Serum 25-Hydroxyvitamin D Concentrations Are Inversely Correlated with Hepatic Lipid Content in Male Collegiate Football Athletes
Abstract
:1. Introduction
2. Materials and Methods
Subjects
3. Procedures
3.1. Anthropometric Measurements
3.2. Magnetic Resonance Imaging and Spectroscopy
3.3. Analysis of Blood Samples
3.4. Cardiorespiratory Fitness
3.5. Dietary Intake
3.6. Statistical Analysis
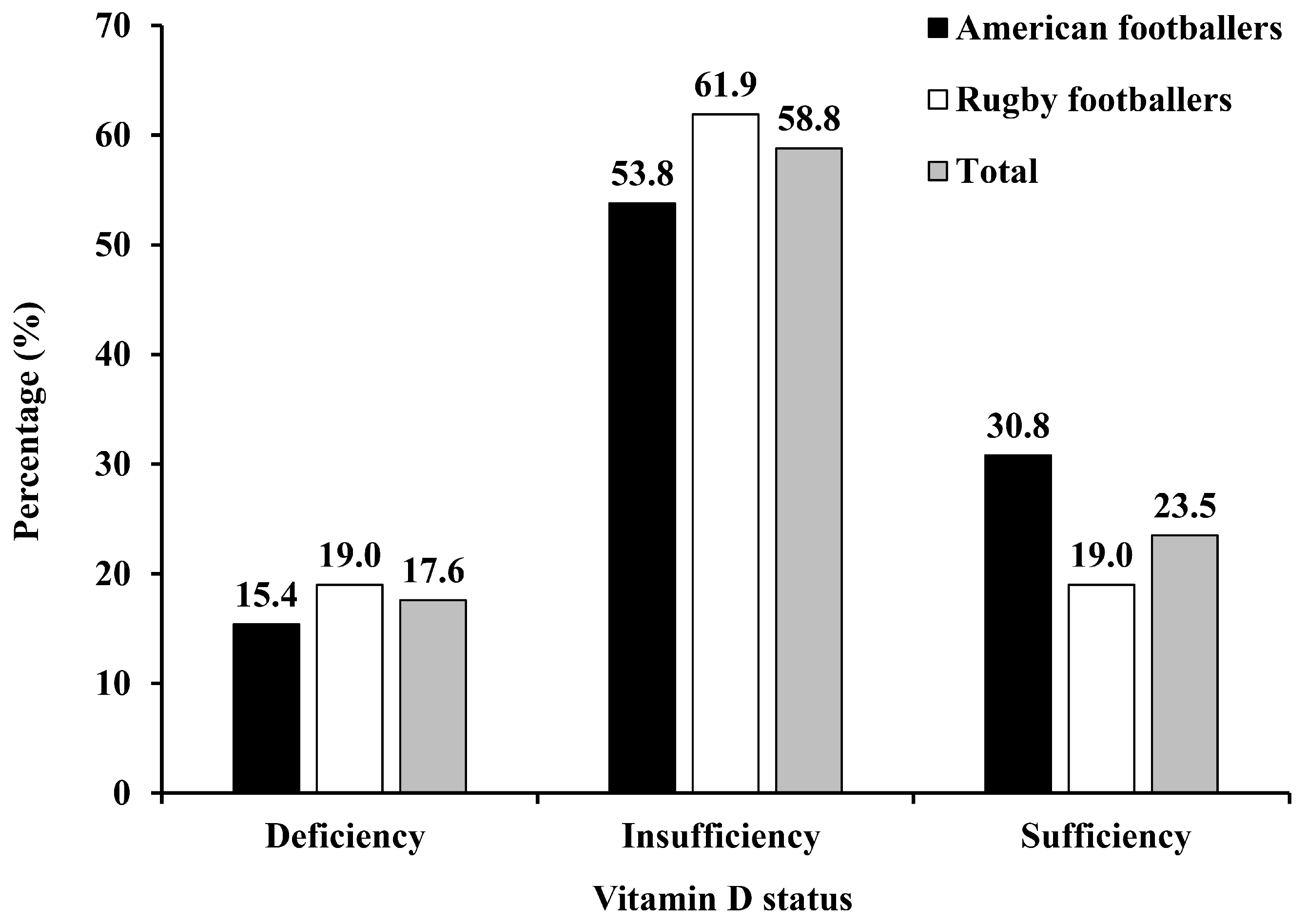
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Saydah, S.; Bullard, K.M.; Cheng, Y.; Ali, M.K.; Gregg, E.W.; Geiss, L.; Imperatore, G. Trends in cardiovascular disease risk factors by obesity level in adults in the United States, NHANES 1999–2010. Obesity 2014, 22, 1888–1895. [Google Scholar] [CrossRef] [PubMed]
- Shulman, G.I. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N. Engl. J. Med. 2014, 371, 2237–2238. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhang, X.; Wang, L.; Guo, Y.; Xie, M. Prevalence of metabolic syndrome and its components among Chinese professional athletes of strength sports with different body weight categories. PLoS ONE 2013, 8, E79758. [Google Scholar] [CrossRef] [PubMed]
- Murata, H.; Oshima, S.; Torii, S.; Taguchi, M.; Higuchi, M. Characteristics of body composition and cardiometabolic risk of Japanese male heavyweight Judo athletes. J. Physiol. Anthropol. 2016, 35, 10. [Google Scholar] [CrossRef] [PubMed]
- Selden, M.A.; Helzberg, J.H.; Waeckerle, J.F.; Browne, J.E.; Brewer, J.H.; Monaco, M.E.; Tang, F.; O´keefe, J.H. Cardiometabolic abnormalities in current National Football League players. Am. J. Cardiol. 2009, 103, 969–971. [Google Scholar] [CrossRef] [PubMed]
- Sevastianova, K.; Santos, A.; Kotronen, A.; Hakkarainen, A.; Makkonen, J.; Silander, K.; Peltonen, M.; Romeo, S.; Lundbom, J.; Lundbom, N.; et al. Effect of short-term carbohydrate overfeeding and long-term weight loss on liver fat in overweight humans. Am. J. Clin. Nutr. 2012, 96, 727–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iggman, D.; Rosqvist, F.; Larsson, A.; Amlov, J.; Beckman, L.; Rudling, M.; Riserus, U. Role of dietary fats in modulating cardiometabolic risk during moderate weight gain: A randomized double-blind overfeeding trial (LIPOGAIN Study). J. Am. Heart Assoc. 2014, 3, e001095. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D. Nonclassic actions of vitamin D. J. Clin. Endocrinol. Metab. 2009, 94, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Pollock, N.; Stallmann-Jorgensen, I.S.; Gutin, B.; Lan, L.; Chen, T.C.; Keeton, D.; Petty, K.; Holick, M.F.; Zhu, H. Low 25-hydroxyvitamin D levels in adolescents: Race, season, adiposity, physical activity, and fitness. Pediatrics 2010, 125, 1104–1111. [Google Scholar] [CrossRef] [PubMed]
- Kremer, R.; Campbell, P.P.; Reinhardt, T.; Gilsanz, V. Vitamin D status and its relationship to body fat, final height, and peak bone mass in young women. J. Clin. Endocrinol. Metab. 2009, 94, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Sulistyoningrum, D.C.; Green, T.J.; Lear, S.A.; Devlin, AM. Ethnic-specific differences in vitamin D status is associated with adiposity. PLoS ONE 2012, 7, e43159. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Cao, Z.B.; Tanisawa, K.; Ito, T.; Oshima, S.; Ishimi, Y.; Tabata, I.; Higuchi, M. Associations between the serum 25(OH)D concentration and lipid profiles in Japanese men. J. Atheroscler. Thromb. 2015, 22, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Wortsman, J.; Matsuoka, L.Y.; Chen, T.C.; Lu, Z.; Holick, M.F. Decreased bioavailability of vitamin D in obesity. Am. J. Clin. Nutr. 2000, 72, 690–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vimaleswaran, K.S.; Vimaleswaran, K.S.; Berry, D.J.; Lu, C.; Tikkanen, E.; Pilz, S.; Hiraki, L.T.; Cooper, J.D.; Dastani, Z.; Li, R.; et al. Causal relationship between obesity and vitamin D status: Bi-directional Mendelian randomization analysis of multiple cohorts. PLoS Med. 2013, 10, e1001383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Q.; Sergeev, I.N. Calcium and vitamin D in obesity. Nutr. Res. Rev. 2012, 25, 130–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sergeev, L.N.; Song, Q. High vitamin D and calcium intakes reduce diet-induced obesity in mice by increasing adipose tissue apoptosis. Mol. Nutr. Food Res. 2014, 58, 1342–1348. [Google Scholar] [CrossRef] [PubMed]
- Mai, X.M.; Chen, Y.; Camargo, C.A.; Langhammer, A. Cross-sectional and prospective cohort study of serum 25-hydroxyvitamin D level and obesity in Adults. Am. J. Epidemiol. 2012, 175, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; So, W.Y.; Zhang, D.; Cheng, Q.; Boucher, B.J.; Leung, P.S. Calcitriol reduces hepatic triglyceride accumulation and glucose output through Ca2+/CaMKK beta/AMPK activation under insulin resistant conditions in type 2 diabetes. Curr. Mol. Med. 2016, 16, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Borges, C.C.; Salles, A.F.; Bringhenti, I.; Mandarim-De-Lacerda, C.A.; Aguila, M.B. Vitamin D deficiency increases lipogenesis and reduces Beta-oxidation in the liver of diet-induced obese mice. J. Nutr. Sci. Vitaminol. 2018, 64, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Cassity, E.P.; Redzic, M.; Teager, C.R.; Thomas, D.T. The effect of body composition and BMI on 25(OH)D response in vitamin D-supplemented athletes. Eur. J. Sport Sci. 2016, 16, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Heller, J.E.; Thomas, J.J.; Hollis, B.W.; Larson-Meyer, D.E. Relation between vitamin D status and body composition in collegiate athletes. Int. J. Sport Nutr. Exerc. Metab. 2015, 25, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Barcal, J.N.; Thomas, J.T.; Hollis, B.W.; Austin, K.J.; Alexander, B.M.; Larson-Meyer, D.E. Vitamin D and weight cycling: Impact on injury, illness, and inflammation in collegiate wrestlers. Nutrients 2016, 8, 775. [Google Scholar] [CrossRef] [PubMed]
- Gilsanz, V.; Kremer, A.; Mo, A.O.; Wren, T.A.L.; Kremer, R. Vitamin D status and its relation to muscle mass and muscle fat in young women. J. Clin. Endocr. Metab. 2010, 95, 1595–1601. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.; Joham, A.; Teede, H.; Gibson-Helm, M.; Harrison, C.; Cassar, S.; Hutchison, S.; Ebeling, P.R.; Stepto, N. Associations of vitamin D with inter- and intra-muscular adipose tissue and insulin resistance in women with and without polycystic ovary syndrome. Nutrients 2016, 8, 774. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Lin, H.; Xia, M.; Aleteng, Q.; Li, X.; Ma, H.; Pan, B.; Gao, J.; Gao, X. Vitamin D levels are inversely associated with liver fat content and risk of non-alcoholic fatty liver disease in a Chinese middle-aged and elderly population: The Shanghai Changfeng Study. PLoS ONE 2016, 11, e0157515. [Google Scholar]
- Dube, J.J.; Amati, F.; Stefanovic-Racic, M.; Toledo, F.G.S.; Sauers, S.E.; Goodpaster, B.H. Exercise-induced alterations in intramyocellular lipids and insulin resistance: The athlete's paradox revisited. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E882–E888. [Google Scholar] [CrossRef] [PubMed]
- Sunami, A.; Sasaki, K.; Suzuki, Y.; Oguma, N.; Ishihara, J.; Nakai, A.; Yasuda, J.; Yokoyama, Y.; Yoshizaki, T.; Tada, Y.; et al. Validity of a semi-quantitative food frequency questionnaire for collegiate athletes. J. Epidemiol. 2016, 26, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Cao, Z.B.; Tanisawa, K.; Ito, T.; Oshima, S.; Higuchi, M. Vitamin D supplementation reduces insulin resistance in Japanese adults: A secondary analysis of a double-blind, randomized, placebo-controlled trial. Nutr. Res. 2016, 36, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhu, Y.; Guo, P.; Chen, Z.; Xie, F.; Liu, X.; He, S. Serum 25(OH)D and lipid levels in Chinese Obese and normal weight males before and after oral vitamin D supplementation. Biomed. Environ. Sci. 2013, 26, 801–807. [Google Scholar] [PubMed]
- Sun, X.; Cao, Z.B.; Taniguchi, H.; Tanisawa, K.; Higuchi, M. Effect of an acute bout of endurance exercise on serum 25(OH)D concentrations in young adults. J. Clin. Endocrinol. Metab. 2017, 102, 3937–3944. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Cao, Z.B.; Tanisawa, K.; Taniguchi, H.; Kubo, T.; Higuchi, M. Effects of chronic endurance exercise training on serum 25(OH)D concentrations in elderly Japanese men. Endocrine 2018, 59, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Berglund, L.; Sundgot-Borgen, J.; Berglund, B. Adipositas athletica: A group of neglected conditions associated with medical risks. Scand. J. Med. Sci. Sports 2011, 21, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Ma, X.; Shen, Y.; Ni, J.; Luo, Y.; Xiao, Y.; Bao, Y.; Jia, W. Associations of serum 25-hydroxyvitamin D3 levels with visceral adipose tissue in Chinese men with normal glucose tolerance. PLoS ONE 2014, 9, e86773. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.H.; Lee, J.H.; Kim, J.W.; Cho, J.H.; Choi, Y.H.; Ko, S.H.; Zimmet, P.; Son, H.Y. Epidemic obesity and type 2 diabetes in Asia. Lancet 2016, 368, 1681–1688. [Google Scholar] [CrossRef]
- Bosma, M. Lipid droplet dynamics in skeletal muscle. Exp. Cell Res. 2016, 340, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; He, J.; Watkins, S.; Kelley, D.E. Skeletal muscle lipid content and insulin resistance: Evidence for a paradox in endurance-trained athletes. J. Clin. Endocr. Metab. 2001, 86, 5755–5761. [Google Scholar] [CrossRef] [PubMed]

| Variable | Total (n = 34) | American Football (n = 13) | Rugby Football (n = 21) |
|---|---|---|---|
| Age (years) | 21.0 (20.0–21.0) | 21.0 (20.0–22.0) | 21.0 (20.0–21.0) |
| Height (cm) | 175.0 ± 4.8 | 176.1 ± 4.5 | 174.4 ± 5.0 |
| Weight (kg) | 90.4 ± 10.9 | 95.5 ± 9.3 | 87.3 ± 10.8 * |
| BMI (kg/m2) | 29.5 ± 3.0 | 30.7 ± 2.0 | 28.7 ± 3.3 |
| WC (cm) | 90.3 ± 8.0 | 94.8 ± 6.4 | 87.5 ± 7.8 * |
| % Body fat | 16.6 ± 4.1 | 19.4 ± 2.6 | 14.8 ± 3.9 a,* |
| SFA (cm2) | 168.0 (89.5–222.0) | 207.0 (170.0–275.0) | 112.5 (72.3–183.0) a,* |
| VFA (cm2) | 82.8 ± 32.4 | 95.1 ± 31.6 | 74.8 ± 31.2 a |
| VO2peakFFM (mL/kg/min) | 48.7 ± 5.6 | 46.5 ± 5.4 | 50.1 ± 5.4 a |
| IHCL | 0.037 (0.017–0.082) | 0.047 (0.023–0.177) | 0.024 (0.008–0.064) |
| IMCL | 0.013 ± 0.006 | 0.014 ± 0.008 | 0.012 ± 0.005 |
| EMCL | 0.017 (0.012–0.030) | 0.019 (0.012–0.032) | 0.015 (0.012–0.028) |
| AST (IU/L) | 24.5 (21.8–36.0) | 25.0 (22.5–34.0) | 24.0 (21.0–36.5) |
| ALT (IU/L) | 34.1 ± 16.4 | 37.4 ± 20.8 | 32.1 ± 13.1 |
| γ-GTP (IU/L) | 28.0 (24.5–38.0) | 29.0 (24.0–35.0) | 27.0 (24.0–43.0) |
| 25(OH)D (nmol/L) | 61.3 (54.2–74.7) | 62.3 (53.9–79.0) | 60.3 (52.8–73.0) |
| Total energy intake (kcal) | 3895.8 (3534.5–4365.0) | 4071.7 (3662.8–5089.1) | 3840.7 (3476.1–4232.5) b |
| Carbohydrate intake (g) | 511.6 (461.9–621.0) | 522.6 (479.3–658.9) | 501.0 (450.2–571.1) b |
| Fat intake (g) | 130.9 ± 35.0 | 143.0 ± 32.0 | 123.1 ± 35.3 b |
| Protein intake (g) | 143.2 ± 33.8 | 155.0 ± 37.5 | 135.5 ± 29.6 b |
| Vitamin D intake (μg/day) | 10.4 ± 4.1 | 11.2 ± 4.9 | 9.7 ± 12.4 b |
| Calcium intake (mg/day) | 1185.4 ± 442.0 | 1284.8 ± 402.3 | 1120.8 ± 464.3 b |
| Variables | 25(OH)D | 25(OH)D * | ||
|---|---|---|---|---|
| r | p-Value | r | p-Value | |
| BMI (kg/m2) | −0.231 | 0.189 | −0.012 | 0.952 |
| WC (cm) | −0.279 | 0.110 | −0.200 | 0.307 |
| % Body fat | −0.036 | 0.841 | 0.164 | 0.405 |
| SFA (cm2) | −0.138 | 0.444 | −0.026 | 0.895 |
| VFA (cm2) | −0.307 | 0.083 | −0.218 | 0.265 |
| IHCL | −0.372 | 0.030 | −0.378 | 0.047 |
| IMCL | 0.032 | 0.860 | −0.080 | 0.686 |
| EMCL | 0.019 | 0.913 | 0.125 | 0.526 |
| ALT (IU/L) | −0.098 | 0.583 | −0.048 | 0.806 |
| AST (IU/L) | 0.174 | 0.324 | 0.217 | 0.268 |
| γ-GTP (IU/L) | −0.297 | 0.088 | −0.317 | 0.100 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Cao, Z.-B.; Tanisawa, K.; Oshima, S.; Higuchi, M. Serum 25-Hydroxyvitamin D Concentrations Are Inversely Correlated with Hepatic Lipid Content in Male Collegiate Football Athletes. Nutrients 2018, 10, 942. https://doi.org/10.3390/nu10070942
Sun X, Cao Z-B, Tanisawa K, Oshima S, Higuchi M. Serum 25-Hydroxyvitamin D Concentrations Are Inversely Correlated with Hepatic Lipid Content in Male Collegiate Football Athletes. Nutrients. 2018; 10(7):942. https://doi.org/10.3390/nu10070942
Chicago/Turabian StyleSun, Xiaomin, Zhen-Bo Cao, Kumpei Tanisawa, Satomi Oshima, and Mitsuru Higuchi. 2018. "Serum 25-Hydroxyvitamin D Concentrations Are Inversely Correlated with Hepatic Lipid Content in Male Collegiate Football Athletes" Nutrients 10, no. 7: 942. https://doi.org/10.3390/nu10070942





