Bioavailability of a Novel Form of Microencapsulated Bovine Lactoferrin and Its Effect on Inflammatory Markers and the Gut Microbiome: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Approval
2.2. Study Population
2.3. Study Design
2.4. Study Intervention
2.5. Outcome Measures
2.5.1. Lactoferrin Absorption
2.5.2. CD4+/CD8+/CD69+ Analysis
2.5.3. Gut Microbiome
2.6. Statistical Analysis
3. Results
3.1. Participants
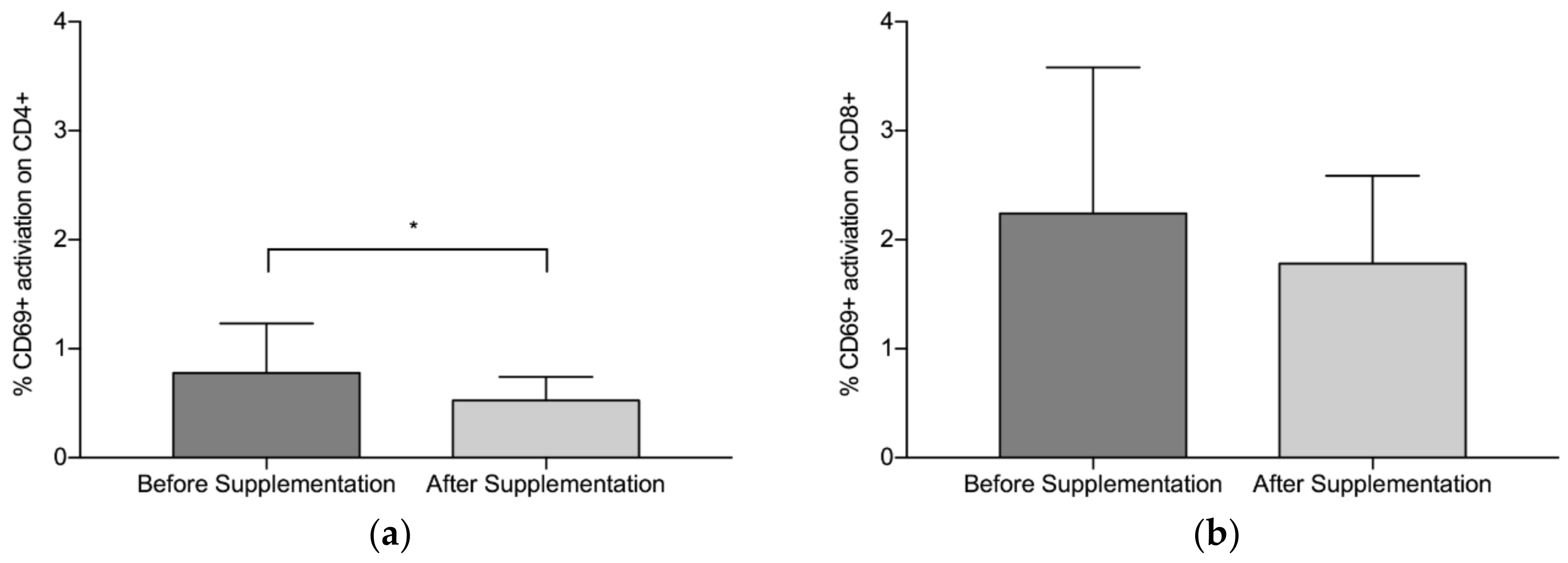
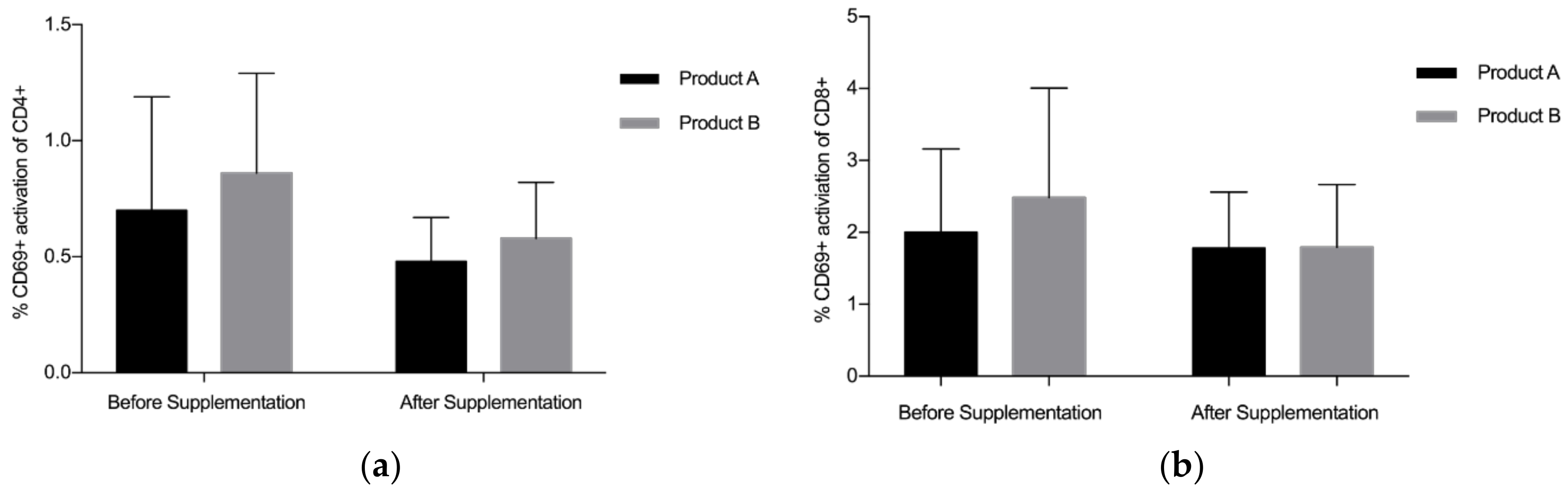
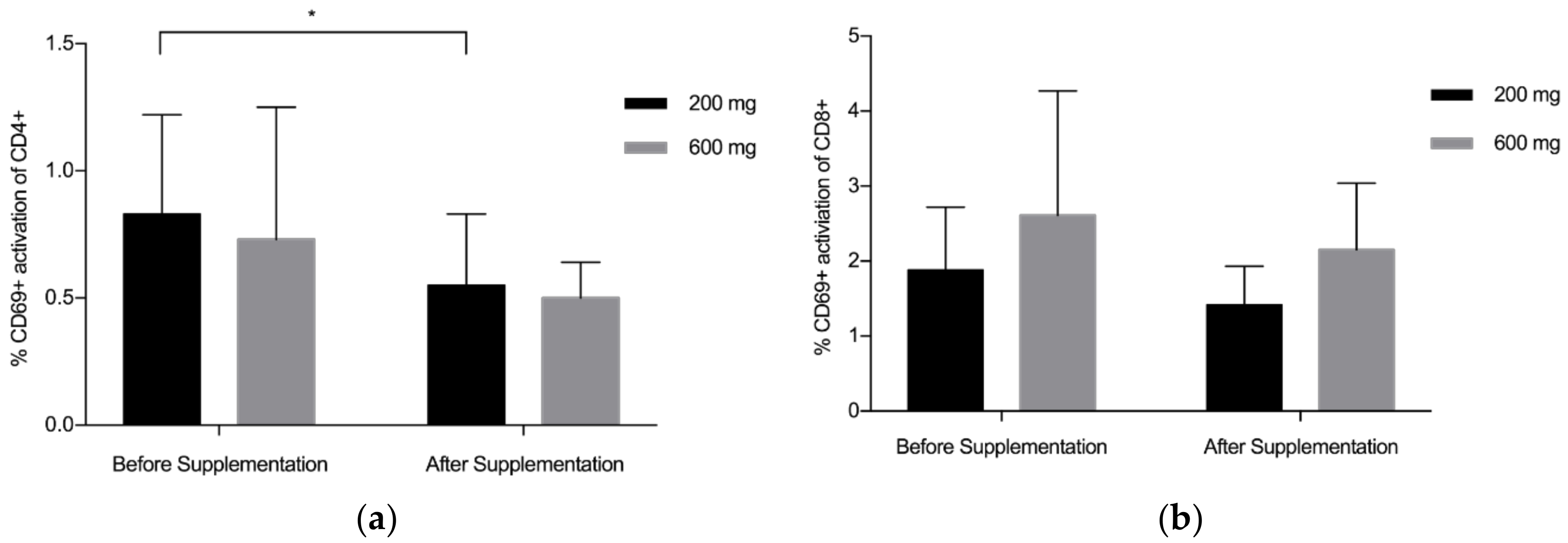
3.2. CD4+/CD8+/CD69+ Analysis
3.3. Lactoferrin Absorption
3.3.1. Faecal Samples
3.3.2. Serum Samples
3.4. Gut Microbiome
3.4.1. Metagenomic Sequencing
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lönnerdal, B.; Lyer, S. Lactoferrin: molecular structure and biological function. Annu. Rev. Nutr. 1995, 15, 93–110. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, L.; Calvo, M.; Brock, J.H. Biological role of lactoferrin. Arch. Dis. Child. 1992, 67, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Clare, D.A.; Catignani, G.L.; Swaisgood, H.E. Biodefense properties of milk: The role of antimicrobial proteins and peptides. Curr. Pharm. Des. 2003, 9, 1239–1255. [Google Scholar] [CrossRef] [PubMed]
- Baveye, S.; Elass, E.; Mazurier, J.; Spik, G.; Legrand, D. Lactoferrin: A multifunctional glycoprotein involved in the modulation of the inflammatory process. Clin. Chem. Lab. Med. 1999, 37, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Baynes, R.D.; Bezwoda, W.R. Lactoferrin and the inflammatory response. Adv. Exp. Med. Biol. 1994, 357, 133–141. [Google Scholar] [PubMed]
- Kruzel, M.L.; Harari, Y.; Mailman, D.; Actor, J.K.; Zimecki, M. Differential effects of prophylactic, concurrent and therapeutic lactoferrin treatment on LPS-induced inflammatory responses in mice. Clin. Exp. Immunol. 2002, 130, 25–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Legrand, D. Overview of Lactoferrin as a Natural Immune Modulator. J. Pediatr. 2016, 173, S10–S15. [Google Scholar] [CrossRef] [PubMed]
- Actor, J.K.; Hwang, S.H.; Kruzel, M.L. Lactoferrin as a natural immune modulator. Curr. Pharm. Des. 2009, 15, 1956–1973. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Timilsena, Y.P.; Blanch, E.; Adhikari, B. Lactoferrin: Structure, function, denaturation and digestion. Crit. Rev. Food Sci. Nutr. 2017, 21, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Bokkhim, H.; Bansal, N.; Grøndahl, L.; Bhandari, B. In-vitro digestion of different forms of bovine lactoferrin encapsulated in alginate micro-gel particles. Food Hydrocoll. 2016, 52, 231–242. [Google Scholar] [CrossRef] [Green Version]
- Bokkhim, H.; Bansal, N.; Grøndahl, L.; Bhandari, B. Characterization of alginate—lactoferrin beads prepared by extrusion gelation method. Food Hydrocoll. 2016, 53, 270–276. [Google Scholar] [CrossRef]
- Ching, S.H.; Bansal, N.; Bhandari, B. Alginate gel particles—A review of production techniques and physical properties. Crit. Rev. Food Sci. Nutr. 2017, 57, 1133–1152. [Google Scholar] [CrossRef] [PubMed]
- Ching, S.H.; Bansal, N.; Bhandari, B. Physical stability of emulsion encapsulated in alginate microgel particles by the impinging aerosol technique. Food Res. Int. 2015, 75, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Ammendolia, M.G.; Agamennone, M.; Pietrantoni, A.; Lannutti, F.; Siciliano, R.A.; De Giulio, B.; Amici, C.; Superti, F. Bovine lactoferrin-derived peptides as novel broad-spectrum inhibitors of influenza virus. Pathog. Glob. Health 2012, 106, 12–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jegasothy, H.; Weerakkody, R.; Selby-Pham, S.; Bennett, L. In vitro heme and non-heme iron capture from hemoglobin, myoglobin and ferritin by bovine lactoferrin and implications for suppression of reactive oxygen species in vivo. Biometals 2014, 27, 1371–1382. [Google Scholar] [CrossRef] [PubMed]
- Salama, M.A.; Alaa, B.I.; Manal, B.M.; Abdel-Kader, A.E. Development of New Strategy for Non-Antibiotic Therapy: Bovine Lactoferrin Has a Potent Antimicrobial and Immunomodulator Effects. Adv. Infect. Diseases 2013, 3, 185–192. [Google Scholar]
- Puddu, P.; Latorre, D.; Carollo, M.; Catizone, A.; Ricci, G.; Valenti, P.; Gessani, S.; Kaushal, D. Bovine Lactoferrin Counteracts Toll-Like Receptor Mediated Activation Signals in Antigen Presenting Cells. PLoS ONE 2011, 6, e22504. [Google Scholar] [CrossRef] [PubMed]
- Arciniega-Martínez, I.; Campos-Rodríguez, R.; Drago-Serrano, M.; Sánchez-Torres, L.; Cruz-Hernández, T.; Reséndiz-Albor, A. Modulatory Effects of Oral Bovine Lactoferrin on the IgA Response at Inductor and Effector Sites of Distal Small Intestine from BALB/c Mice. Arch. Immunol. Ther. Exp. (Warsz) 2016, 64, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.Y.; Comstock, S.S.; Shunk, J.M.; Monaco, M.H.; Donovan, S.M. Natural killer cell populations and cytotoxic activity in pigs fed mother’s milk, formula, or formula supplemented with bovine lactoferrin. Pediatr. Res. 2013, 74, 402–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spagnuolo, P.A.; Bird, R.P.; Hoffman-Goetz, L. Effect of short-term dietary intake of bovine lactoferrin on intestinal lymphocyte apoptosis in healthy mice. Nutrition 2007, 23, 812–817. [Google Scholar] [CrossRef] [PubMed]
- Valenti, P.; Frioni, A.; Rossi, A.; Ranucci, S.; De Fino, I.; Cutone, A.; Rosa, L.; Bragonzi, A.; Berlutti, F. Aerosolized bovine lactoferrin reduces neutrophils and pro-inflammatory cytokines in mouse models of Pseudomonas aeruginosa lung infections 1. Biochem Cell Biol. 2017, 95, 41–47. [Google Scholar] [CrossRef] [PubMed]
- King, J.C.; Cummings, G.E.; Guo, N.; Trivedi, L.; Readmond, B.X.; Keane, V.; Feigelman, S.; de Waard, R. A double-blind, placebo-controlled, pilot study of bovine lactoferrin supplementation in bottle-fed infants. J. Pediatr. Gastroenterol. Nutr. 2007, 44, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Manzoni, P.; Rinaldi, M.; Cattani, S.; Pugni, L.; Romeo, M.G.; Messner, H.; Stolfi, I.; Decembrino, L.; Laforgia, N.; Vagnarelli, F.; et al. Bovine lactoferrin supplementation for prevention of late-onset sepsis in very low-birth-weight neonates: a randomized trial. JAMA. 2009, 302, 1421–1428. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, J.T.; Zegarra, J.J.; Cam, J.L.; Llanos, J.R.; Pezo, J.A.; Cruz, J.K.; Zea-Vera, J.A.; Cárcamo, J.C.; Campos, J.M.; Bellomo, J.S. Randomized controlled trial of lactoferrin for prevention of sepsis in peruvian neonates less than 2500 g. Pediatr. Infect. Disease J. 2015, 34, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Sherman, M.P.; Sherman, J.; Arcinue, R.; Niklas, V. Randomized Control Trial of Human Recombinant Lactoferrin: A Substudy Reveals Effects on the Fecal Microbiome of Very Low Birth Weight Infants. J. Pediatr. 2016, 173, S37–S42. [Google Scholar] [CrossRef] [PubMed]
- Mulder, A.M.; Connellan, P.A.; Oliver, C.J.; Morris, C.A.; Stevenson, L.M. Bovine lactoferrin supplementation supports immune and antioxidant status in healthy human males. Nutr. Res. 2008, 28, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Lönnerdal, B. Transcriptomic profiling of intestinal epithelial cells in response to human, bovine and commercial bovine lactoferrins. Biometals 2014, 27, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Troost, F.J.; Saris, W.H.M.; Brummer, R.J.M. Recombinant human lactoferrin ingestion attenuates indomethacin-induced enteropathy in vivo in healthy volunteers. Eur. J. Clin. Nutr. 2003, 57, 1579–1585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- HIV.GOV. What is a CD4 count and why is it important. Available online: https://www.aids.gov/hiv-aids-basics/just-diagnosed-with-hiv-aids/understand-your-test-results/cd4-count/ (accessed on 20 September 2016).
- Janeway, C.; Travers, P.; Walport, M.; Shlomchik, M.; Schlomchik, M. Immunobiology: The Immune System in Health and Disease, 5th ed.; Garland Publishing: New York, NY, USA, 2001. [Google Scholar]
- Simms, P.E.; Ellis, T.M. Utility of flow cytometric detection of CD69 expression as a rapid method for determining poly- and oligoclonal lymphocyte activation. Clin. Diagn. Lab. Immunol. 1996, 3, 301–304. [Google Scholar] [PubMed]
- Horz, H.P.; Conrads, G. The Discussion Goes on: What Is the Role of in Humans? Archaea 2010. [Google Scholar] [CrossRef] [PubMed]
- Kielak, A.M.; Barreto, C.C.; Kowalchuk, G.A.; van Veen, J.A.; Kuramae, E.E. The Ecology of Acidobacteria: Moving beyond Genes and Genomes. Front Microbiol. 2016, 7, 744. [Google Scholar] [CrossRef] [PubMed]
- Campbell, A.G.; Schwientek, P.; Vishnivetskaya, T.; Woyke, T.; Levy, S.; Beall, C.J.; Griffen, A.; Leys, E.; Podar, M. Diversity and genomic insights into the uncultured C hloroflexi from the human microbiota. Environ. Microbiol. 2014, 16, 2635–2643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmes, A.J.; Tujula, N.A.; Holley, M.; Contos, A.; James, J.M.; Rogers, P.; Gillings, M.R. Phylogenetic structure of unusual aquatic microbial formations in Nullarbor caves, Australia. Environ. Microbiol. 2001, 3, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Raghoebarsing, A.A.; Pol, A.; van de Pas-Schoonen, K.T.; Smolders, A.J.; Ettwig, K.F.; Rijpstra, W.I.; Schouten, S.; Damste, J.S.; Op den Camp, H.J.; Jetten, M.S.; et al. A microbial consortium couples anaerobic methane oxidation to denitrification. Nature 2006, 440, 918–921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lücker, S.; Wagner, M.; Maixner, F.; Pelletier, E.; Koch, H.; Vacherie, B.; Rattei, T.; Sinninghe Damsté, J.; Spieck, E.; Paslier, D.L.; et al. A Nitrospira metagenome illuminates the physiology and evolution of globally important nitrite-oxidizing bacteria. Proc. Natl. Acad. Sci. 2010, 107, 13479–13484. [Google Scholar] [Green Version]
- Bäckhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, E.M.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA. 2004, 101, 15718–15723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semova, I.; Carten, J.D.; Stombaugh, J.; Mackey, L.C.; Knight, R.; Farber, S.A.; Rawls, J.F. Microbiota regulate intestinal absorption and metabolism of fatty acids in the zebrafish. Cell Host Microbe 2012, 12, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA. 2005, 102, 11070–11075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costello, E.K.; Gordon, J.I.; Secor, S.M.; Knight, R. Postprandial remodeling of the gut microbiota in Burmese pythons. ISME J. 2010, 4, 1375–1385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mastromarino, P.; Capobianco, D.; Campagna, G.; Laforgia, N.; Drimaco, P.; Dileone, A.; Baldassarre, M. Correlation between lactoferrin and beneficial microbiota in breast milk and infant’s feces. Biometals 2014, 27, 1077–1086. [Google Scholar] [CrossRef] [PubMed]
- Donovan, S.M. The Role of Lactoferrin in Gastrointestinal and Immune Development and Function: A Preclinical Perspective. J. Pediatr. 2016, 173, S16–S28. [Google Scholar] [CrossRef] [PubMed]
| Regime | n | Arm 1 (4 Weeks) | Arm 2 (4 Weeks) | |
|---|---|---|---|---|
| 1 | 3 | 200 mg Product B | Washout (2 weeks) | 200 mg Product A |
| 2 | 3 | 200 mg Product A | 200 mg Product B | |
| 3 | 3 | 600 mg Product B | 600 mg Product A | |
| 4 | 3 | 600 mg Product A | 600 mg Product B |
| Sample | Pre-Supplementation | Post-Supplementation | p-Value |
|---|---|---|---|
| Product A 200 mg | 0.53 (1.18) | 0.03 (0.06) | 0.373 |
| Product A 600 mg | 0.04 (0.10) | 0.03 (0.05) | 0.771 |
| Product B 200 mg | 0.08 (0.15) | 0.08 (0.18) | 0.654 |
| Product B 600 mg | 0.11 (0.27) | 2.45 (2.41) | 0.055 |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dix, C.; Wright, O. Bioavailability of a Novel Form of Microencapsulated Bovine Lactoferrin and Its Effect on Inflammatory Markers and the Gut Microbiome: A Pilot Study. Nutrients 2018, 10, 1115. https://doi.org/10.3390/nu10081115
Dix C, Wright O. Bioavailability of a Novel Form of Microencapsulated Bovine Lactoferrin and Its Effect on Inflammatory Markers and the Gut Microbiome: A Pilot Study. Nutrients. 2018; 10(8):1115. https://doi.org/10.3390/nu10081115
Chicago/Turabian StyleDix, Clare, and Olivia Wright. 2018. "Bioavailability of a Novel Form of Microencapsulated Bovine Lactoferrin and Its Effect on Inflammatory Markers and the Gut Microbiome: A Pilot Study" Nutrients 10, no. 8: 1115. https://doi.org/10.3390/nu10081115





