Bacillus clausii for the Treatment of Acute Diarrhea in Children: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
:1. Introduction
2. Methods
2.1. Criteria for Considering Studies for this Review
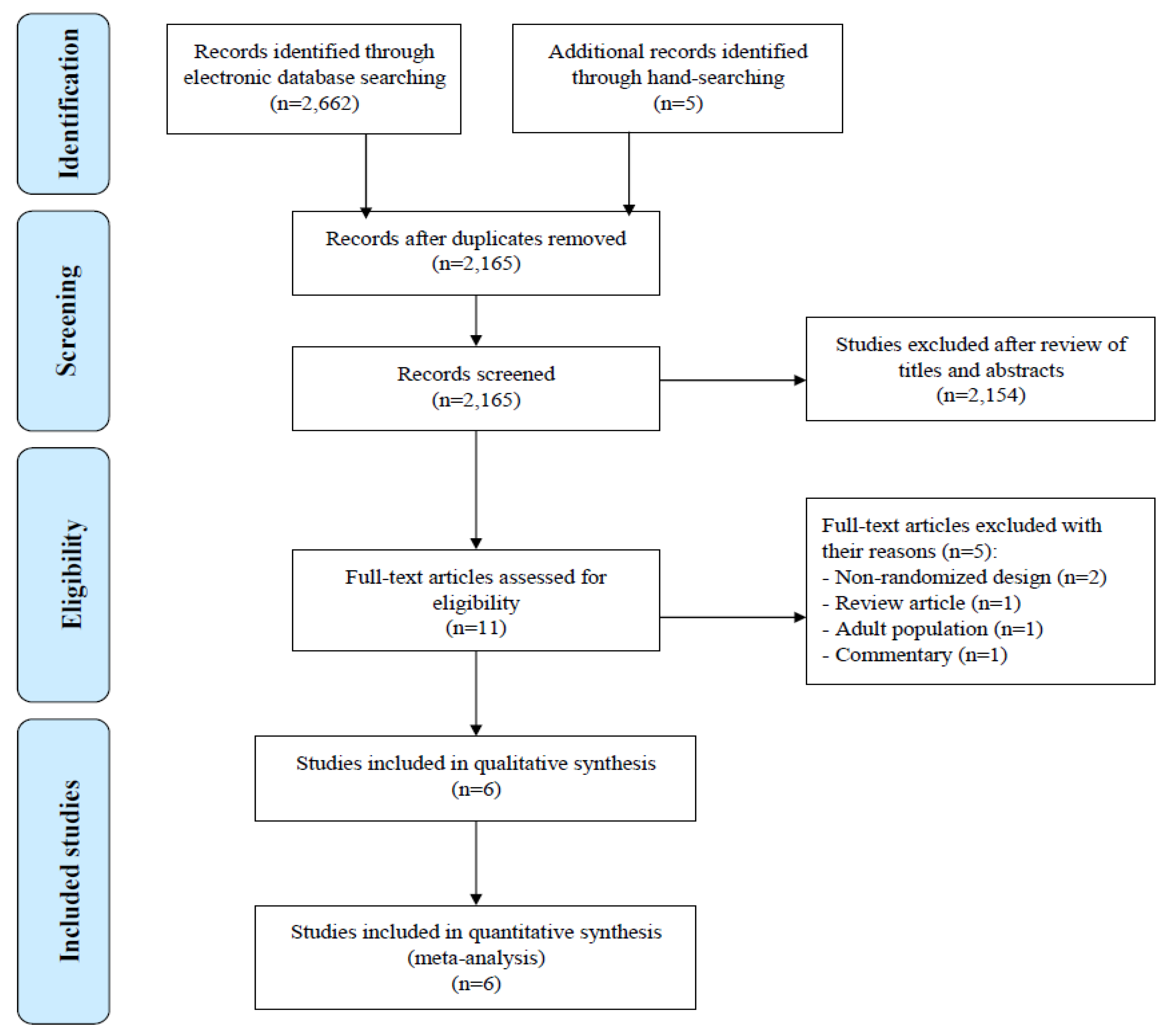
2.2. Search Strategy for Identification of Studies
2.3. Study Selection
2.4. Data Extraction
2.5. Quality Assessment
2.6. Statistical Methods
3. Results
3.1. Characteristics of Included Studies
3.2. Risk of Bias within Included Studies
3.3. Primary Findings
3.4. Secondary Findings
3.5. Publication Bias
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Diarrhoeal Disease: Fact Sheet. 2017. Available online: http://www.who.int/mediacentre/factsheets/fs330/en/ (accessed on 2 March 2018).
- Beaugerie, L.; Sokol, H. Acute infectious diarrhea in adults: Epidemiology and management. Presse Med. 2013, 42, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Farthing, M.; Salam, M.A.; Lindberg, G.; Dite, P.; Khalif, I.; Salazar-Lindo, E.; Ramakrishna, B.S.; Goh, K.L.; Thomson, A.; Khan, A.G.; et al. Acute diarrhea in adults and children: A global perspective. J. Clin. Gastroenterol. 2013, 47, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Navaneethan, U.; Giannella, R.A. Mechanisms of infectious diarrhea. Nat. Clin. Pract. Gastroenterol. Hepatol. 2008, 5, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Guarner, F.; Schaafsma, G.J. Probiotics. Int. J. Food Microbiol. 1998, 39, 237–238. [Google Scholar] [CrossRef]
- Matsuzaki, T.; Chin, J. Modulating immune responses with probiotic bacteria. Immunol. Cell. Biol. 2000, 78, 67–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheikhyoussef, A.; Pogori, N.; Chen, W.; Zhang, H. Antimicrobial proteinaceous compounds obtained from bifidobacteria: From production to their application. Int. J. Food Microbiol. 2008, 125, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Abedi, D.; Feizizadeh, S.; Akbari, V.; Jafarian-Dehkordi, A. In vitro anti-bacterial and antiadherence effects of Lactobacillus delbrueckii subsp bulgaricus on Escherichia coli. Res. Pharm. Sci. 2013, 8, 260–268. [Google Scholar] [PubMed]
- Duc, L.H.; Hong, H.A.; Barbosa, T.M.; Henriques, A.O.; Cutting, S.M. Characterization of Bacillus probiotics available for human use. Appl. Environ. Microbiol. 2004, 70, 2161–2171. [Google Scholar] [CrossRef]
- Nista, E.C.; Candelli, M.; Cremonini, F.; Cazzato, I.A.; Zocco, M.A.; Franceschi, F.; Cammarota, G.; Gasbarrini, G.; Gasbarrini, A. Bacillus clausii therapy to reduce side-effects of anti-Helicobacter pylori treatment: Randomized, double-blind, placebo controlled trial. Aliment. Pharmacol. Ther. 2004, 20, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Sudha, M.R.; Bhonagiri, S.; Kumar, M.A. Efficacy of Bacillus clausii strain UBBC-07 in the treatment of patients suffering from acute diarrhoea. Benef. Microbes 2013, 4, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Guarino, A.; Ashkenazi, S.; Gendrel, D.; Lo Vecchio, A.; Shamir, R.; Szajewska, H. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition; European Society for Pediatric Infectious Diseases. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition/European Society for Pediatric Infectious Diseases evidence-based guidelines for the management of acute gastroenteritis in children in Europe: Update 2014. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 132–152. [Google Scholar] [PubMed]
- Ciffo, F. Determination of the spectrum of antibiotic resistance of the "Bacillus subtilis" strains of Enterogermina. Chemioterapia 1984, 3, 45–52. [Google Scholar] [PubMed]
- Centre for Reviews and Dissemination, University of York. CRD’s Guidance for Undertaking Reviews in Health Care. 2009. Available online: https://www.york.ac.uk/media/crd/Systematic_Reviews.pdf (accessed on 2 March 2018).
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Smith, G.D.; Altman, D.G. Systematic Reviews in Health Care: Meta-Analysis in Context, 2nd ed.; BMJ: London, UK, 2001. [Google Scholar]
- Viechtbauer, W. Conducting Meta-Analyses in R with the metafor Package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- Canani, R.B.; Cirillo, P.; Terrin, G.; Cesarano, L.; Spagnuolo, M.I.; De Vincenzo, A.; Albano, F.; Passariello, A.; De Marco, G.; Manguso, F.; et al. Probiotics for treatment of acute diarrhoea in children: Randomised clinical trial of five different preparations. BMJ 2007, 335, 340. [Google Scholar] [CrossRef] [PubMed]
- Maugo, B.M. Effectiveness of Bacillus clausii in Reducing Duration of Illness in Acute Diarrhoea in Children 6–59 months of Age Admitted with Severe Dehydration. Available online: http://erepository.uonbi.ac.ke/bitstream/handle/11295/8325/DR._MAUGO_BRIAN_MAUGO_M.MED_PAEDS_2012.pdf?sequence=1 (accessed on 19 April 2018).
- Urtula, R.P.; Dacula, C.E. Bacillus clausii an Adjunct Treatment for Pediatric Patients with Acute Non-Bloody Diarrhea: Randomized, Controlled Clinical Trial [Abstract]. Available online: http://arl4.library.sk/arl-sllk/en/detail-sllk_un_cat-0013574-Bacillus-clausii-an-adjunct-treatment-for-pediatric-patients-with-acute-nonbloody-diarrhea-a-rando/ (accessed on 19 April 2018).
- Lahiri, K.R. GMA-CO Clinical Study Report: ENTER_L_01486. Sanofi-Aventis. Available online: https://www.sanofi.com/media/Project/One-Sanofi-Web/sanofi-com/common/docs/clinical-study-results/ENTER_L._01486_summary.pdf (accessed on 19 April 2018).
- Lahiri, K.; Jadhav, K.; Gahlowt, P.; Najmuddin, F. Bacillus Clausii As An Adjuvant Therapy In Acute Childhood Diarrhoea. IOSR-JDMS 2015, 14, 74–76. Available online: http://www.iosrjournals.org/iosr-jdms/papers/Vol14-issue5/Version-1/S014517476.pdf (accessed on 19 April 2018).
- Lahiri, K.; D’Souza, J.; Gahlowt, P. Beneficial Role of Probiotic in Acute Childhood Diarrhea. J. Harmoniz. Res. Med. Health Sci. 2015, 2, 26–30. Available online: https://www.johronline.com/issue/20150614-174258.724.pdf (accessed on 19 April 2018).
- The Cochrane Collaboration. Cochrane Reviewers’ Handbook 4.2.2. Available online: https://www.iecs.org.ar/cochrane/guias/Handbook_4-2-2.pdf (accessed on 19 April 2018).
- Mendelsohn, A.S.; Asirvatham, J.R.; Mkaya Mwamburi, D.; Sowmynarayanan, T.V.; Malik, V.; Muliyil, J.; Kang, G. Estimates of the economic burden of rotavirus-associated and all-cause diarrhoea in Vellore, India. Trop. Med. Int. Health 2008, 13, 934–942. [Google Scholar] [CrossRef] [PubMed]
- Burke, R.M.; Rebolledo, P.A.; Embrey, S.R.; Wagner, L.D.; Cowden, C.L.; Kelly, F.M.; Smith, E.R.; Iñiguez, V.; Leon, J.S. The burden of pediatric diarrhea: A cross-sectional study of incurred costs and perceptions of cost among Bolivian families. BMC Public Health 2013, 13, 708. [Google Scholar] [CrossRef] [PubMed]
- Urdaci, M.C.; Bressollier, P.; Pinchuk, I. Bacillus clausii probiotic strains: Antimicrobial and immunomodulatory activities. J. Clin. Gastroenterol. 2004, 38, S86–S90. [Google Scholar] [CrossRef] [PubMed]
- Cenci, G.; Trotta, F.; Caldini, G. Tolerance to challenges miming gastrointestinal transit by spores and vegetative cells of Bacillus clausii. J. Appl. Microbiol. 2006, 101, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- Ripert, G.; Racedo, S.M.; Elie, A.M.; Jacquot, C.; Bressollier, P.; Urdaci, M.C. Secreted Compounds of the Probiotic Bacillus clausii Strain O/C Inhibit the Cytotoxic Effects Induced by Clostridium difficile and Bacillus cereus Toxins. Antimicrob. Agents Chemother. 2016, 60, 3445–3454. [Google Scholar] [CrossRef] [PubMed]
- Salvetti, S.; Celandroni, F.; Ghelardi, E.; Baggiani, A.; Senesi, S. Rapid determination of vitamin B2 secretion by bacteria growing on solid media. J. Appl. Microbiol. 2003, 95, 1255–1260. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Authors, Publication Year (Country) | Study Design | Number of Treated Patients (I/C) | M/F (In %) | Age | Intervention vs. Comparator (Dosage and Duration) | Outcome Measures | Follow-Up | Main Results |
|---|---|---|---|---|---|---|---|---|
| Canani et al., 2007 (Italy) [19] | Prospective, multicenter, single-blind, randomized, controlled | 100/92 | 47/53 | Median: 18 months | 1 × 109 CFU of Bacillus clausii bid for 5 days + ORS for 3 to 6 h vs. ORS for 3 to 6 h (followed by full strength formula of lactose or cows’ milk, depending on age, in both groups) | Total duration of diarrhea, number of stools/day and their consistency, incidence and median duration of vomiting, fever (>37.5 °C), number of hospital admissions, safety and tolerability | Day 1 to day 7 | Median duration of diarrhea in patients receiving Bacillus clausii (118 h) similar to control group (115 h), with an estimated difference of 1 h between both groups (p = 0.76). All other outcomes were also similar in both groups. Bacillus clausii was well tolerated, with no observed adverse events. |
| Lahiri, 2008 (India) [22] | Phase III, controlled, open-label, randomized, parallel-group, multicenter, comparative | 132/132 | 54.5/45.5 | Mean (SD): 1.6 (1.0) years | 2 × 109 CFU of Bacillus clausii bid + ORS + 20 mg/day of zinc supplement, for 5 days vs. ORS + 20 mg/day of zinc supplement, for 5 days | Duration of diarrhea, mean number of daily stools, effect on consistency of stools, vomiting episodes per day, reported adverse events, parents’ overall global assessment of tolerability at end of treatment period | Day 6 to day 10 (after end of study treatment) | Mean (SD) duration of diarrhea lower in the experimental group (48.6 (38.2) h), vs. control group (56.1 (40) h; p = 0.13). Difference in the mean (SD) number of stools until recovery statistically not significant (p = 0.19); trend favoring the experimental group (7.4 (6.5) motions vs. 8.6 (6.5) motions in control group). |
| Lahiri, Jadhav et al., 2015 (India) [23] | Open-label, prospective, randomized, controlled | 69/62 | 63.4/36.6 | 6 months to 12 years | 2 × 109 CFU of Bacillus clausii bid + ORS + zinc, for 5 days vs. ORS + zinc for 5 days | Mean duration of diarrhea, mean duration of hospitalization, frequency of diarrhea, direct and indirect costs | At 6, 12, 24, 36, 48, 60, and 72 h | Mean duration of diarrhea 22.64 h and mean duration of hospital stay 2.78 days in the Bacillus clausii group vs. 47.05 h and 4.30 days, respectively, in the control group (p < 0.01 for diarrhea duration). Treatment with Bacillus clausii reduced total treatment costs by 472 Indian rupees compared to ORS alone. |
| Lahiri, D’Souza et al., 2015 (India) [24] | Open-label, prospective, randomized, controlled | 80/80 | 52.5/47.5 | Up to 6 years | 2 × 109 CFU of Bacillus clausii bid + ORS + zinc, for 5 days vs. ORS + zinc for 5 days | Mean duration of diarrhea, mean stool frequency, % of children with no dehydration, % of children benefiting from breastfeeding | At 6, 12, 24, 36, 48, 60, and 72 h | Mean (SD) duration of diarrhea 22.26 h and mean stool frequency 1.15 in the Bacillus clausii group vs. 34.16 h and 1.70, respectively in control group (p < 0.05). |
| Maugo, 2012 (Kenya) [20] | Randomized, double-blind, placebo- controlled | 51/51 | 51.1/48.9 | Mean (SD): Bacillus clausii group: 11.3 (5.3) and control group: 11.9 (6.4) months | 2 × 109 CFU of Bacillus clausii bid + ORS + zinc sulfate, for 5 days vs. zinc sulfate + ORS + 1 vial bid of a placebo packaged in identical looking vials containing sterile water, for 5 days | Mean duration of diarrhea, mean duration of hospitalization, mean reduction of the number of diarrheal episodes per day | Day 1 to day 7 | Mean (SD) duration of diarrhea in Bacillus clausii group was shorter (77.59 (34.10) h) than placebo group (86.74 (40.16) h), with mean absolute difference between groups of 9.15 h (p = 0.248). Significant decrease in mean number of diarrheal motions on day 3 (2.74 (1.81) motions in the Bacillus clausii group vs. 3.80 (2.70) motions in placebo group, mean absolute difference = 1.05 motions; p = 0.033) and day 4 (1.45 (1.13) motions in the Bacillus clausii group vs. 2.35 (2.19) motions in placebo group, mean absolute difference = 0.9 motions; p = 0.018) in the Bacillus clausii group vs. placebo group. |
| Urtula and Dacula, 2008 (The Philippines) [21] | Monocentric, randomized, controlled | 35/35 | NR | NR | 2 × 109 or 4×109 CFU of Bacillus clausii per day, depending on the age of the children + ORS, for 3 days vs. ORS for 3 days | Mean duration of diarrhea, mean duration of hospitalization, mean frequency of stools | After day 3 of therapy, and upon discharge | Mean (SD) duration of diarrhea significantly shorter in the Bacillus clausii group (69.84 (16.84) h) than in control group (83.76 (22.05) h) (p = 0.005), with absolute difference of duration of diarrhea between groups of 13.92 h. Mean duration of hospital stay was also shorter favoring Bacillus clausii group (59.0 h vs. 76.8 h) (p = 0.063). |
| Authors and Publication Year | Was Randomization Carried Out Appropriately? | Was the Concealment of Treatment Allocation Adequate? | Were the Groups Similar at the Outset of the Study in Terms of Prognostic Factors? | Were the Care Providers, Participants and Outcome Assessors Blind to Treatment Allocation? | Were There any Unexpected Imbalances in Drop-Outs between Groups? | Is There any Evidence to Suggest that the Authors Measured More Outcomes than They Reported? | Did the Analysis Include an Intention-To-Treat Analysis? If So, Was This Appropriate and Were Appropriate Methods Used to Account for Missing Data? | Overall Study Quality |
|---|---|---|---|---|---|---|---|---|
| Canani et al., 2007 [19] | Yes | Yes | Yes | No | No | No | Yes/Yes | Good |
| Lahiri, 2008 [22] | Unclear | Unclear | Unclear | No | No | Unclear | Yes/Yes | Fair |
| Lahiri, Jadhav et al., 2015 [23] | Unclear | Unclear | Unclear | No | Unclear | No | Unclear | Poor * |
| Lahiri, D’Souza et al., 2015 [24] | Unclear | Unclear | Unclear | No | Unclear | No | Unclear | Poor * |
| Maugo, 2012 [20] | Yes | Yes | Yes | Yes | No | No | No | Good |
| Urtula and Dacula, 2008 [21] | Yes | Yes | Yes | Unclear | Unclear | No | Unclear | Fair |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ianiro, G.; Rizzatti, G.; Plomer, M.; Lopetuso, L.; Scaldaferri, F.; Franceschi, F.; Cammarota, G.; Gasbarrini, A. Bacillus clausii for the Treatment of Acute Diarrhea in Children: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2018, 10, 1074. https://doi.org/10.3390/nu10081074
Ianiro G, Rizzatti G, Plomer M, Lopetuso L, Scaldaferri F, Franceschi F, Cammarota G, Gasbarrini A. Bacillus clausii for the Treatment of Acute Diarrhea in Children: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients. 2018; 10(8):1074. https://doi.org/10.3390/nu10081074
Chicago/Turabian StyleIaniro, Gianluca, Gianenrico Rizzatti, Manuel Plomer, Loris Lopetuso, Franco Scaldaferri, Francesco Franceschi, Giovanni Cammarota, and Antonio Gasbarrini. 2018. "Bacillus clausii for the Treatment of Acute Diarrhea in Children: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" Nutrients 10, no. 8: 1074. https://doi.org/10.3390/nu10081074





