Effects of Genistein on Differentiation and Viability of Human Visceral Adipocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation of Pre-Adipocyte from Adipose Tissue
2.2. Culture, Expansion and Treatment of Pre-Adipocytes
2.3. Oil-Red O Staining
2.4. Cell Viability
2.5. Mitochondrial Membrane Potential Measurement
2.6. ROS Quantification
2.7. Cell Lysates
2.8. Western Blotting
2.9. Statistical Analysis
3. Results
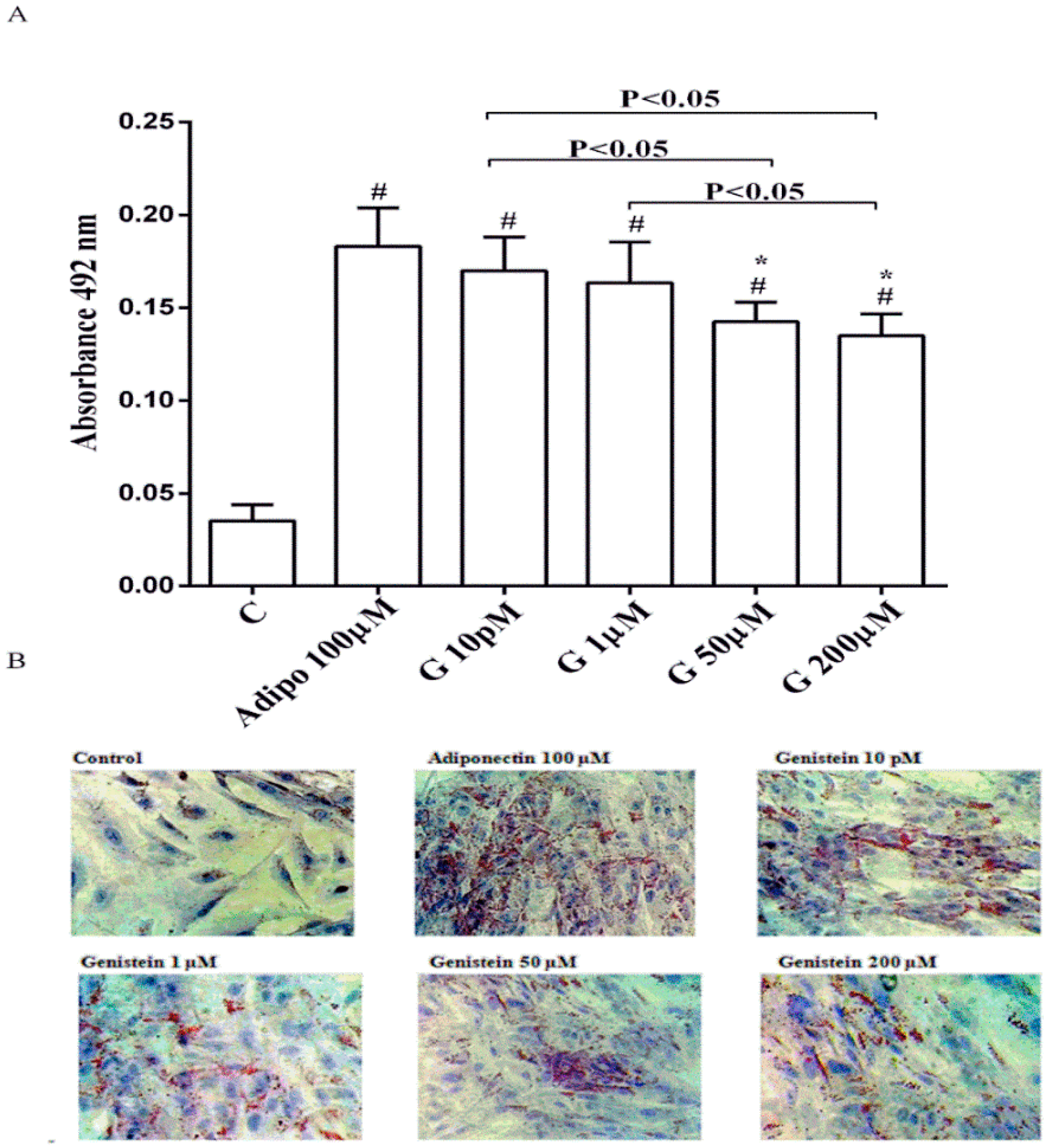
3.1. Effects of Genistein on Human Visceral Pre-Adipocytes Differentiation in Physiological Conditions
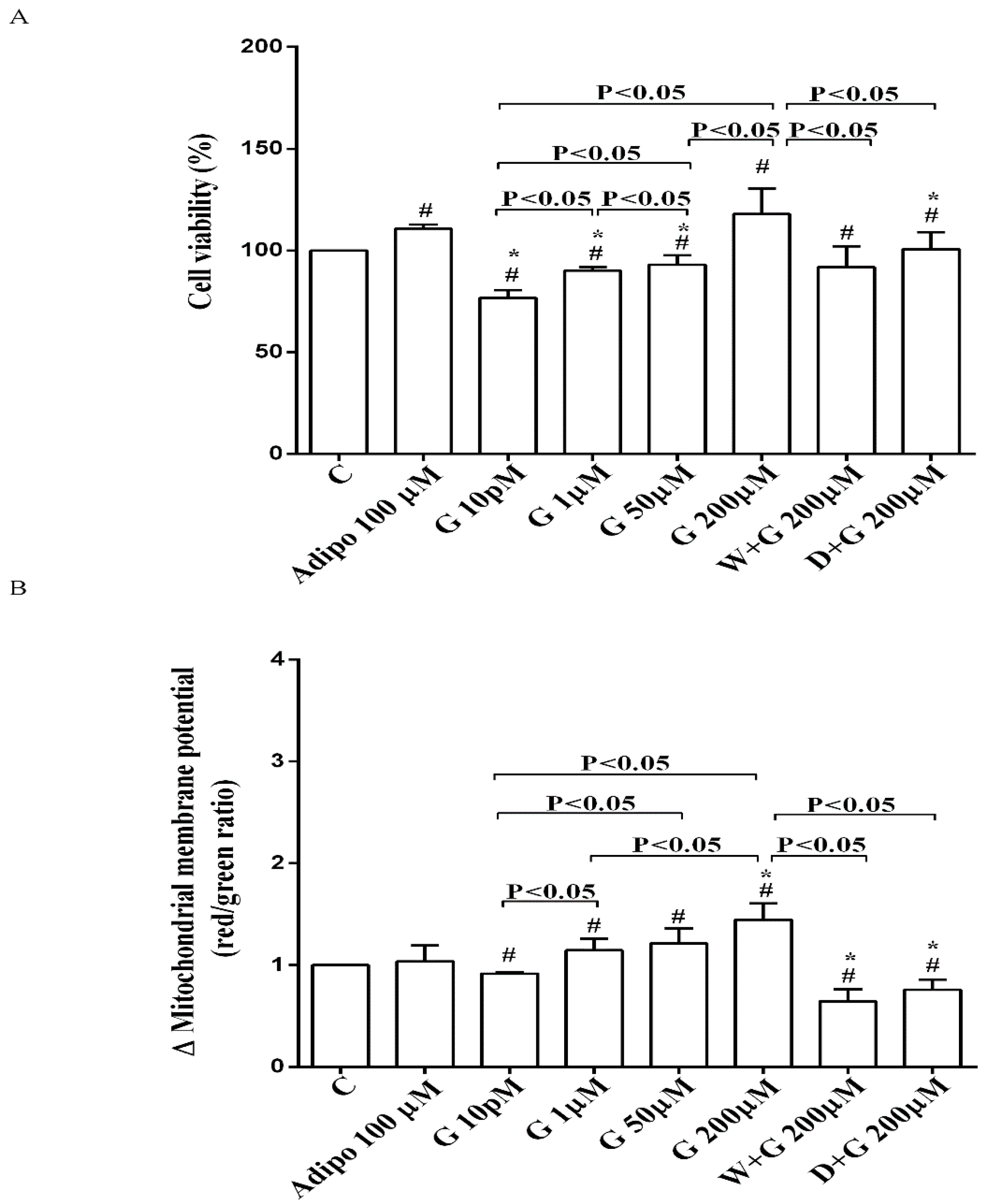
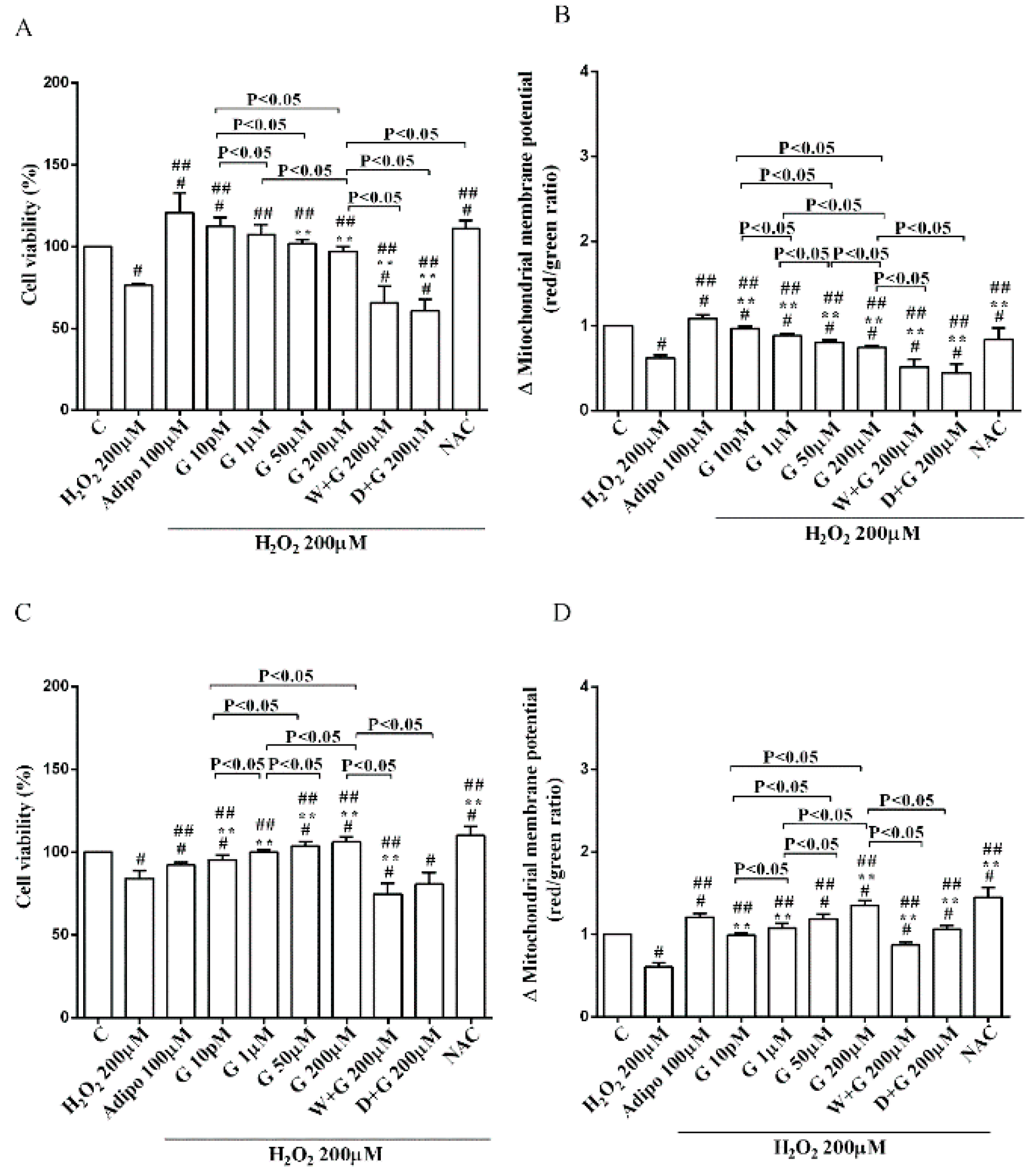
3.2. Effects of Genistein on Cell Viability and Mitochondrial Membrane Potential in Human Visceral Pre-Adipocytes/Adipocytes Cultured in Physiological and Peroxidative Conditions
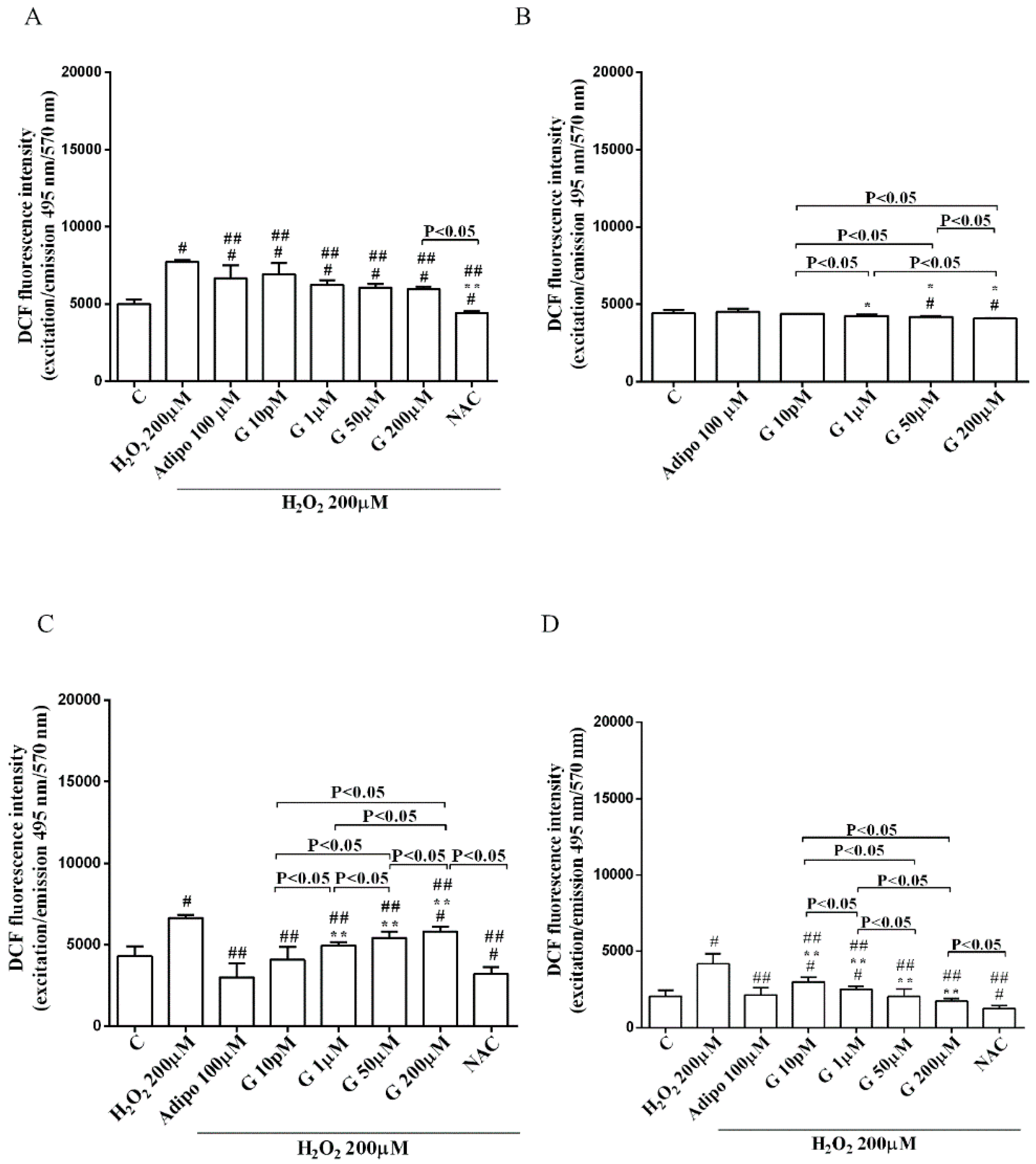
3.3. Effects of Genistein on ROS Production in Human Visceral Pre-Adipocytes/Adipocytes Cultured in Peroxidative Conditions
3.4. Expression of UCP1 in Human Visceral Pre-Adipocytes/Adipocytes Cultured in Physiological and Peroxidative Conditions
3.5. Involvement of Akt, Mfn2, and AMPKα/β in the Effects of Genistein in Human Visceral Pre-Adipocytes/Adipocytes Cultured in Physiological and Peroxidative Conditions
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hwang, J.T.; Park, I.J.; Shin, J.I.; Lee, Y.K.; Lee, S.K.; Baik, H.W.; Ha, J.; Park, O.J. Genistein, EGCG, and capsaicin inhibit adipocyte differentiation process via activating AMP–activated protein kinase. Biochem. Biophys. Res. Commun. 2005, 16, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Nouredine, B.; Guanzhong, W. Genistein: A promising therapeutic agent for obesity and diabetes treatment. Eur. J. Pharmacol. 2013, 698, 31–38. [Google Scholar]
- Rayalam, S.; Della-Fera, M.A.; Yang, J.Y.; Park, H.J.; Ambati, S.; Baile, C.A. Resveratrol potentiates genistein’s antiadipogenic and proapoptotic effects in 3T3-L1 adipocytes. J. Nutr. 2007, 137, 2668–2673. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.; Jain, S.K. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, D.A.; Prior, S.L.; Barry, J.D.; Caplin, S.; Baxter, J.N.; Stephens, J.W. Changes in markers of oxidative stress and DNA damage in human visceral adipose tissue from subjects with obesity and type 2 diabetes. Diabetes Res. Clin. Pract. 2014, 106, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Johannes, K.; Mathias, F.; Moriko, I.; Bradford, B.L.; Manuel, B.; Ronald, K. β3-Adrenergic Stimulation Differentially Inhibits Insulin Signaling and Decreases Insulin-induced Glucose Uptake in Brown Adipocytes. J. Biol. Chem. 1999, 274, 34795–34802. [Google Scholar]
- Martinez, J.A. Mitochondrial oxidative stress and inflammation: An slalom to obesity and insulin resistance. J. Physiol. Biochem. 2006, 62, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.I.; Naz, L.; Yasmeen, G. Obesity: An independent risk factor for systemic oxidative stress. Pak. J. Pharm. Sci. 2006, 19, 62–65. [Google Scholar] [PubMed]
- Ku, C.R.; Cho, Y.H.; Hong, Z.Y.; Lee, H.; Lee, S.J.; Hong, S.S.; Lee, E.J. The Effects of High Fat Diet and Resveratrol on Mitochondrial Activity of Brown Adipocytes. Endocrinol. Metab. 2016, 31, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Giordano, A.; Smorlesi, A.; Frontini, A.; Barbatelli, G.; Cinti, S. White, brown and pink adipocytes: The extraordinary plasticity of the adipose organ. Eur. J. Endocrinol. 2014, 170, R159–R171. [Google Scholar] [CrossRef] [PubMed]
- Aziz, S.A.; Wakeling, L.A.; Miwa, S.; Alberdi, G.; Hesketh, J.E.; Ford, D. Metabolic programming of a beige adipocyte phenotype by genistein. Mol. Nutr. Food Res. 2017, 61, 1600574. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P. Perspective: Does brown fat protect against diseases of aging? Ageing Res. Rev. 2010, 9, 69–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martini, C.N.; Gabrielli, M.; Brandani, J.N.; Vila Mdel, C. Glyphosate Inhibits PPAR Gamma Induction and Differentiation of Preadipocytes and is able to Induce Oxidative Stress. J. Biochem. Mol. Toxicol. 2016, 30, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Dang, Z.C. Dose-dependent effects of soy phyto-oestrogen genistein on adipocytes: Mechanisms of action. Obes. Rev. 2009, 10, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Kurrat, A.; Blei, T.; Kluxen, F.M.; Mueller, D.R.; Piechotta, M.; Soukup, S.T.; Kulling, S.E.; Diel, P. Lifelong exposure to dietary isoflavones reduces risk of obesity in ovariectomized Wistar rats. Mol. Nutr. Food Res. 2015, 59, 2407–2418. [Google Scholar] [CrossRef] [PubMed]
- Ganai, A.A.; Khan, A.A.; Malik, Z.A.; Farooqi, H. Genistein modulates the expression of NF-κB and MAPK (p-38 and ERK1/2), thereby attenuating d-Galactosamine induced fulminant hepatic failure in Wistar rats. Toxicol. Appl. Pharmacol. 2015, 283, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Liu, H.; Jiang, Z. Genistein Ameliorates Fat Accumulation Through AMPK Activation in Fatty Acid-Induced BRL Cells. J. Food Sci. 2017, 82, 2719–2725. [Google Scholar] [CrossRef] [PubMed]
- Borrás, C.; Gambini, J.; Gómez-Cabrera, M.C.; Sastre, J.; Pallardó, F.V.; Mann, G.E.; Vi-a, J. Genistein, a soy isoflavone, up-regulates expression of antioxidant genes: Involvement of estrogen receptors, ERK1/2, and NFκB. FASEB J. 2006, 20, 2136–2138. [Google Scholar] [CrossRef] [PubMed]
- Surico, D.; Ercoli, A.; Farruggio, S.; Raina, G.; Filippini, D.; Mary, D.; Minisini, R.; Surico, N.; Pirisi, M.; Grossini, E. Modulation of Oxidative Stress by 17 β-Estradiol and Genistein in Human Hepatic Cell Lines In Vitro. Cell Physiol. Biochem. 2017, 42, 1051–1062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szkudelska, K.; Nogowski, L.; Szkudelski, T. Resveratrol and genistein as adenosine triphosphate-depleting agents in fat cells. Metabolism 2011, 60, 720–729. [Google Scholar] [CrossRef] [PubMed]
- Bunnell, B.A.; Flaat, M.; Gagliardi, C.; Patel, B.; Rippol, C. Adipose-derived stem cells: Isolation, expansion and differentiation. Sci. Direct. Methods 2008, 45, 115–120. [Google Scholar] [CrossRef] [PubMed]
- De Cillà, S.; Farruggio, S.; Vujosevic, S.; Raina, G.; Filippini, D.; Gatti, V.; Clemente, N.; Mary, D.; Vezzola, D.; Casini, G.; et al. Anti-Vascular Endothelial Growth Factors Protect Retinal Pigment Epithelium Cells Against Oxidation by Modulating Nitric Oxide Release and Autophagy. Cell Physiol. Biochem. 2017, 42, 1725–1738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Surico, D.; Farruggio, S.; Marotta, P.; Raina, G.; Mary, D.; Surico, N.; Vacca, G.; Grossini, E. Human Chorionic Gonadotropin Protects Vascular Endothelial Cells from Oxidative Stress by Apoptosis Inhibition, Cell Survival Signalling Activation and Mitochondrial Function Protection. Cell Physiol. Biochem. 2015, 36, 2108–2120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valli, V.; Heilmann, K.; Danesi, F.; Bordoni, A.; Gerhäuser, C. Modulation of Adipocyte Differentiation and Proadipogenic Gene Expression by Sulforaphane, Genistein, and Docosahexaenoic Acid as a First Step to Counteract Obesity. Oxid. Med. Cell Longev. 2018, 2018, 1617202. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, B.; Liu, S.; Si, H. Antiadipogenic Effects and Mechanisms of Combinations of Genistein, Epigallocatechin-3-Gallate, and/or Resveratrol in Preadipocytes. J. Med. Food 2017, 20, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Liao, Q.C.; Li, Y.L.; Qin, Y.F.; Quarles, L.D.; Xu, K.K.; Li, R.; Zhou, H.H.; Xiao, Z.S. Inhibition of adipocyte differentiation by phytoestrogen genistein through a potential downregulation of extracellular signal-regulated kinases 1/2 activity. J. Cell Biochem. 2008, 104, 1853–1864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirota, K.; Morikawa, K.; Hanada, H.; Nonaka, M.; Nakajima, Y.; Kobayashi, M.; Nakajima, R. Effect of genistein and daidzein on the proliferation and differentiation of human preadipocyte cell line. J. Agric. Food Chem. 2010, 58, 5821–5827. [Google Scholar] [CrossRef] [PubMed]
- Sienkiewicz, P.; Surazyński, A.; Pałka, J.; Miltyk, W. Nutritional concentration of genistein protects human dermal fibroblasts from oxidative stress-induced collagen biosynthesis inhibition through IGF-I receptor-mediated signaling. Acta. Pol. Pharm. 2008, 65, 203–211. [Google Scholar] [PubMed]
- Nascimento, E.B.M.; Sparks, L.M.; Divoux, A.; Van Gisbergen, M.W.; Broeders, E.P.M.; Jörgensen, J.A.; Schaart, G.; Bouvy, N.D.; Van Marken Lichtenbelt, W.D.; Schrauwen, P. Genetic Markers of Brown Adipose Tissue Identity and In Vitro Brown Adipose Tissue Activity in Humans. Obesity (Silver Spring) 2018, 26, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Jin, W.; Pan, D. Ifi27 is indispensable for mitochondrial function and browning in adipocytes. Biochem. Biophys. Res. Commun. 2018, 501, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Boutant, M.; Kulkarni, S.S.; Joffraud, M.; Ratajczak, J.; Valera-Alberni, M.; Combe, R.; Zorzano, A.; Cantó, C. Mfn2 is critical for brown adipose tissue thermogenic function. EMBO J. 2017, 36, 1543–1558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soriano, F.X.; Liesa, M.; Bach, D.; Chan, D.C.; Palacín, M.; Zorzano, A. Evidence for a mitochondrial regulatory pathway defined by peroxisome proliferator-activated receptor-gamma coactivator-1 alpha, estrogen-related receptor-alpha, and mitofusin 2. Diabetes 2006, 55, 1783–1791. [Google Scholar] [CrossRef] [PubMed]
- Chan, X.H.D.; Balasundaram, G.; Attia, A.B.E.; Goggi, J.L.; Ramasamy, B.; Han, W.; Olivo, M.; Sugii, S. Multimodal Imaging Approach to Monitor Browning of Adipose Tissue In Vivo. J. Lipid. Res. 2018, 59, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G.; Carling, D.; Carlson, M. The AMP-activated/SNF1 protein kinase subfamily: Metabolic sensors of the eukaryotic cell? Annu. Rev. Biochem. 1998, 67, 821–855. [Google Scholar] [CrossRef] [PubMed]
- Kemp, B.E.; Stapleton, D.; Campbell, D.J.; Chen, Z.P.; Murthy, S.; Walter, M.; Gupta, A.; Adams, J.J.; Katsis, F.; van Denderen, B.; et al. AMP-activated protein kinase, super metabolic regulator. Biochem. Soc. Trans. 2003, 31, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Saha, A.K.; Xiang, X.; Ruderman, N.B. AMPK, the metabolic syndrome and cancer. Trends Pharmacol. Sci. 2005, 26, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.C.; Ding, Y.; Kim, J.; Kim, E.A.; Fernando, I.P.S.; Heo, S.J.; Lee, S.H. 3-Chloro-4,5-dihydroxybenzaldehyde inhibits adipogenesis in 3T3-L1 adipocytes by regulating expression of adipogenic transcription factors and AMPK activation. Chem. Biol. Interact. 2018, 287, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Mak, J.C.W.; Lee, M.Y.K.; Xu, A.; Ip, M.S.M. Low-Frequency Intermittent Hypoxia Promotes Subcutaneous Adipogenic Differentiation. Oxid. Med. Cell Longev. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Luo, N.; Klein, R.L.; Garvey, W.T. Adiponectin promotes adipocyte differentiation, insulin sensitivity, and lipid accumulation. J. Lipid Res. 2005, 46, 1369–1379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daval, M.; Foufelle, F.; Ferré, P. Functions of AMP-activated protein kinase in adipose tissue. J. Physiol. 2006, 574, 55–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gan, L.; Yan, J.; Liu, Z.; Feng, M.; Sun, C. Adiponectin prevents reduction of lipid-induced mitochondrial biogenesis via AMPK/ACC2 pathway in chicken adipocyte. J. Cell Biochem. 2015, 116, 1090–1100. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.; Choi, J.M.; Park, S.E.; Rhee, E.J.; Lee, W.Y.; Oh, K.W.; Park, S.W.; Park, C.Y. Adiponectin deletion impairs insulin signaling in insulin-sensitive but not insulin-resistant 3T3-L1 adipocytes. Life Sci. 2015, 132, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, M.; Lam, K.S.; Xu, A. Protective roles of adiponectin in obesity-related fatty liver diseases: Mechanisms and therapeutic implications. Arq. Bras. Endocrinol. Metabol. 2009, 53, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Shibata, M.; Hakuno, F.; Yamanaka, D.; Okajima, H.; Fukushima, T.; Hasegawa, T.; Ogata, T.; Toyoshima, Y.; Chida, K.; Kimura, K.; et al. Akt Paraquat-induced oxidative stress represses phosphatidylinositol 3-kinase activities leading to impaired glucose uptake in 3T3-L1 adipocytes. J. Biol. Chem. 2010, 285, 20915–20925. [Google Scholar] [CrossRef] [PubMed]
- Tatsumi, F.; Kaneto, H.; Hashiramoto, M.; Tawaramoto, K.; Obata, A.; Kimura, T.; Shimoda, M.; Hamamoto, S.; Kanda-Kimura, Y.; Kamei, S.; et al. Anti-hypertensive azelnidipine preserves insulin signaling and glucose uptake against oxidative stress in 3T3-L1 adipocytes. Endocr. J. 2015, 62, 741–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antonopoulos, A.S.; Margaritis, M.; Coutinho, P.; Shirodaria, C.; Psarros, C.; Herdman, L.; Sanna, F.; De Silva, R.; Petrou, M.; Sayeed, R.; et al. Adiponectin as a link between type 2 diabetes and vascular NADPH oxidase activity in the human arterial wall: the regulatory role of perivascular adipose tissue. Diabetes 2015, 64, 2207–2219. [Google Scholar] [CrossRef] [PubMed]
- Grossini, E.; Farruggio, S.; Qoqaiche, F.; Raina, G.; Camillo, L.; Sigaudo, L.; Mary, D.; Surico, N.; Surico, D. Monomeric adiponectin increases cell viability in porcine aortic endothelial cells cultured in normal and high glucose conditions: Data on kinases activation. Data Brief 2016, 10, 1381–1386. [Google Scholar] [CrossRef] [PubMed]
- Pidoux, G.; Witczak, O.; Jarnæss, E.; Myrvold, L.; Urlaub, H.; Stokka, A.J.; Küntziger, T.; Taskén, K. Optic atrophy 1 is an A-kinase anchoring protein on lipid droplets that mediates adrenergic control of lipolysis. EMBO J. 2011, 30, 4371–4386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hahn, W.S.; Kuzmicic, J.; Burrill, J.S.; Donoghue, M.A.; Foncea, R.; Jensen, M.D.; Lavandero, S.; Arriaga, E.A.; Bernlohr, D.A. Proinflammatory cytokines differentially regulate adipocyte mitochondrial metabolism, oxidative stress, and dynamics. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E1033–E1045. [Google Scholar] [CrossRef] [PubMed]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grossini, E.; Farruggio, S.; Raina, G.; Mary, D.; Deiro, G.; Gentilli, S. Effects of Genistein on Differentiation and Viability of Human Visceral Adipocytes. Nutrients 2018, 10, 978. https://doi.org/10.3390/nu10080978
Grossini E, Farruggio S, Raina G, Mary D, Deiro G, Gentilli S. Effects of Genistein on Differentiation and Viability of Human Visceral Adipocytes. Nutrients. 2018; 10(8):978. https://doi.org/10.3390/nu10080978
Chicago/Turabian StyleGrossini, Elena, Serena Farruggio, Giulia Raina, David Mary, Giacomo Deiro, and Sergio Gentilli. 2018. "Effects of Genistein on Differentiation and Viability of Human Visceral Adipocytes" Nutrients 10, no. 8: 978. https://doi.org/10.3390/nu10080978



