Does a Supplemental Low-Protein Diet Decrease Mortality and Adverse Events After Commencing Dialysis? A Nationwide Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source
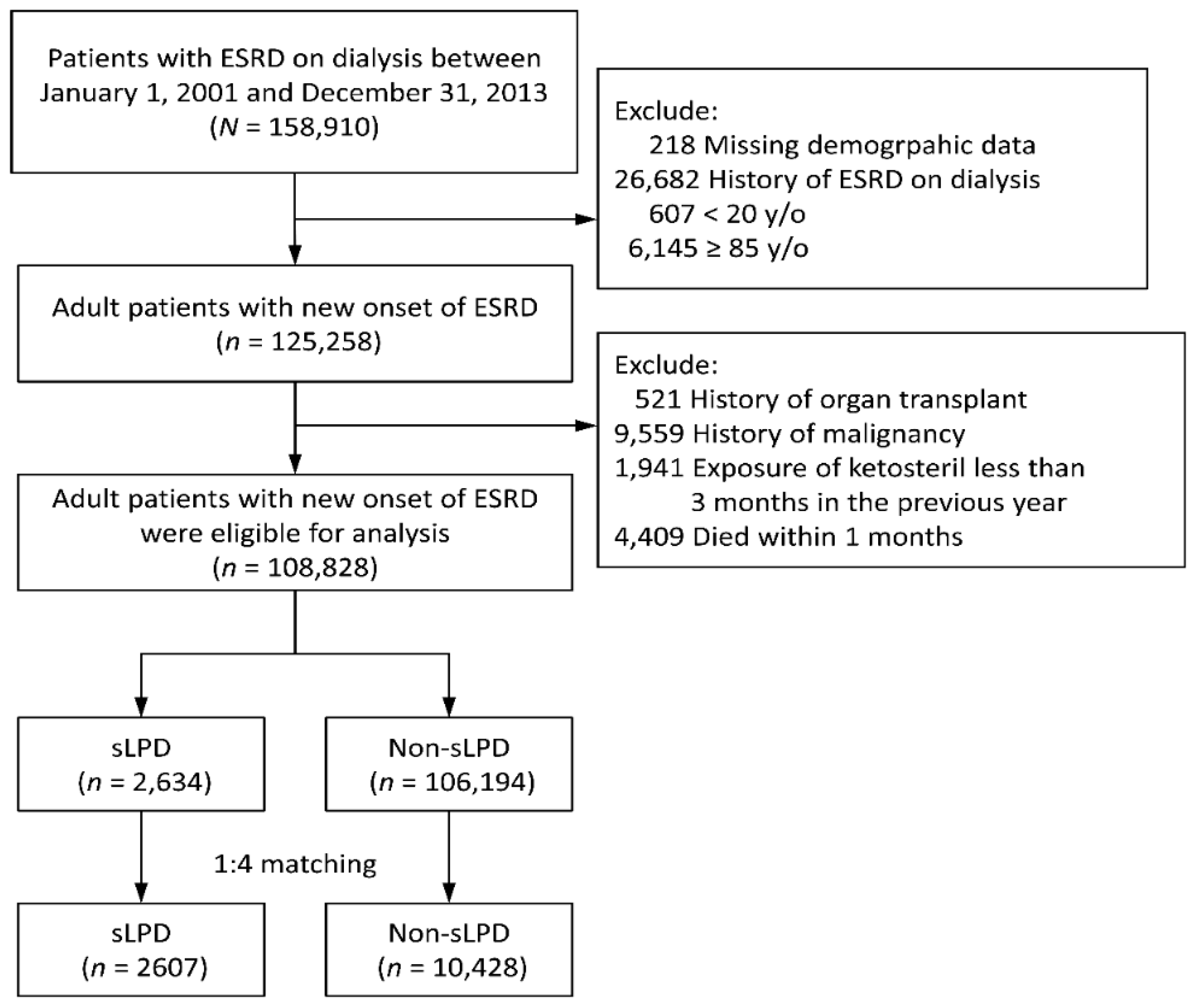
2.2. Patient Selection and Study Design
2.3. Covariates and Study Outcomes
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
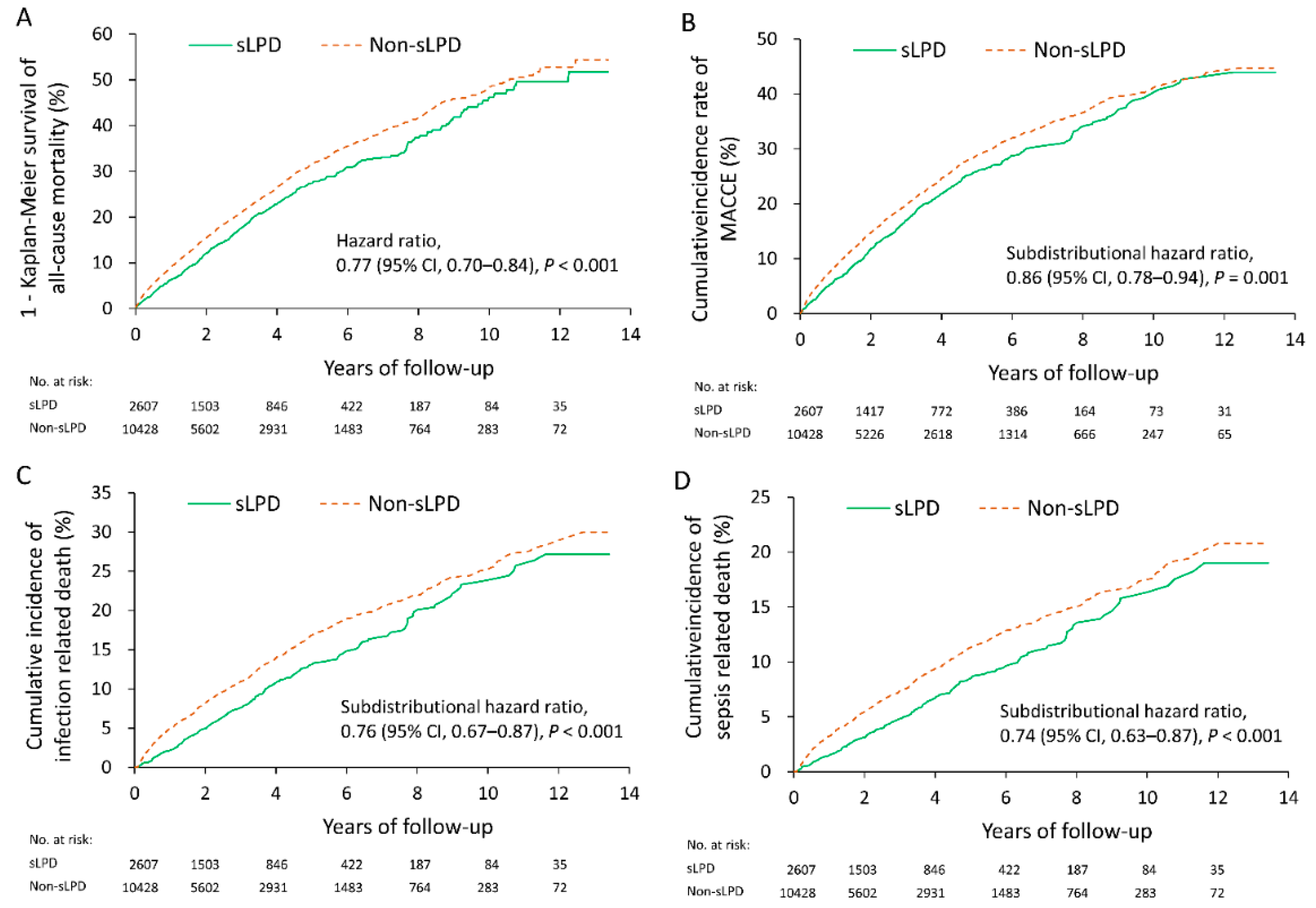
3.2. Follow-Up Outcomes
3.3. Subgroup Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Cuppari, L.; Meireles, M.S.; Ramos, C.I.; Kamimura, M.A. Subjective global assessment for the diagnosis of protein-energy wasting in nondialysis-dependent chronic kidney disease patients. J. Ren. Nutr. 2014, 24, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Fukagawa, M.; Drüeke, T.B. Introduction: expanding concepts of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int. Suppl. 2013, 3, 419. [Google Scholar] [CrossRef] [PubMed]
- Isakova, T.; Nickolas, T.L.; Denburg, M.; Yarlagadda, S.; Weiner, D.E.; Gutiérrez, O.M.; Bansal, V.; Rosas, S.E.; Nigwekar, S.; Yee, J.; et al. KDOQI US Commentary on the 2017 KDIGO Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Am. J. Kidney Dis. 2017, 70, 737–751. [Google Scholar] [CrossRef] [PubMed]
- Akchurin, O.M.; Kaskel, F. Update on inflammation in chronic kidney disease. Blood. Purif. 2015, 39, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Jagadeswaran, D.; Indhumathi, E.; Hemamalini, A.J.; Sivakumar, V.; Soundararajan, P.; Jayakumar, M. Inflammation and nutritional status assessment by malnutrition inflammation score and its outcome in pre-dialysis chronic kidney disease patients. Clin. Nutr. 2018. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, L.; Liabeuf, S.; Oliveira, R.B.; Louvet, L.; Kamel, S.; Lemke, H.D.; Vanholder, R.; Choukroun, G.; Massy, Z.A.; European Uremic Toxin (EUTox) Work Group. Uremic toxicity and sclerostin in chronic kidney disease patients. Nephrol. Ther. 2014, 10, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Komaba, H.; Koizumi, M.; Kakuta, T.; Fukagawa, M. Role of uremic toxins and oxidative stress in the development of chronic kidney disease-mineral and bone disorder. J. Ren. Nutr. 2012, 22, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Addis, T.; Lew, W. Diet and Death in Acute Uremia. J. Clin. Investig. 1939, 18, 773–775. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.S. On the Influence of a Diet with High Protein Content on the Kidney. Can. Med. Assoc. J. 1921, 11, 682–683. [Google Scholar] [PubMed]
- Walser, M. Does dietary therapy have a role in the predialysis patient? Am. J. Clin. Nutr. 1980, 33, 1629–1637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cianciaruso, B.; Pota, A.; Pisani, A.; Torraca, S.; Annecchini, R.; Lombardi, P.; Capuano, A.; Nazzaro, P.; Bellizzi, V.; Sabbatini, M. Metabolic effects of two low protein diets in chronic kidney disease stage 4–5—A randomized controlled trial. Nephrol. Dial. Transplant. 2008, 23, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Walser, M.; Mitch, W.E.; Maroni, B.J.; Kopple, J.D. Should protein intake be restricted in predialysis patients? Kidney Int. 1999, 55, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Kasiske, B.L.; Lakatua, J.D.; Ma, J.Z.; Louis, T.A. A meta-analysis of the effects of dietary protein restriction on the rate of decline in renal function. Am. J. Kidney Dis. 1998, 31, 954–961. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, F.; Alberti, D.; Graziani, G.; Buccianti, G.; Redaelli, B.; Giangrande, A. Prospective, randomised, multicentre trial of effect of protein restriction on progression of chronic renal insufficiency. Lancet 1991, 337, 1299–1304. [Google Scholar] [CrossRef]
- Kidney Disease Outcomes Quality Initiative. Clinical practice guidelines for nutrition in chronic renal failure. Am. J. Kidney Dis. 2000, 35, S1–S140. [Google Scholar]
- Prakash, S.; Pande, D.P.; Sharma, S.; Sharma, D.; Bal, C.S.; Kulkarni, H. Randomized, double-blind, placebo-controlled trial to evaluate efficacy of ketodiet in predialytic chronic renal failure. J. Ren. Nutr. 2004, 14, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Walser, M.; Lund, P.; Ruderman, N.B.; Coulter, A.W. Synthesis of essential amino acids from their alpha-keto analogues by perfused rat liver and muscle. J. Clin. Investig. 1973, 52, 2865–2877. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.W.; Tungsanga, K.; Walser, M. Effect of the level of dietary protein on the utilization of alpha-ketoisocaproate for protein synthesis. Am. J. Clin. Nutr. 1986, 43, 504–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walser, M. Ketoacids in the treatment of uremia. Clin. Nephrol. 1975, 3, 180–186. [Google Scholar] [PubMed]
- Teplan, V.; Schück, O.; Votruba, M.; Poledne, R.; Kazdova, L.; Skibova, J.; Malý, J. Metabolic effects of keto acid—Amino acid supplementation in patients with chronic renal insufficiency receiving a low-protein diet and recombinant human erythropoietin—A randomized controlled trial. Wien. Klin. Wochenschr. 2001, 113, 661–669. [Google Scholar]
- Jiang, N.; Qian, J.; Sun, W.; Lin, A.; Cao, L.; Wang, Q.; Ni, Z.; Wan, Y.; Linholm, B.; Axelsson, J. Better preservation of residual renal function in peritoneal dialysis patients treated with a low-protein diet supplemented with keto acids: A prospective, randomized trial. Nephrol. Dial. Transplant. 2009, 24, 2551–2558. [Google Scholar] [CrossRef] [PubMed]
- Mircescu, G.; Gârneaţă, L.; Stancu, S.H.; Căpuşă, C. Effects of a supplemented hypoproteic diet in chronic kidney disease. J. Ren. Nutr. 2007, 17, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Brunori, G.; Viola, B.F.; Parrinello, G.; De Biase, V.; Como, G.; Franco, V.; Garibotto, G.; Zubani, R.; Cancarini, G.C. Efficacy and safety of a very-low-protein diet when postponing dialysis in the elderly: A prospective randomized multicenter controlled study. Am. J. Kidney Dis. 2007, 49, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Kopple, J.D.; Levey, A.S.; Greene, T.; Chumlea, W.C.; Gassman, J.J.; Hollinger, D.L.; Maroni, B.J.; Merrill, D.; Scherch, L.K.; Schulman, G.; et al. Effect of dietary protein restriction on nutritional status in the Modification of Diet in Renal Disease Study. Kidney Int. 1997, 52, 778–791. [Google Scholar] [CrossRef] [PubMed]
- Kovesdy, C.P.; Kopple, J.D.; Kalantar-Zadeh, K. Management of protein-energy wasting in non-dialysis-dependent chronic kidney disease: reconciling low protein intake with nutritional therapy. Am. J. Clin. Nutr. 2013, 97, 1163–1177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menon, V.; Kopple, J.D.; Wang, X.; Beck, G.J.; Collins, A.J.; Kusek, J.W.; Greene, T.; Levey, A.S.; Sarnak, M.J. Effect of a very low-protein diet on outcomes: long-term follow-up of the Modification of Diet in Renal Disease (MDRD) Study. Am. J. Kidney Dis. 2009, 53, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.C.; Hwang, S.J.; Taiwan Society of Nephrology. Incidence, prevalence and mortality trends of dialysis end-stage renal disease in Taiwan from 1990 to 2001: The impact of national health insurance. Nephrol. Dial. Transplant. 2008, 23, 3977–3982. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.S.; Lai, M.S.; Gau, S.S.F.; Wang, S.C.; Tsai, H.J. Concordance between patient self-reports and claims data on clinical diagnoses, medication use, and health system utilization in Taiwan. PLoS ONE 2014, 9, e112257. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.Y.; Chen, C.H.; Li, C.Y.; Lai, M.L. Validating the diagnosis of acute ischemic stroke in a National Health Insurance claims database. J. Formos. Med. Assoc. 2015, 114, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.L.; Lee, C.H.; Chen, P.S.; Li, Y.H.; Lin, S.J.; Yang, Y.H.K. Validation of acute myocardial infarction cases in the national health insurance research database in Taiwan. J. Epidemiol. 2014, 24, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.L.; Kao, Y.H.Y.; Lin, S.J.; Lee, C.H.; Lai, M.L. Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol. Drug Saf. 2011, 20, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.L.; Chien, H.C.; Lee, C.H.; Lin, S.J.; Yang, Y.H.K. Validity of in-hospital mortality data among patients with acute myocardial infarction or stroke in National Health Insurance Research Database in Taiwan. Int. J. Cardiol. 2015, 201, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.C.; Wang, I.K.; Wei, C.C.; Lin, C.L.; Tsai, C.T.; Hsia, T.C.; Sung, F.C.; Kao, C.H. The Risk of Septicemia in End-Stage Renal Disease with and without Renal Transplantation: A Propensity-Matched Cohort Study. Medicine 2015, 94, e1437. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.L.; Li, C.Y.; Hsieh, T.H.; Chang, C.M.; Lee, H.C.; Lee, N.Y.; Wu, C.J.; Lee, C.C.; Shih, H.I.; Ko, W.C. Epidemiology, disease spectrum and economic burden of non-typhoidal Salmonella infections in Taiwan, 2006–2008. Epidemiol. Infect. 2012, 140, 2256–2263. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm. Stat. 2011, 10, 150–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Normand, S.L.T.; Landrum, M.B.; Guadagnoli, E.; Ayanian, J.Z.; Ryan, T.J.; Cleary, P.D.; McNeil, B.J. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: A matched analysis using propensity scores. J. Clin. Epidemiol. 2001, 54, 387–398. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, M.J.; Fine, J. A proportional hazards regression model for the subdistribution with right-censored and left-truncated competing risks data. Stat. Med. 2011, 30, 1933–1951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Austin, P.C. Comparing paired vs non-paired statistical methods of analyses when making inferences about absolute risk reductions in propensity-score matched samples. Stat. Med. 2011, 30, 1292–1301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malvy, D.; Maingourd, C.; Pengloan, J.; Bagros, P.; Nivet, H. Effects of severe protein restriction with ketoanalogues in advanced renal failure. J. Am. Coll. Nutr. 1999, 18, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H.; Yang, Y.W.; Hung, S.C.; Kuo, K.L.; Wu, K.D.; Wu, V.C.; Hsieh, T.C.; National Taiwan University Study Group on Acute Renal Failure. Ketoanalogues supplementation decreases dialysis and mortality risk in patients with anemic advanced chronic kidney disease. PLoS ONE 2017, 12, e0176847. [Google Scholar]
- Bellizzi, V.; Chiodini, P.; Cupisti, A.; Viola, B.F.; Pezzotta, M.; De Nicola, L.; Minutolo, R.; Barsotti, G.; Piccoli, G.B.; Di Iorio, B. Very low-protein diet plus ketoacids in chronic kidney disease and risk of death during end-stage renal disease: A historical cohort controlled study. Nephrol. Dial. Transplant. 2015, 30, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Córdoba, J.; López-Hellı́n, J.; Planas, M.; Sabı́n, P.; Sanpedro, F.; Castro, F.; Esteban, R.; Guardia, J. Normal protein diet for episodic hepatic encephalopathy: Results of a randomized study. J. Hepatol. 2004, 41, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, T.; Izumi, N.; Charlton, M.R.; Sata, M. Branched-chain amino acids as pharmacological nutrients in chronic liver disease. Hepatology 2011, 54, 1063–1070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hidaka, H.; Nakazawa, T.; Kutsukake, S.; Yamazaki, Y.; Aoki, I.; Nakano, S.; Asaba, N.; Minamino, T.; Takada, J.; Tanaka, Y.; et al. The efficacy of nocturnal administration of branched-chain amino acid granules to improve quality of life in patients with cirrhosis. J. Gastroenterol. 2013, 48, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Bellizzi, V.; Di Iorio, B.R.; De Nicola, L.; Minutolo, R.; Zamboli, P.; Trucillo, P.; Catapano, F.; Cristofano, C.; Scalfi, L.; Conte, G. Very low protein diet supplemented with ketoanalogs improves blood pressure control in chronic kidney disease. Kidney Int. 2007, 71, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Teplan, V.; Schück, O.; Knotek, A.; Hajný, J.; Horáčková, M.; Kvapil, M. Enhanced metabolic effect of erythropoietin and keto acids in CRF patients on low-protein diet: Czech multicenter study. Am. J. Kidney Dis. 2003, 41, S26–S30. [Google Scholar] [CrossRef] [PubMed]
- Chauveau, P.; Combe, C.; Rigalleau, V.; Vendrely, B.; Aparicio, M. Restricted protein diet is associated with decrease in proteinuria: Consequences on the progression of renal failure. J. Ren. Nutr. 2007, 17, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Bernard, S.; Fouque, D.; Laville, M.; Zech, P. Effects of low-protein diet supplemented with ketoacids on plasma lipids in adult chronic renal failure. Miner. Electrolyte. Metab. 1996, 22, 143–146. [Google Scholar] [PubMed]
- Lameire, N.; Van Biesen, W.; Vanholder, R. Did 20 years of technological innovations in hemodialysis contribute to better patient outcomes? Clin. J. Am. Soc. Nephrol. 2009, 4, S30–S40. [Google Scholar] [CrossRef] [PubMed]
- Hiroshige, K.; Sonta, T.; Suda, T.; Kanegae, K.; Ohtani, A. Oral supplementation of branched-chain amino acid improves nutritional status in elderly patients on chronic haemodialysis. Nephrol. Dial. Transplant. 2001, 16, 1856–1862. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, M.; Bellizzi, V.; Chauveau, P.; Cupisti, A.; Ecder, T.; Fouque, D.; Garneata, L.; Lin, S.; Mitch, W.E.; Teplan, V.; et al. Protein-restricted diets plus keto/amino acids—A valid therapeutic approach for chronic kidney disease patients. J. Ren. Nutr. 2012, 22, S1–S21. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.H.; Kim, D.K.; Park, J.T.; Kang, E.W.; Yoo, T.H.; Kim, B.S.; Choi, K.H.; Lee, H.Y.; Han, D.S.; Shin, S.K. Influence of ketoanalogs supplementation on the progression in chronic kidney disease patients who had training on low-protein diet. Nephrology 2009, 14, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, G.B.; Ventrella, F.; Capizzi, I.; Vigotti, F.N.; Mongilardi, E.; Grassi, G.; Loi, V.; Cabiddu, G.; Avagnina, P.; Versino, E. Low-Protein Diets in Diabetic Chronic Kidney Disease (CKD) Patients: Are They Feasible and Worth the Effort? Nutrients 2016, 8, 649. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.; Gul, A.; Sarnak, M.J. Cardiovascular risk factors in chronic kidney disease. Kidney Int. 2005, 68, 1413–1418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minakuchi, H.; Wakino, S.; Hayashi, K.; Inamoto, H.; Itoh, H. Serum creatinine and albumin decline predict the contraction of nosocomial aspiration pneumonia in patients undergoing hemodialysis. Ther. Apher. Dial. 2014, 18, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Van Zuijdewijn, C.L.D.R.; ter Wee, P.M.; Chapdelaine, I.; Bots, M.L.; Blankestijn, P.J.; van den Dorpel, M.A.; Nubé, M.J.; Grooteman, M.P. A Comparison of 8 Nutrition-Related Tests to Predict Mortality in Hemodialysis Patients. J. Ren. Nutr. 2015, 25, 412–419. [Google Scholar] [CrossRef] [PubMed]

| Before Matching | After Matching | |||||
|---|---|---|---|---|---|---|
| Characteristic | sLPD (n = 2634) | Non-sLPD (n = 106,194) | ASMD | sLPD (n = 2607) | Non-sLPD (n = 10,428) | ASMD |
| Age | 60.9 ± 12.9 | 62.1 ± 13.6 | 0.09 | 60.9 ± 12.9 | 61.1 ± 13.9 | 0.01 |
| Age ≥ 65 years, n (%) | 1074 (40.8) | 49,145 (46.3) | 0.11 | 1068 (41.0) | 4304 (41.3) | 0.01 |
| Male gender, n (%) | 1343 (51.0) | 54,473 (51.3) | 0.01 | 1331 (51.1) | 5359 (51.4) | 0.01 |
| Comorbidity in the previous year, n (%) | ||||||
| Hypertension | 2304 (87.5) | 93,064 (87.6) | 0.00 | 2279 (87.4) | 9196 (88.2) | 0.02 |
| Diabetes mellitus | 1074 (40.8) | 62,480 (58.8) | 0.37 | 1073 (41.2) | 4265 (40.9) | 0.01 |
| Dyslipidemia | 653 (24.8) | 28,077 (26.4) | 0.04 | 648 (24.9) | 2529 (24.3) | 0.01 |
| Atrial fibrillation | 52 (2.0) | 3249 (3.1) | 0.07 | 52 (2.0) | 190 (1.8) | 0.01 |
| Peripheral arterial disease | 70 (2.7) | 3871 (3.6) | 0.05 | 70 (2.7) | 292 (2.8) | 0.01 |
| Liver cirrhosis | 60 (2.3) | 4285 (4.0) | 0.10 | 60 (2.3) | 219 (2.1) | 0.01 |
| Dementia | 58 (2.2) | 3191 (3.0) | 0.05 | 58 (2.2) | 226 (2.2) | 0.00 |
| Charlson Comorbidity Index score | 3.7 ± 1.8 | 4.6 ± 2.0 | 0.47 | 3.8 ± 1.8 | 3.7 ± 1.7 | 0.02 |
| Hospitalization history, n (%) | ||||||
| Heart failure | 361 (13.7) | 27,567 (26.0) | 0.31 | 361 (13.8) | 1415 (13.6) | 0.01 |
| Stroke | 278 (10.6) | 18,395 (17.3) | 0.19 | 278 (10.7) | 1079 (10.3) | 0.01 |
| Myocardial infarction | 100 (3.8) | 7378 (6.9) | 0.14 | 100 (3.8) | 402 (3.9) | 0.01 |
| Infection-related hospitalization | 1230 (46.7) | 64,470 (60.7) | 0.28 | 1223 (46.9) | 4971 (47.7) | 0.02 |
| Initial dialysis type, n (%) | ||||||
| Hemodialysis | 2028 (77.0) | 94,647 (89.1) | 0.33 | 2015 (77.3) | 8115 (77.8) | 0.01 |
| Peritoneal dialysis | 606 (23.0) | 11,547 (10.9) | 0.33 | 592 (22.7) | 2313 (22.2) | 0.01 |
| Medication, n (%) | ||||||
| Aspirin/Clopidogrel | 573 (21.8) | 29,267 (27.6) | 0.13 | 570 (21.9) | 2306 (22.1) | 0.00 |
| ACEI/ARB | 1314 (49.9) | 48,098 (45.3) | 0.09 | 1300 (49.9) | 5142 (49.3) | 0.01 |
| Other antihypertensive agents | 2147 (81.5) | 82,373 (77.6) | 0.10 | 2122 (81.4) | 8510 (81.6) | 0.01 |
| Loop diuretics | 1341 (50.9) | 58,310 (54.9) | 0.08 | 1334 (51.2) | 5415 (51.9) | 0.01 |
| K-sparing diuretics | 40 (1.5) | 2387 (2.2) | 0.05 | 40 (1.5) | 178 (1.7) | 0.02 |
| OHA | 606 (23.0) | 34,346 (32.3) | 0.21 | 606 (23.2) | 2441 (23.4) | 0.00 |
| Insulin | 403 (15.3) | 23,087 (21.7) | 0.17 | 403 (15.5) | 1592 (15.3) | 0.01 |
| PPI | 377 (14.3) | 16,756 (15.8) | 0.04 | 374 (14.3) | 1520 (14.6) | 0.01 |
| NSAID (including COX2) | 297 (11.3) | 15,193 (14.3) | 0.09 | 297 (11.4) | 1184 (11.4) | 0.00 |
| Statin | 548 (20.8) | 22,687 (21.4) | 0.01 | 543 (20.8) | 2253 (21.6) | 0.02 |
| Fibrate or Gemfibrozil | 65 (2.5) | 4352 (4.1) | 0.09 | 65 (2.5) | 252 (2.4) | 0.01 |
| Iron supplement | 639 (24.3) | 17,960 (16.9) | 0.18 | 634 (24.3) | 2584 (24.8) | 0.01 |
| Pentoxifylline | 666 (25.3) | 11,794 (11.1) | 0.37 | 644 (24.7) | 2533 (24.3) | 0.01 |
| Vitamin D therapy | 485 (18.4) | 10,066 (9.5) | 0.26 | 472 (18.1) | 1800 (17.3) | 0.02 |
| Sodium bicarbonate | 640 (24.3) | 7686 (7.2) | 0.48 | 615 (23.6) | 2296 (22.0) | 0.04 |
| Calcium supplementation | 1058 (40.2) | 36,447 (34.3) | 0.12 | 1041 (39.9) | 4122 (39.5) | 0.01 |
| Steroid | 256 (9.7) | 8463 (8.0) | 0.06 | 253 (9.7) | 1057 (10.1) | 0.01 |
| Follow-up (years) | 3.3 ± 2.8 | 4.0 ± 3.3 | 0.24 | 3.3 ± 2.8 | 3.0 ± 2.7 | 0.09 |
| Event No. (%) | sLPD vs Non-sLPD | |||
|---|---|---|---|---|
| Outcome # | sLPD (n = 2607) | Non-sLPD (n = 10,428) | HR (95% CI) | p-Value |
| All-cause mortality | 603 (23.1) | 2877 (27.6) | 0.77 (0.70–0.84) | <0.001 |
| Cardiovascular composite adverse event § | 500 (19.2) | 2240 (21.5) | 0.86 (0.78–0.94) | 0.001 |
| Acute myocardial infarction | 87 (3.3) | 327 (3.1) | 1.05 (0.83–1.33) | 0.695 |
| Acute ischemic stroke | 114 (4.4) | 525 (5.0) | 0.86 (0.70–1.05) | 0.135 |
| Intracerebral hemorrhage | 34 (1.3) | 181 (1.7) | 0.74 (0.51–1.07) | 0.107 |
| Heart failure | 90 (3.5) | 418 (4.0) | 0.85 (0.68–1.07) | 0.156 |
| Cardiovascular death | 310 (11.9) | 1366 (13.1) | 0.88 (0.78–0.99) | 0.039 |
| Infection-related hospitalization | 1009 (38.7) | 4479 (43.0) | 0.83 (0.78–0.89) | <0.001 |
| Infection death | 259 (9.9) | 1308 (12.5) | 0.76 (0.67–0.87) | <0.001 |
| Sepsis-related hospitalization | 415 (15.9) | 2188 (21.0) | 0.71 (0.64–0.79) | <0.001 |
| Sepsis death | 171 (6.6) | 890 (8.5) | 0.74 (0.63–0.87) | <0.001 |
| Disability | 788 (30.2) | 3274 (31.4) | 0.94 (0.87–1.01) | 0.098 |
| PRBC (admission) | 1451 (55.7) | 6004 (57.6) | 0.91 (0.86–0.96) | 0.001 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yen, C.-L.; Tu, K.-H.; Lin, M.-S.; Chang, S.-W.; Fan, P.-C.; Hsiao, C.-C.; Chen, C.-Y.; Hsu, H.-H.; Tian, Y.-C.; Chang, C.-H. Does a Supplemental Low-Protein Diet Decrease Mortality and Adverse Events After Commencing Dialysis? A Nationwide Cohort Study. Nutrients 2018, 10, 1035. https://doi.org/10.3390/nu10081035
Yen C-L, Tu K-H, Lin M-S, Chang S-W, Fan P-C, Hsiao C-C, Chen C-Y, Hsu H-H, Tian Y-C, Chang C-H. Does a Supplemental Low-Protein Diet Decrease Mortality and Adverse Events After Commencing Dialysis? A Nationwide Cohort Study. Nutrients. 2018; 10(8):1035. https://doi.org/10.3390/nu10081035
Chicago/Turabian StyleYen, Chieh-Li, Kun-Hua Tu, Ming-Shyan Lin, Su-Wei Chang, Pei-Chun Fan, Ching-Chung Hsiao, Chao-Yu Chen, Hsiang-Hao Hsu, Ya-Chun Tian, and Chih-Hsiang Chang. 2018. "Does a Supplemental Low-Protein Diet Decrease Mortality and Adverse Events After Commencing Dialysis? A Nationwide Cohort Study" Nutrients 10, no. 8: 1035. https://doi.org/10.3390/nu10081035





