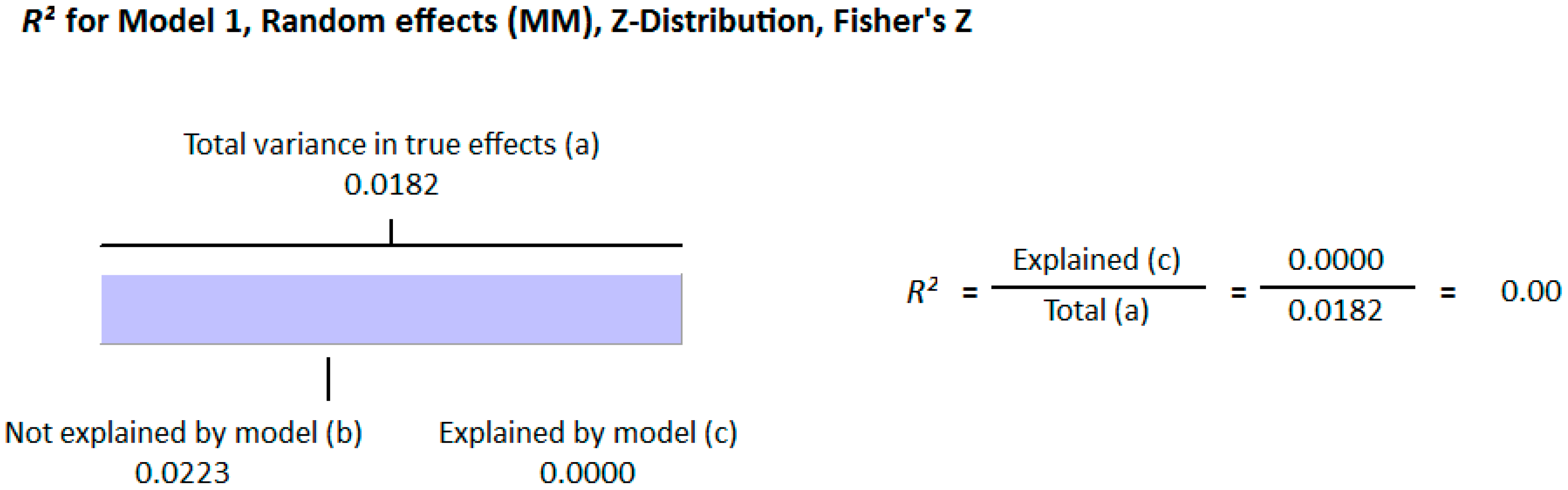
Meta Regression Analysis
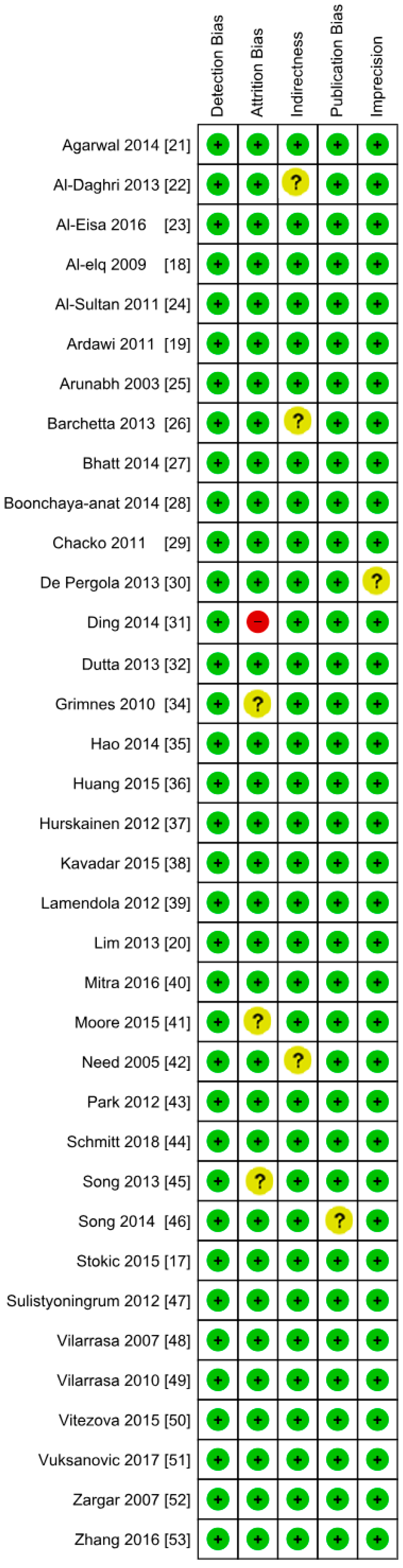
3.2. Excluded Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kabadi, S.M.; Liu, L.; Auchincloss, A.H.; Zakeri, I.F. Multivariate path analysis of serum 25-hydroxyvitamin D concentration, inflammation, and risk of type 2 diabetes mellitus. Dis. Mark. 2013, 35, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Hebebrand, J.; Hinney, A. Environmental and genetic risk factors in obesity. Child Adolesc. Psychiatr. Clin. N. Am. 2009, 18, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.; Hashmi, O.; Dutton, D.; Mavrodaris, A.; Stranges, S.; Kandala, S.B.; Clarke, A.; Franco, O.H. Levels of vitamin D and cardiometabolic disorders: Systematic review and meta-analysis. Maturitas 2010, 65, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Braun, A.B.; Gibbons, F.K.; Litonjua, A.A.; Giovannucci, E.; Christopher, K.B. Low serum 25-hydroxyvitamin D at critical care initiation is associated with increased mortality. Crit. Care Med. 2012, 40, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Roomi, M.A.; Farooq, A.; Ullah, E.; Lone, K.P. Hypovitaminosis D and its association with life style factors. Pak. J. Med. Sci. 2015, 31, 1236–1240. [Google Scholar] [CrossRef] [PubMed]
- Cashman, K.D.; Dowling, K.G.; Skrabakova, Z.; Gross, M.G.; Valtuena, J.; Henauw, S.D.; Moreno, L.; Daamsgaard, C.T.; Michaelsen, K.F.; Molgaard, C.; et al. Vitamin D Deficiency in Europe: Pandemic? Am. J. Clin. Nutr. 2016, 103, 1033–1044. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.S.; Ajani, U.A.; McGuire, L.C.; Liu, S. Concentrations of serum vitamin D and metabolic syndrome among US adults. Diabetes Care 2005, 28, 1228–1230. [Google Scholar] [CrossRef] [PubMed]
- Chiu, K.C.; Chu, A.; Go, V.L.; Saad, M.F. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am. J. Clin. Nutr. 2004, 79, 820–825. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.L.; Ng, Y.M.; Kang, P.S.; Lim, S.K. Association between serum 25-hydroxyvitamin D and glycated hemoglobin levels in type 2 diabetes patients with chronic kidney disease. J. Diabetes Investig. 2017, 9, 375–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, K.; DeLuca, H.F. Cloning of the human 1α, 25dihydroxyvitamin D-3 24-hydroxylase gene promoter and identification of two vitamin D-responsive elements. Biochim. Biophys. Acta 1995, 1263, 1–9. [Google Scholar] [CrossRef]
- Clark, S.A.; Stumpf, W.E.; Sar, M.; DeLuca, H.F.; Tanaka, Y. Target cells for 1,25 dihydroxyvitamin D3 in the pancreas. Cell Tissue Res. 1980, 209, 515–520. [Google Scholar]
- Simpson, R.U.; Thomas, G.A.; Arnold, A.J. Identification of 1,25-dihydroxyvitamin D3 receptors and activities in muscle. J. Biol. Chem. 1985, 260, 8882–8891. [Google Scholar] [PubMed]
- Nimitphong, H.; Holick, M.F.; Fried, S.K.; Lee, M.J. 25-Hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 promote the differentiation of human subcutaneous preadipocytes. PLoS ONE 2012, 7, e52171. [Google Scholar] [CrossRef] [PubMed]
- Blum, M.; Dolnikowski, G.; Seyoum, E.; Harris, S.S.; Booth, S.L.; Peterson, J.; Saltzman, E.; Dawson-Hughes, B. Vitamin D (3) in fat tissue. Endocrine 2008, 33, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, S.; Jeppesen, P.B. Is hypovitaminosis d related to incidence of type 2 diabetes and high fasting glucose level in healthy subjects: A systematic review and meta-analysis of observational studies. Nutrients 2018, 10, 59. [Google Scholar] [CrossRef] [PubMed]
- Wamberg, L.; Christiansen, T.; Paulsen, S.K.; Fisker, S.; Rask, P.; Rejnmark, L.; Richelsen, B.; Pedersen, S.B. Expression of vitamin D metabolizing enzymes in human adipose tissue: The effect of obesity and diet induced weight loss. Int. J. Obes. 2013, 37, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Stokic, E.; Kupusinac, A.; Dragana, T.; Branka, K.Z.; Milena, M.; Dragana, S.; Soskic, S.; Isenovic, E. Obesity and vitamin D deficiency: Trends to promote a more proatherogenic cardiometabolic risk profile. Angiology 2015, 66, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Al-Elq, A.H.; Sadat-Ali, M.; Al-Turki, H.A.; Al-Mulhim, F.A.; Al-Ali, A.K. Is there a relationship between body mass index and serum vitamin D levels? Saudi Med. J. 2009, 30, 1542–1546. [Google Scholar] [PubMed]
- Ardawi, M.S.M.; Qari, M.H.; Rouzi, A.A.; Maimani, A.A.; Raddadi, R.M. Vitamin D status in relation to obesity, bone mineral density, bone turnover markers and vitamin D receptor genotypes in healthy Saudi pre- and postmenopausal women. Osteoporos. Int. 2011, 22, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Kim, M.J.; Choi, S.H.; Shin, C.S.; Park, K.S.; Jang, H.C.; Billings, L.K.; Meigs, J.B. Association of vitamin D deficiency with incidence of type 2 diabetes in high-risk Asian subjects. Am. J. Clin. Nutr. 2013, 97, 524–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, N.; Mithal, A.; Kaur, P.; Dhingra, V.; Godbole, M.M.; Shukla, M. Vitamin D and insulin resistance in postmenopausal Indian women. Indian. J. Endocr. Metab. 2014, 18, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Al-Daghri, N.M.; Al-Attas, O.S.; Alokail, M.S.; Alkharfy, K.M.; Al-Othman, A.; Draz, H.M.; Yakout, S.M.; Al-Saleh, Y.; Al-Yousef, M.; Sabico, S.; et al. Hypovitaminosis D associations with adverse metabolic parameters are accentuated in patients with Type 2 diabetes mellitus: A body mass index-independent role of adiponectin? J. Endocrinol. Investig. 2013, 36, 1–6. [Google Scholar]
- Al-Eisa, E.S.; Alghadir, A.H.; Gabr, S.A. Correlation between vitamin D levels and muscle fatigue risk factors based on physical activity in healthy older adults. Clin. Interv. Aging 2016, 11, 513. [Google Scholar] [PubMed] [Green Version]
- Al-Sultan, A.I.; Amin, T.T.; Abou-Seif, M.A.; Al Naboli, M.R. Vitamin D, parathyroid hormone levels and insulin sensitivity among obese young adult Saudis. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 135–147. [Google Scholar] [PubMed]
- Arunabh, S.; Pollack, S.; Yeh, J.; Aloia, J.F. Body Fat Content and 25-Hydroxyvitamin D Levels in Healthy Women. J. Clin. Endocrinol. Metab. 2003, 88, 157–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barchetta, I.; de Bernardinis, M.; Capoccia, D.; Baroni, M.G.; Fontana, M.; Fraioli, A.; Morini, S.; Leonetti, F.; Cavallo, G. Hypovitaminosis d is independently associated with metabolic syndrome in obese patients. PLoS ONE 2013, 8, e68689. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.P.; Misra, A.; Sharma, M.; Guleria, R.; Pandey, R.M.; Luthra, K.; Vikram, N.K. Vitamin D insufficiency is associated with abdominal obesity in urban asian indians without diabetes in north india. Diabetes Technol. Ther. 2014, 16, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Boonchaya-anant, P.; Holick, F.M.; Caroline, M. ApovianSerum 25-Hydroxyvitamin D Levels and Metabolic Health Status in Extremely Obese Individuals. Obesity 2014, 22, 2539–2543. [Google Scholar] [CrossRef] [PubMed]
- Chacko, S.A.; Song, Y.; Manson, J.E.; Horn, L.V.; Eaton, C.; Martin, L.W.; McTiernan, A.; Curb, J.D.; Wylie-Rosett, J.; Phillips, L.S.; et al. Serum 25-hydroxyvitamin D concentrations in relation to cardiometabolic risk factors and metabolic syndrome in postmenopausal women. Am. J. Clin. Nutr. 2011, 94, 209–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Pergola, G.; Nitti, A.; Bartolomeo, N.; Gesuita, A.; Giagulli, V.A.; Triggiani, V.; Guastamacchia, E.; Silvestris, F. Possible role of hyperinsulinemia and insulin resistance in lower vitamin D levels in overweight and obese patients. Biomed. Res. Int. 2013, 2013, 921348. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Wang, C.; Ma, H.; Tian, Y.; Lu, Y.; Pang, S. The Study of Serum Vitamin D and Insulin Resistance in Chinese Populations with Normal Glucose Tolerance. Int. J. Endocrinol. 2014, 2014, 870235. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.; Maisnam, I.; Shrivastava, A.; Sinha, A.; Ghosh, S.; Mukhopadhyay, P.; Mukhopadhyay, S.; Chowdhury, S. Serum vitamin-D predicts insulin resistance in individuals with prediabetes. Indian J. Med. Res. 2013, 138, 853–860. [Google Scholar] [PubMed]
- Farrell, S.W.; Willis, B.L. Cardiorespiratory Fitness, Adiposity, and Serum 25-Dihydroxyvitamin D Levels in Women: The cooper center longitudinal study. J. Womens Health 2012, 21, 1. [Google Scholar] [CrossRef] [PubMed]
- Grimnes, G.; Emaus, N.; Joakimsen, R.M.; Figenschau, Y.; Jenssen, T.; Njølstad, I.; Schirmer, H.; Jorde, R. Baseline serum 25-hydroxyvitamin D concentrations in the Tromsø Study 1994–95 and risk of developing type 2 diabetes mellitus during 11 years of follow-up. Diabet. Med. 2010, 27, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Ma, X.; Shen, Y.; Ni, J.; Luo, Y.; Xiao, Y.; Bao, Y.; Jia, W. Associations of Serum 25-Hydroxyvitamin D3 Levels with Visceral Adipose Tissue in Chinese Men with Normal Glucose Tolerance. PLoS ONE 2014, 9, e86773. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Chang, H.; Lu, C.; Tseng, F.; Lee, L.; Huang, K. Vitamin D status and risk of metabolic syndrome among non-diabetic young adults. Clin. Nutr. 2015, 34, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Hurskainen, A.R.; Virtanen, J.K.; Tuomainen, T.P.; Nurmi, T.; Voutilainen, S. Association of serum 25-hydroxyvitamin D with type 2 diabetes and markers of insulin resistance in a general older population in Finland. Diabetes Metab. Res. Rev. 2012, 28, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Kavadar, G.; Demircioglu, D.T.; Ozgonenel, L.; Emre, T.Y. The relationship between vitamin D status, physical activity and insulin resistance in overweight and obese subjects. Bosn. J. Basic Med. Sci. 2015, 15, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Lamendola, C.A.; Ariel, D.; Feldman, D.; Reaven, G.M. Relations between obesity, insulin resistance, and 25-hydroxyvitamin D. Am. J. Clin. Nutr. 2012, 95, 1055–1059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitra, S.; Nayak, P.K.; Agrawal, S.; Sahoo, J.P.; Kamalanathan, S.K.; Nanda, R. Vitamin D Status and Cardio-Metabolic Risk in Indian Postmenopausal Women. J. Clin. Diagn. Res. 2016, 10, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.; Hochner, H.; Sitlani, C.M.; Williams, M.A.; Hoofnagle, A.N.; de Boer, I.H.; Kestenbaum, B.; Siscovic, D.S.; Friedlander, Y.; Enquobahrie, D.A. Plasma vitamin D is associated with fasting insulin and homeostatic model assessment of insulin resistance in young adult males, but not females, of the Jerusalem Perinatal Study. Public Health Nutr. 2015, 18, 1324–1331. [Google Scholar] [CrossRef] [PubMed]
- Need, A.G.; O’Loughlin, P.D.; Horowitz, M.; Nordin, B.E.C. Relationship between fasting serum glucose, age, body mass index and serum 25 hydroxyvitamin D in postmenopausal women. Clin. Endocrinol. 2005, 62, 738–741. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Lim, Y.; Kim, J.H.; Bae, S.; Oh, S.; Hong, Y. Association of serum 25-hydroxyvitamin d levels with markers for metabolic syndrome in the elderly: A repeated measure analysis. J. Korean Med. Sci. 2012, 27, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, E.B.; Nahas-Neto, J.; Bueloni-Dias, F.; Poloni, P.F.; Orsatti, C.L.; Nahas, E.A.P. Vitamin D deficiency is associated with metabolic syndrome in postmenopausal women. Maturitas 2018, 107, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Song, H.R.; Park, C.H. Low serum vitamin D level is associated with high risk of metabolic syndrome in post-menopausal women. J. Endocrinol. Investig. 2013, 36, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Song, B.M.; Rhee, Y.; Kim, C.O.; Youm, Y.; Kim, K.M.; Lee, E.Y.; Lee, J.M.; Yoon, Y.M.; Kim, H.C. Urban-rural differences explain the association between serum 25-hydroxyvitamin D level and insulin resistance in Korea. Nutrients 2014, 6, 5806–5818. [Google Scholar] [CrossRef] [PubMed]
- Sulistyoningrum, D.C.; Green, T.J.; Lear, S.A.; Devlin, A.M. Ethnic-specific differences in vitamin d status is associated with adiposity. PLoS ONE 2012, 7, e43159. [Google Scholar] [CrossRef] [PubMed]
- Vilarrasa, N.; Maravall, J.; Estepa, A.; Sanchez, R.; Masdevall, C.; Navarro, M.A.; Alia, P.; Soler, J.; Gomez, J.M. Low 25-hydroxyvitamin D concentrations in obese women: Their clinical significance and relationship with anthropometric and body composition variables. J. Endocrinol. Investig. 2007, 30, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Vilarrasa, N.; Vendrell, J.; Maravall, J.; Elio, I.; Solano, E.; Jose, P.S.; Garcia, I.; Virgili, N.; Soler, J.; Gomez, J.M. Is plasma 25(OH) D related to adipokines, inflammatory cytokines and insulin resistance in both a healthy and morbidly obese population? Endocrine 2010, 38, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Vitezova, A.; Zillikens, M.C.; Herpt, T.T.W.V.; Sijbrands, E.J.G.; Hofman, A.; Uitterlinden, A.G.; Franco, O.H.; Jong, J.C.K. Vitamin D status and metabolic syndrome in the elderly: The Rotterdam Study. European J. Endocrinol. 2015, 172, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Vuksanovic, M.; Mihajlovic, G.; Zivkovic, T.B.; Gavrilovic, A.; Arsenovic, B.; Svorcan, J.Z.; Petkovic, M.M.; Vujovic, S. Cross-talk between muscle and bone in postmenopausal women with hypovitaminosis D. Climacteric 2017, 20, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Zargar, A.H.; Ahmad, S.; Masoodi, S.R.; Wani, A.I.; Bashir, M.I.; Laway, B.A.; Shah, Z.A. Vitamin D status in apparently healthy adults in Kashmir Valley of Indian subcontinent. Postgrad. Med. J. 2007, 83, 713–716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, F.F.; Al Hooti, S.; Al Zenki, S.; Alomirah, H.; Jamil, K.M.; Rao, A.; Al Jahmah, N.; Saltzman, E.; Ausman, L.M. Vitamin D deficiency is associated with high prevalence of diabetes in Kuwaiti adults: Results from a national survey. BMC Public Health 2016, 16, 100. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.; Kim, K.; Kim, B.; Kim, C.; Kang, S.K.; Mok, J. Relationship between vitamin D status and vascular complications in patients with type 2 diabetes mellitus. Nutr. Res. 2015, 36, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Kavaric, S.; Vuksanovic, M.; Bozovic, D.; Jovanovic, M.; Jeremic, V.; Radojici, Z.; Peki, S.; Popovic, V. Body weight and waist circumference as predictors of vitamin D deficiency in patients with type 2 diabetes and cardiovascular disease. Vojnosanit. Pregl. 2013, 70, 163–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmadieh, H.; Azar, S.T.; Lakkis, N.; Arabi, A. Hypovitaminosis d in patients with type 2 diabetes mellitus: A relation to disease control and complications. ISRN Endocrinol. 2013, 2013, 641098. [Google Scholar] [CrossRef] [PubMed]
- Bonakdaran, S.; Varasteh, A. Correlation between serum 25 (OH) vitamin D3 and laboratory risk markers of cardiovascular disease in type 2 diabetic patients. Saudi Med. J. 2009, 30, 509–514. [Google Scholar] [PubMed]
- Cai, X.; Hu, Z.; Chen, L.; Han, X.; Ji, L. Analysis of the associations between Vitamin D and albuminuria or beta-Cell function in Chinese type 2 Diabetes. Biomed. Res. Int. 2014, 2014, 640909. [Google Scholar] [CrossRef] [PubMed]
- Cigolini, M.; Iagulli, M.; Miconi, V.; Galiotto, M.; Lombardi, S.; Targher, G. Serum 25-hydroxyvitamin D3 concentrations and prevalence of cardiovascular disease among type 2 diabetic patients. Diabetes Care 2006, 29, 722–724. [Google Scholar] [CrossRef] [PubMed]
- Dalgard, C.; Petersen, M.S.; Weihe, P.; Grandjean, P. Vitamin D status in relation to glucose metabolism and type 2 diabetes in septuagenarians. Diabetes Care 2011, 34, 1284–1288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.; Wu, X.; Fu, Q.; Li, Y.; Yang, T.; Tang, W. The relationship between serum 25-hydroxyvitamin D and insulin sensitivity and beta cell function in newly diagnosed Type 2 Diabetes. J. Diabetes Res. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Haidari, F.; Zakerkish, M.; Karandish, M.; Saki, A.; Pooraziz, S. Association between serum vitamin d level and glycemic and inflammatory markers in non-obese patients with type 2 Diabetes. Iran. J. Med. Sci. 2016, 41, 367–373. [Google Scholar]
- Kondo, M.; Toyoda, M.; Miyatake, H.; Tanaka, E.; Koizumi, M.; Komaba, H.; Kimura, M.; Umezono, T.; Fukagawa, M. The Prevalence of 25-hydroxyvitamin D Deficiency in Japanese patients with diabetic nephropathy. Intern. Med. 2016, 55, 2555–2562. [Google Scholar] [CrossRef] [PubMed]
- Maser, R.E.; Lenhard, M.J.; Pohlig, R.T. Vitamin D insufficiency is associated with reduced parasymthetic nerve function in type 2 diabetes. Endocr. Pract. 2015, 21, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Minambres, I.; Sánchez-Quesada, J.L.; Vinagre, I.; Sánchez-Hernández, J.; Urgell, E.; de Leiva, A.; Pérez, A. Hypovitaminosis D in type 2 diabetes: Relation with features of the metabolic syndrome and glycemic control. Endocr. Res. 2015, 40, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.T.; Guo, J.F.; Mei, S.; Zhan, M.; Hu, Z.; Zhong, C.; Zeng, C.; Liu, X.; Ma, Q.; Li, B.; et al. Vitamin D deficiency in relation to risk of metabolic syndrome in middle aged and elderly patients with type 2 diabetes Mellitus. J. Nutr. Sci. Vitaminol. 2016, 62, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Raskajr, I.; Raskova, M.; Zikan, V.; Skrha, J. High Prevalence of Hypovitaminosis D in postmenopausal women with type 2 diabetes mellitus. Prague Med. Rep. 2016, 117, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Sadiya, A.; Ahmed, S.M.; Skaria, S.; Abusnana, S. Vitamin D status and its relationship with metabolic markers in persons with obesity and type 2 diabetes in the UAE: A cross-sectional study. J. Diabetes Res. 2014, 2014, 869307. [Google Scholar] [CrossRef] [PubMed]
- Sheth, J.J.; Shah, A.; Sheth, F.J.; Trivedi, S.; Lele, M.; Shah, N.; Thakor, P.; Vaidya, R. Does vitamin D play a significant role in type 2diabetes? BMC Endocr. Disord. 2015, 2015, 15. [Google Scholar]
- Yilmaz, H.; Kaya, M.; Sahin, M.; Delibasi, T. Is vitamin D status a predictor glycaemic regulation and cardiac complication in type 2 diabetes mellitus patients? Diabetes Metab. Syndr. Clin. Res. Rev. 2012, 6, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Bischof, M.G.; Heinze, G.; Vierhapper, H. Vitamin D status and its relation to age and body mass index. Horm. Res. 2006, 66, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Coney, P.; Demers, L.M.; Dodson, W.C.; Kunselman, A.R.; Ladson, G.; Legro, R.S. Determination of vitamin D in relation to body mass index and race in a defined population of black and white women. Int. J. Gynaecol. Obstet. 2012, 119, 21–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, X.; Wang, Y.; Hou, Y.; Han, F.; Ren, J.; Hu, Z. Vitamin D deficiency and related risk factors in patients with diabetic nephropathy. J. Int. Med. Res. 2016, 44, 673–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Didriksen, A.; Grimnes, G.; Hutchinson, K.M.; Svartberg, J.; Ragnar, M.; Joakimsen, R.; Rolf, J. The serum 25-hydroxyvitamin D response to vitamin D supplementation is related to genetic factors, BMI, and baseline levels. Eur. J. Endocrinol. 2013, 169, 559–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guasch, A.; Bullo, M.; Rabassa, A.; Bonada, A.; Castillo, D.D.; Sabench, F.; Salas-Salvado, J. Plasma vitamin D and parathormone are associated with obesity and atherogenic dyslipidemia: A cross-sectional study. Cardiovasc. Diabetol. 2012, 11, 149. [Google Scholar] [CrossRef] [PubMed]
- Konradsen, S.; Ag, H.; Lindberg, F.; Hexeberg, S.; Jorde, R. Serum 1,25-dihydroxy vitamin D is inversely associated with body mass index. Eur. J. Nutr. 2008, 47, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Lagunov, A.; Porojnicu, A.C.; Lindberg, F.; Hexeberg, S.; Moan, J. The dependency of vitamin D status on body mass index, gender, age and season. Anticancer Res. 2009, 29, 3713–3720. [Google Scholar] [CrossRef]
- LeBlanc, E.S.; Rizzo, J.H.; Pedula, K.L.; Ensrud, K.E.; Cauley, J.; Hochberg, M.; Hillier, T.A. Associations between 25-hydroxyvitamin D and weight gain in elderly women. J. Womens Health 2012, 21, 10. [Google Scholar] [CrossRef] [PubMed]
- Mai, X.; Chen, Y.; Camargo, C.A.; Langhammer, A. Cross-sectional and prospective cohort study of serum 25-hydroxyvitamin D level and obesity in adults. Am. J. Epidemiol. 2012, 175, 10. [Google Scholar] [CrossRef] [PubMed]
- Araghi, S.O.; Dijk, S.C.V.; Ham, A.C.; Brouwer-brolsma, E.M.; Enneman, A.W.; Sohl, E.; Swart, K.M.A.; Zwaluw, N.L.V.D.; Wijngaarden, J.P.V.; Dhonukshe-rutten, R.A.M.; et al. Bmi and body fat mass is inversely associated with vitamin d levels in older individuals. J. Nutr. Health Aging 2015, 19, 980–985. [Google Scholar] [CrossRef]
- Ou, H.; Karnchanasorn, R.; Leen, L.Z.; Chiu, K.C. 2011 interaction of BMI with vitamin D and insulin Sensitivity. Eur. J. Clin. Investig. 2011, 41, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Palacios, C.; Cynthia, K.G.; Perez, M.; Joshipura, K. Determinants of vitamin D status among overweight and obese puerto rican adults. Ann. Nutr. Metab. 2012, 60, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Li, L. Serum 25-hydroxyvitamin D level and diabetic nephropathy in patients with type 2 diabetes mellitus. Int. Urol. Nephrol. 2015, 47, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Perez-Diaz, I.; Sebastian-Barajas, G.; Hernandez-Flores, Z.G.; Rivera-Moscoso, R.; Osorio-Landa, H.K.; Flores-Rebollar, A. The impact of vitamin D levels on glycemic control and bone mineral density in postmenopausal women with type 2 diabetes. J. Endocrinol. Investig. 2015, 38, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
- Shinkov, A.; Borissova, A.M.; Dakovska, L.; Vlahov, J.; Kassabova, L.; Svinarov, D. Winter 25-hydroxyvitamin D levels in young urban adults are affected by smoking, body mass index and educational level. Eur. J. Clin. Nutr. 2015, 69, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.S.; Weinheimer-Haus, E.M.; Fleet, J.C.; Peacock, M.; Campbell, W.W. The apparent relation between plasma 25-hydroxyvitamin D and insulin resistance is largely attributable to central adiposity in overweight and obese adults. J. Nutr. 2015, 145, 2683–2689. [Google Scholar] [CrossRef] [PubMed]
- Godala, M.; Materek-Kusmierkiewicz, I.; Moczulski, D.; Rutkowski, M.; Szatko, F.; Gaszynska, E.; Tokarski, S.; Kowalski, J. Metabolic Syndrome Increases the Risk of Plasma Vitamin A, C, E and D Deficiency. Endocrinol. Metab. Syndr. 2016, 5, 5. [Google Scholar]
- Forouhi, N.G.; Luan, J.; Cooper, A.; Boucher, B.J.; Wareham, N.J. Baseline serum 25-hydroxy vitamin D is predictive of future glycemic status and insulin resistance. Diabetes 2008, 57, 2619–2625. [Google Scholar] [CrossRef] [PubMed]
- Han, S.S.; Kim, M.H.; Lee, S.M.; Lee, J.P.; Kim, S.; Joo, K.W.; Lim, C.S.; Kim, Y.S.; Kim, D.K. Association between body fat and vitamin D status in Korean adults. Asia Pac. J. Clin. Nutr. 2014, 23, 65–75. [Google Scholar] [PubMed]
- Looker, A.C. Body fat and vitamin D status in black versus white women. J. Clin. Endocrinol. Metab. 2005, 90, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Mason, C.; Xiao, L.; Imayama, I.; Duggan, C.R.; Bain, C.; Foster-Schubert, K.E.; Kong, A.; Campbell, K.L.; Wang, C.; Neuhouser, M.L.; et al. Effects of weight loss on serum vitamin D in postmenopausal women. Am. J. Clin. Nutr. 2011, 94, 95–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pilz, S.; Hurk, K.V.D.; Nijpels, G.; Stehouwer, C.D.A.; Riet, E.V.; Kienreich, K.; Tomaschitz, A.; Dekker, J.M. Vitamin D status, incident diabetes and prospective changes in glucose metabolism in older subjects: The Hoorn study. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Sergi, G.; Rui, M.D.; Bolzetta, F.; Toffanello, E.D.; Zambon, S.; Corti, M.; Sartori, L.; Musacchio, E.; Baggio, G.; et al. Serum 25-Hydroxyvitamin D and incidence of diabetes in elderly people: The PRO.V.A. study. J. Clin. Endocrinol. Metab. 2014, 99, 2351–2358. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, H.M.; Mavroeidi, A.; Barr, R.J.; Black, A.J.; Fraser, W.; Reid, D.M. Vitamin D status in postmenopausal women living at higher latitudes in the UK in relation to bone health, overweight, sunlight exposure and dietary vitamin D. Bone 2008, 42, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Mansuri, S.; Badawi, A.; Kayaniyil, S.; Cole, D.; Harris, S.B.; Mamakeesick, M.; Maguire, J.; Zinman, B.; Connelly, P.W.; Hanley, A.J. Associations of circulating 25(OH)D with cardiometabolic disorders underlying type 2 diabetes mellitus in an Aboriginal Canadian community. Diabetes Res. Clin. Pract. 2015, 109, 440–449. [Google Scholar] [CrossRef] [PubMed]
- McGill, A.; Stewart, J.M.; Lithander, F.E.; Strik, C.M.; Poppitt, S.D. Relationships of low serum vitamin D3 with anthropometry and markers of the metabolic syndrome and diabetes in overweight and obesity. Nutr. J. 2008, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Moy, F.; Bulgiba, A. High prevalence of vitamin D insufficiency and its association with obesity and metabolic syndrome among Malay adults in Kuala Lumpur, Malaysia. Moy and Bulgiba. BMC Public Health 2011, 11, 735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellan, M.; Guzzaloni, G.; Rinaldi, M.; Merlotti, E.; Ferrari, C.; Tagliaferri, A.; Pirisi, M.; Aimaretti, G.; Scacchi, M.; Marzullo, P. Altered glucose metabolism rather than naïve type 2 diabetes mellitus (T2DM) is related to vitamin D status in severe obesity. Cardiovasc. Diabetol. 2014, 13, 57. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.; Hong, S. Vitamin D status and its association with cardiometabolic risk factors in Korean adults based on a 2008-2010 Korean national health and nutrition examination survey nutrition research and practice. Nutr. Res. Pract. 2013, 7, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Botella-Carretero, J.I.; Alvarez-Blasco, F.; Villafruela, J.J.; Balsa, J.A.; Vazquez, C.; Escobar-Morreale, H.F. Vitamin D deficiency is associated with the metabolic syndrome in morbid obesity. Clin. Nutr. 2007, 26, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.M.; Rutter, M.K.; Neill, T.W.O.; Boonen, S.; vanderschueren, D.; Bouillon, R.; Bartfai, G.; Casanueva, F.F.; Finn, J.D.; Forti, G.; et al. The European male ageing study group. Vitamin D, parathyroid hormone and the metabolic syndrome in middle-aged and older European men. Eur. J. Endocrinol. 2009, 161, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Yu, Z.; Pan, A.; Hu, F.B.; Franco, O.H.; Li, H.; Li, X.; Yang, X.; Chen, Y.; Lin, X. Plasma 25-hydroxyvitamin D concentration and metabolic syndrome among middle-aged and elderly Chinese individuals. Diabetes Care 2009, 32, 1278–1283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreozzi, P.; Verrusio, W.; Viscogliosi, G.; Summa, M.L.; Gueli, N.O.; Cacciafesta, M.; Albanese, C.V. Relationship between vitamin D and body fat distribution evaluated by DXA in postmenopausal women. Nutrition 2016, 32, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D: Important for prevention of osteoporosis, cardiovascular heart disease, type 1 diabetes, autoimmune diseases, and some cancers. South Med. J. 2005, 98, 1024–1027. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.E.; Kong, J.; Zhang, W.; Szeto, F.L.; Ye, H.; Deb, D.K.; Brady, M.J.; Li, Y.C. Targeted expression of human vitamin D receptor in adipocytes decreases energy expenditure and induces obesity in mice. J. Biol. Chem. 2011, 286, 33804–33810. [Google Scholar] [CrossRef] [PubMed]
- Stein, E.M.; Strain, G.; Sinha, N.; Ortiz, D.; Pomp, A.; Dakin, G.; McMahon, D.J.; Bockman, R.; Silverberg, S.J. Vitamin D insufficiency prior to bariatric surgery: Risk factors and a pilot treatment study. Clin. Endocrinol. 2009, 71, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Barnard, W.F.; Saxena, V.K.; Wenny, B.N.; DeLuisi, J.J. Daily surface UV exposure and its relationship to surface pollutant measurements. J. Air Waste Manag. Assoc. 2003, 53, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Elminir, H.K. Sensitivity of ultraviolet solar radiation to anthropogenic air pollutants and weather conditions. Atmos. Res. 2007, 84, 250–264. [Google Scholar] [CrossRef]
- Dahifar, H.; Faraji, A.; Ghorbani, A.; Yassobi, S. Impact of dietary and lifestyle on vitamin D in healthy student girls aged 11–15 years. J. Med. Investig. 2006, 53, 204–208. [Google Scholar] [CrossRef]
- Wang, Y.; Beydoun, M.A. The obesity epidemic in the United States–gender, age, socioeconomic, racial/ethnic, and geographic characteristics: A systematic review and meta-regression analysis. Epidemiol. Rev. 2007, 29, 26–28. [Google Scholar] [CrossRef] [PubMed]
- Eartman, C.P.; Beckman, L.M.; Masdkark, K.; Sibley, S.D. The link between obesity and low circulating 25 hydroxy vitamin D concentrations: Considerations and implications. Int. J. Obes. 2012, 36, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Drincic, A.T.; Armas, L.A.; Diest, E.E.V.; Heaney, R.P. Volumetric dilution, rather than sequestration best explains the low vitamin D status of obesity. Obesity 2012, 20, 1444–1448. [Google Scholar] [CrossRef] [PubMed]
- Pathak, K.; Soares, M.J.; Calton, E.K.; Hallet, J. Vitamin D supplementation and body weight status: A systematic review and meta-analysis of randomized controlled trials. Obes. Rev. 2014, 15, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Prodam, F.; Zanetta, S.; Ricotti, R.; Marolda, A.; Giglione, E.; Monzan, A.; Walker, G.E.; Rampone, S.; Castagno, M.; Bellone, S.; et al. Influence of Ultraviolet Radiation on the Association between 25-Hydroxy Vitamin D Levels and Cardiovascular Risk Factors in Obesity. J. Pediatr. 2016, 171, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Welty, F.K.; Alfaddagh, A.; Elajami, T.K. Targeting inflammation in metabolic syndrome. Transl. Res. 2015, 167, 257–280. [Google Scholar] [CrossRef] [PubMed]
- Ghashut, R.A.; Talwar, D.; Kinsella, J.; Duncan, A.; McMillan, D.C. The effect of the systematic inflammatory response on plasma vitamin 25 (OH) D concentrations adjusted for albumen. PLoS ONE 2014, 9, e92614. [Google Scholar] [CrossRef] [PubMed]
- Liefaard, M.C.; Ligtharts, S.; Vitezova, A.; Hofman, A.; Uitterlinden, A.G.; de Jong, J.C.K.; Franco, O.H.; Zillikens, M.C.; Dehghan, A. Vitamin D and C-reactive protein: A Mendelian randomization study. PLoS ONE 2015, 10, e0131740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slusher, A.L.; McAllister, M.J.; Huang, C.J. A therapeutic role of vitamin D on obesity associated inflammation and weight loss intervention. Inflamm. Res. 2015, 64, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Maki, K.C.; Fulgoni, V.L.; Keast, D.R.; Rains, T.M.; Park, K.M.; Rubin, M.R. Vitamin D intake and status are associated with lower prevalence of metabolic syndrome in U.S. adults: National health and nutrition examination surveys 2003–2006. Metab. Syndr. Relat. Disord. 2012, 10, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Wynne, K.S.; McGowan, S.B.; Bloom, S. Appetite control. J. Endocrinol. 2005, 184, 291–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akiyama, M.; Okada, Y.; Kanai, M.; Takahashi, A.; Momozawa, Y.; Ikeda, M.; Iwata, N.; Ikegawa, S.; Hirata, M.; Matsuda, K.; et al. Genome-wide association study identifies 112 new loci for body mass index in the Japanese population. Nat. Genet. 2017, 49, 1458–1467. [Google Scholar] [CrossRef] [PubMed]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rafiq, S.; Jeppesen, P.B. Body Mass Index, Vitamin D, and Type 2 Diabetes: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 1182. https://doi.org/10.3390/nu10091182
Rafiq S, Jeppesen PB. Body Mass Index, Vitamin D, and Type 2 Diabetes: A Systematic Review and Meta-Analysis. Nutrients. 2018; 10(9):1182. https://doi.org/10.3390/nu10091182
Chicago/Turabian StyleRafiq, Shamaila, and Per Bendix Jeppesen. 2018. "Body Mass Index, Vitamin D, and Type 2 Diabetes: A Systematic Review and Meta-Analysis" Nutrients 10, no. 9: 1182. https://doi.org/10.3390/nu10091182




