Anti-Osteoporotic Effects of Polysaccharides Isolated from Persimmon Leaves via Osteoclastogenesis Inhibition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Preparation of Polysaccharides from Persimmon (PLE0)
2.3. Ovariectomized Mouse Model and Estimated Parameters of Bone Loss
2.4. Cell Culture and Osteoclast Formation
2.5. Immunoblotting
2.6. Real-Time Quantitative PCR
2.7. Statistical Analysis
3. Results and Discussion
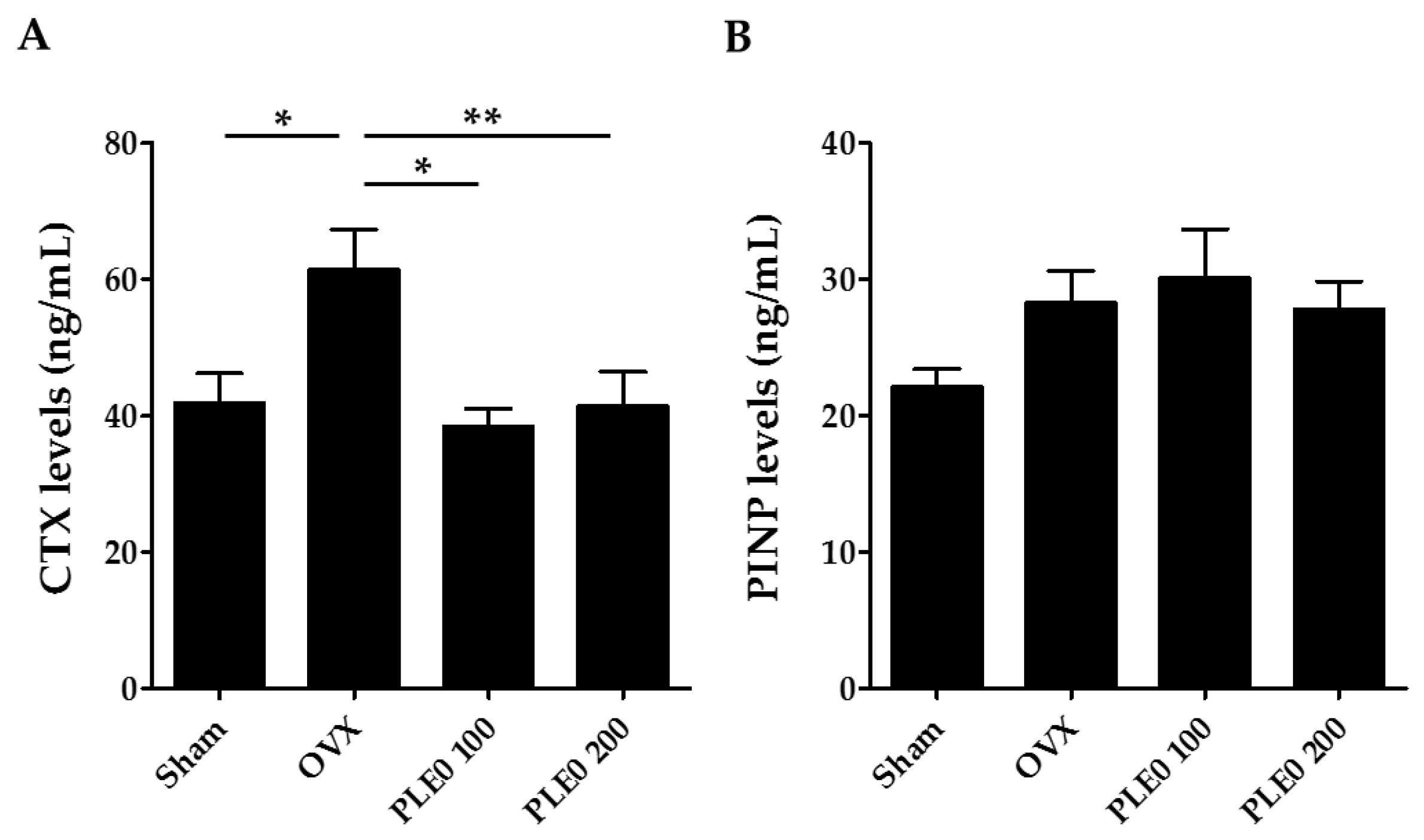
3.1. PLE0 Attenuates OVX-Induced Bone Loss in Mice
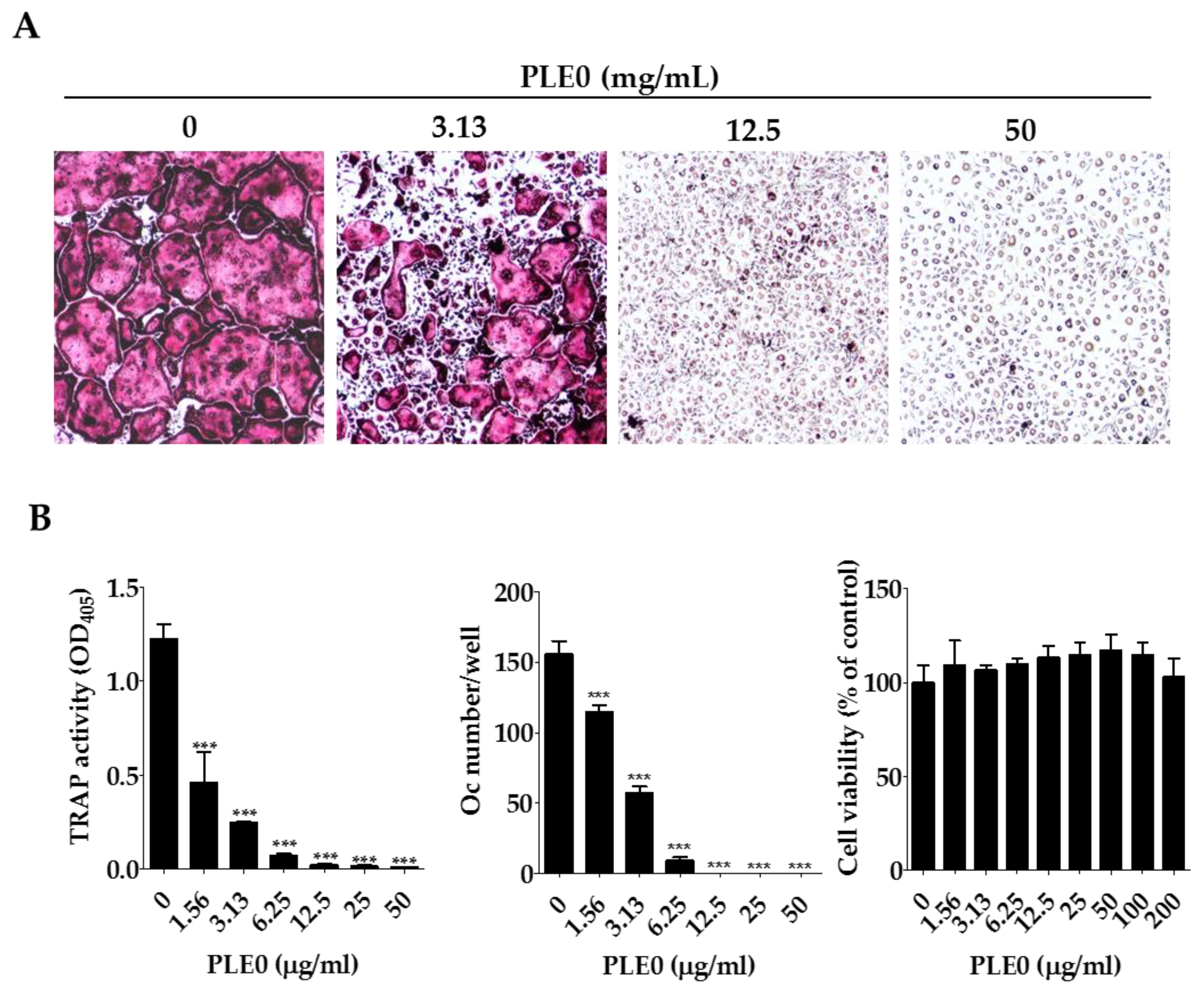
3.2. PLE0 Inhibits Osteoclast Differentiation in BMMs
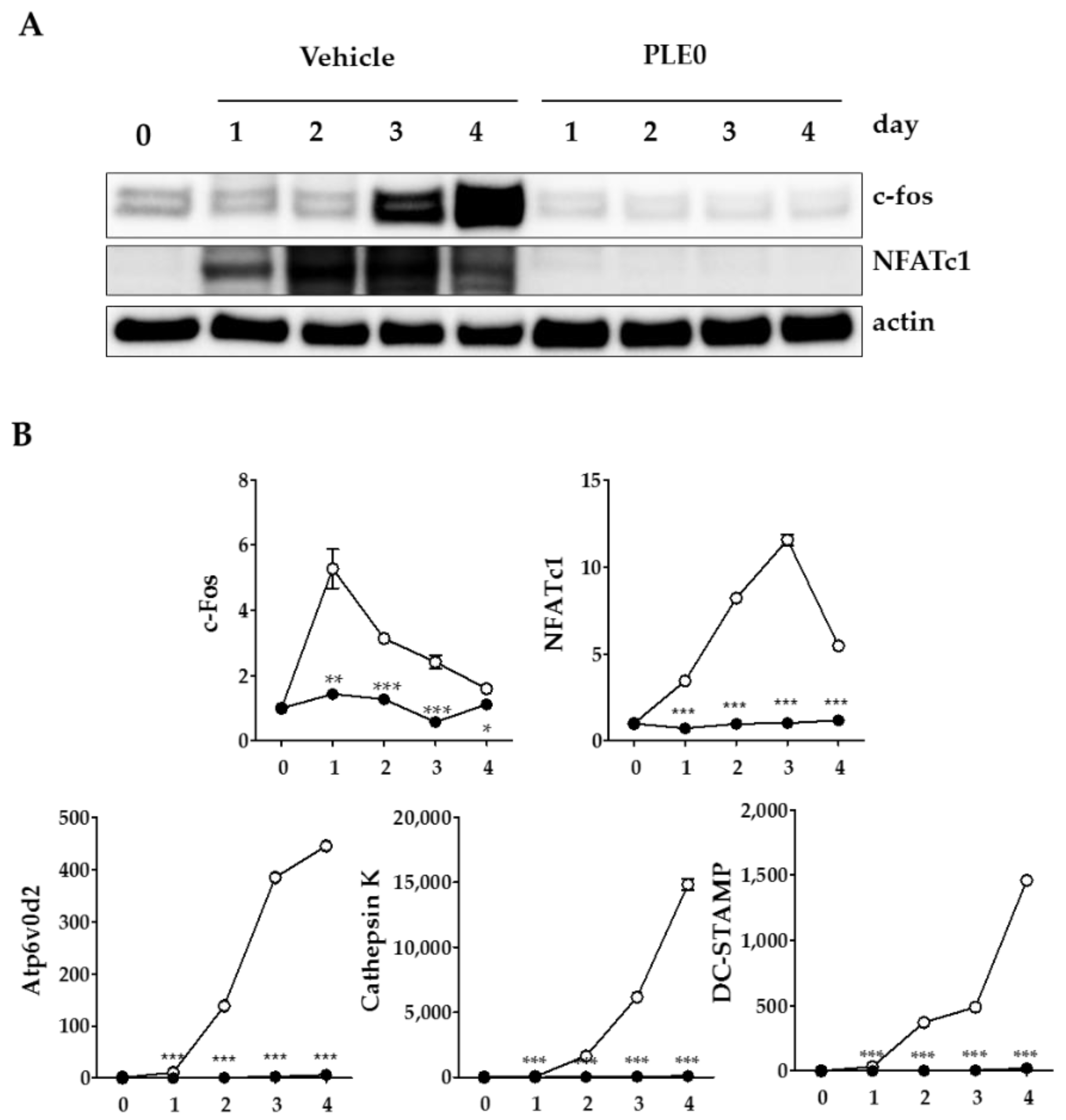
3.3. PLE0 Inhibits RANKL-Induced Expression of c-Fos and NFATc1 in Osteoclast Precusor Cells
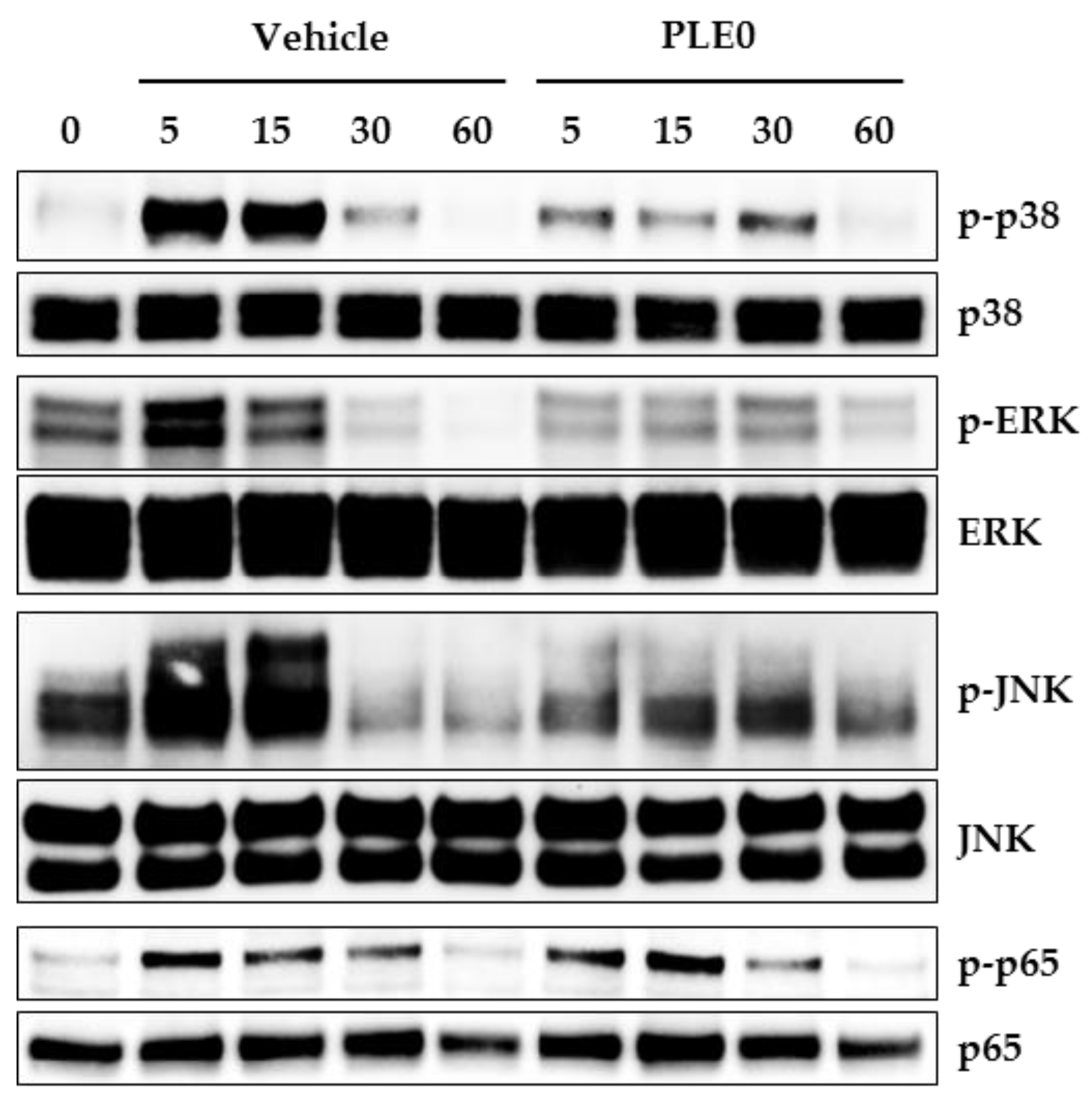
3.4. PLE0 Inhibits RANKL-Induced Early Signaling Pathways
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Seibel, M.J. Biochemical markers of bone turnover: Part i: Biochemistry and variability. Clin. Biochem. Rev. 2005, 26, 97–122. [Google Scholar] [PubMed]
- Asagiri, M.; Sato, K.; Usami, T.; Ochi, S.; Nishina, H.; Yoshida, H.; Morita, I.; Wagner, E.F.; Mak, T.W.; Serfling, E.; et al. Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J. Exp. Med. 2005, 202, 1261–1269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, F.P. M-csf, c-fms, and signaling in osteoclasts and their precursors. Ann. N. Y. Acad. Sci. 2006, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Song, I.; Kim, J.H.; Kim, K.; Jin, H.M.; Youn, B.U.; Kim, N. Regulatory mechanism of NFATc1 in RANKL-induced osteoclast activation. FEBS Lett. 2009, 583, 2435–2440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.; Lee, S.H.; Ha Kim, J.; Choi, Y.; Kim, N. NFATc1 induces osteoclast fusion via up-regulation of Atp6v0d2 and the dendritic cell-specific transmembrane protein (DC-STAMP). Mol. Endocrinol. 2008, 22, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, H.; Kim, S.; Koga, T.; Nishina, H.; Isshiki, M.; Yoshida, H.; Saiura, A.; Isobe, M.; Yokochi, T.; Inoue, J.; et al. Induction and activation of the transcription factor NFATc1 (NFATc2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev. Cell 2002, 3, 889–901. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Guo, Y.; Ma, R.; Fu, M.; Niu, J.; Gao, S.; Zhang, D. Herba epimedii: An ancient Chinese herbal medicine in the prevention and treatment of osteoporosis. Curr. Pharm. Des. 2016, 22, 328–349. [Google Scholar] [CrossRef] [PubMed]
- Shalan, N.A.; Mustapha, N.M.; Mohamed, S. Noni leaf and black tea enhance bone regeneration in estrogen-deficient rats. Nutrition 2017, 33, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, Y.; Xue, L.; Severino, R.P.; Gao, S.; Niu, J.; Qin, L.P.; Zhang, D.; Brömme, D. Salvia miltiorrhiza: an ancient Chinese herbal medicine as a source for anti-osteoporotic drugs. J. Ethnopharmacol. 2014, 155, 1401–1416. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, D.; Yang, D.; Zhen, W.; Zhang, J.; Peng, S. The effect of icariin on bone metabolism and its potential clinical application. Osteoporos. Int. 2018, 29, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Xie, Z.; Xu, X.; Yang, D. Persimmon (Diospyros kaki L.) leaves: A review on traditional uses, phytochemistry and pharmacological properties. J. Ethnopharmacol. 2015, 163, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Tsurunaga, Y.; Takabayashi, Y.; Nishi, M.; Suzuki, Y. Differences in the ascorbic acid, astragalin, and polyphenol contents, and the DPPH radical scavenging activity of 22 commercial persimmon leaf tea products. J. Home Econ. Jpn. 2011, 62, 437–444. [Google Scholar]
- Mallavadhani, U.V.; Panda, A.K.; Rao, Y.R. Review article number 134 pharmacology and chemotaxonomy of diospyros. Phytochemistry 1998, 49, 901–951. [Google Scholar] [CrossRef]
- Sakanaka, S.; Tachibana, Y.; Okada, Y. Preparation and antioxidant properties of extracts of Japanese persimmon leaf tea (kakinoha-cha). Food Chem. 2005, 89, 569–575. [Google Scholar] [CrossRef]
- Asgar, M.A.; Yamauchi, R.; Kato, K. Structural features of pectins from fresh and sun-dried Japanese persimmon fruit. Food Chem. 2004, 87, 247–251. [Google Scholar] [CrossRef]
- Lu, X.; Mo, X.; Guo, H.; Zhang, Y. Sulfation modification and anticoagulant activity of the polysaccharides obtained from persimmon (Diospyros kaki L.) fruits. Int. J. Biol. Macromol. 2012, 51, 1189–1195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lu, X.; Fu, Z.; Wang, Z.; Zhang, J. Sulphated modification of a polysaccharide obtained from fresh persimmon (Diospyros kaki L.) fruit and antioxidant activities of the sulphated derivatives. Food Chem. 2011, 127, 1084–1090. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.G.; Jung, J.Y.; Shin, J.S.; Shin, K.S.; Cho, C.W.; Rhee, Y.K.; Hong, H.D.; Lee, K.T. Immunostimulatory polysaccharide isolated from the leaves of diospyros kaki thumb modulate macrophage via tlr2. Int. J. Biol. Macromol. 2015, 79, 971–982. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.-S.; Lee, H.; Hong, H.-D.; Shin, K.-S. Characterization of immunostimulatory pectic polysaccharide isolated from leaves of Diospyros kaki thumb. (persimmon). J. Funct. Foods 2016, 26, 319–329. [Google Scholar] [CrossRef]
- Ha, H.; An, H.; Shim, K.S.; Kim, T.; Lee, K.J.; Hwang, Y.H.; Ma, J.Y. Ethanol extract of Atractylodes macrocephala protects bone loss by inhibiting osteoclast differentiation. Molecules 2013, 18, 7376–7388. [Google Scholar] [CrossRef] [PubMed]
- Sophocleous, A.; Idris, A.I. Rodent models of osteoporosis. Bonekey Rep. 2014, 3, 614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.H.; Kim, H.N.; Yang, D.; Jung, K.; Kim, H.M.; Kim, H.H.; Ha, H.; Lee, Z.H. Trolox prevents osteoclastogenesis by suppressing RANKL expression and signaling. J. Biol. Chem. 2009, 284, 13725–13734. [Google Scholar] [CrossRef] [PubMed]
- Ha, H.; Shim, K.S.; Kim, T.; An, H.; Lee, C.J.; Lee, K.J.; Ma, J.Y. Water extract of acer tegmentosum reduces bone destruction by inhibiting osteoclast differentiation and function. Molecules 2014, 19, 3940–3954. [Google Scholar] [CrossRef] [PubMed]
- Schepetkin, I.A.; Quinn, M.T. Botanical polysaccharides: Macrophage immunomodulation and therapeutic potential. Int. Immunopharmacol. 2006, 6, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Cao, Z.; Tickner, J.; Qiu, H.; Wang, C.; Chen, K.; Wang, Z.; Guo, C.; Dong, S.; Xu, J. Poria cocos polysaccharide attenuates RANKL-induced osteoclastogenesis by suppressing NFATc1 activity and phosphorylation of ERK and STAT3. Arch. Biochem. Biophys. 2018, 647, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yin, D.; Liu, T.; Chen, F.; Chen, Y.; Wang, X.; Sheng, J. Tea polysaccharide inhibits RANKL-induced osteoclastogenesis in raw264.7 cells and ameliorates ovariectomy-induced osteoporosis in rats. Biomed. Pharmacother. 2018, 102, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Syed, Z.; Khan, A. Bone densitometry: Applications and limitations. J. Obstet. Gynaecol. Can. 2002, 24, 476–484. [Google Scholar] [CrossRef]
- Jakubas-Przewlocka, J.; Przewlocki, P.; Sawicki, A. Assessment of changes due to the long-term effect of estrogen and calcium deficiency in the trabecular bone structure in rats. Clin. Exp. Rheumatol. 2005, 23, 385–388. [Google Scholar] [PubMed]
- Kang, S.J.; Choi, B.R.; Kim, S.H.; Yi, H.Y.; Park, H.R.; Kim, D.C.; Choi, S.H.; Han, C.H.; Park, S.J.; Song, C.H.; et al. Dried pomegranate potentiates anti-osteoporotic and anti-obesity activities of red clover dry extracts in ovariectomized rats. Nutrients 2015, 7, 2622–2647. [Google Scholar] [CrossRef] [PubMed]
- Sims, N.A.; Dupont, S.; Krust, A.; Clement-Lacroix, P.; Minet, D.; Resche-Rigon, M.; Gaillard-Kelly, M.; Baron, R. Deletion of estrogen receptors reveals a regulatory role for estrogen receptors-beta in bone remodeling in females but not in males. Bone 2002, 30, 18–25. [Google Scholar] [CrossRef]
- Martin-Fernandez, M.; Valencia, K.; Zandueta, C.; Ormazabal, C.; Martinez-Canarias, S.; Lecanda, F.; de la Piedra, C. The usefulness of bone biomarkers for monitoring treatment disease: A comparative study in osteolytic and osteosclerotic bone metastasis models. Transl. Oncol. 2017, 10, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Lacey, D.L.; Timms, E.; Tan, H.L.; Kelley, M.J.; Dunstan, C.R.; Burgess, T.; Elliott, R.; Colombero, A.; Elliott, G.; Scully, S.; et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 1998, 93, 165–176. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, N. Regulation of nfatc1 in osteoclast differentiation. J. Bone Metab. 2014, 21, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Wada, T.; Nakashima, T.; Hiroshi, N.; Penninger, J.M. Rankl-rank signaling in osteoclastogenesis and bone disease. Trends Mol. Med. 2006, 12, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.S.; Ghosh, S. Nf-kappab, the first quarter-century: Remarkable progress and outstanding questions. Genes Dev. 2012, 26, 203–234. [Google Scholar] [CrossRef] [PubMed]
- Ha, H.; Shim, K.S.; Kim, T.; An, H.; Ma, J.Y. Water extract of dryopteris crassirhizoma attenuates bone loss by suppressing osteoclast differentiation and function. Evid. Based Complement. Altern. Med. 2013, 2013, 852648. [Google Scholar] [CrossRef] [PubMed]

| Composition/Component | PLE0 1 |
|---|---|
| Chemical composition (%) | |
| Neutral sugar | 58.1 ± 1.66 |
| Uronic acid | 37.0 ± 0.64 |
| 2-keto-3-deoxy-mannooctanoic acid (KDO)-like materials | 4.43 ± 1.51 |
| Protein | 0.48 ± 0.10 |
| Component sugar (mol %) 2 | |
| Fucose | 2.29 ± 0.50 |
| Rhamnose | 4.24 ± 3.80 |
| Arabinose | 19.4 ± 4.10 |
| Galactose | 26.5 ± 1.42 |
| Glucose | 6.77 ± 1.64 |
| Mannose | 2.65 ± 0.62 |
| Xylose | 4.25 ± 0.19 |
| Galacturonic acid | 29.8 ± 1.05 |
| Glucoronic acid | 4.33 ± 0.66 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, Y.-H.; Ha, H.; Kim, R.; Cho, C.-W.; Song, Y.-R.; Hong, H.-D.; Kim, T. Anti-Osteoporotic Effects of Polysaccharides Isolated from Persimmon Leaves via Osteoclastogenesis Inhibition. Nutrients 2018, 10, 901. https://doi.org/10.3390/nu10070901
Hwang Y-H, Ha H, Kim R, Cho C-W, Song Y-R, Hong H-D, Kim T. Anti-Osteoporotic Effects of Polysaccharides Isolated from Persimmon Leaves via Osteoclastogenesis Inhibition. Nutrients. 2018; 10(7):901. https://doi.org/10.3390/nu10070901
Chicago/Turabian StyleHwang, Youn-Hwan, Hyunil Ha, Rajeong Kim, Chang-Won Cho, Young-Ran Song, Hee-Do Hong, and Taesoo Kim. 2018. "Anti-Osteoporotic Effects of Polysaccharides Isolated from Persimmon Leaves via Osteoclastogenesis Inhibition" Nutrients 10, no. 7: 901. https://doi.org/10.3390/nu10070901




