Iodine Status and Consumption of Key Iodine Sources in the U.S. Population with Special Attention to Reproductive Age Women
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Iodine Status
2.3. Food Groups
2.4. Supplements Contributing to Iodine Intake
2.5. Use of Salt at the Table and in Food Prepartaion
2.6. Covariates
2.7. Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zimmermann, M.B.; Jooste, P.L.; Pandav, C.S. Iodine-deficiency disorders. Lancet 2008, 372, 1251–1262. [Google Scholar] [CrossRef]
- Gibson, R.S. Principles of Nutritional Assessement, 2nd ed.; Oxford University Press: New York, NY, USA, 2005. [Google Scholar]
- Zimmermann, M.B.; Trumbo, P.R. Iodine. Adv. Nutr. 2013, 4, 262–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmermann, M.B. The adverse effects of mild-to-moderate iodine deficiency during pregnancy and childhood: A review. Thyroid 2007, 17, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.B. The role of iodine in human growth and development. In Seminars in Cell & Developmental Biology; Academic Press: Cambridge, MA, USA, 2011; Volume 22, pp. 645–652. [Google Scholar]
- Perez-Lopez, F.R. Iodine and thyroid hormones during pregnancy and postpartum. Gynecol. Endocrinol. 2007, 23, 414–428. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, K.A.; Thoma, M.; Copen, C.; Frederiksen, B.; Decker, E.; Moskosky, S. Unintended pregnancy and interpregnancy interval by maternal age, National Survey of Family Growth. Contraception 2018, 98, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Fuge, R.; Johnson, C. Iodine and Human Health, the Role of Environmental Geochemistry and Diet, a Review. Appl. Geochem. 2015, 63. [Google Scholar] [CrossRef]
- Haldimann, M.; Alt, A.; Blanc, A.; Blondeau, K. Iodine Content of Food Groups. J. Food Compos. Anal. 2005, 18, 461–472. [Google Scholar] [CrossRef]
- Murray, C.W.; Egan, S.K.; Kim, H.; Beru, N.; Bolger, P.M. US Food and Drug Administration’s Total Diet Study: Dietary intake of perchlorate and iodine. J. Expo. Sci. Environ. Epidemiol. 2008, 18, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Pearce, E.N.; Pino, S.; He, X.; Bazrafshan, H.R.; Lee, S.L.; Braverman, L.E. Sources of dietary iodine: Bread, cows’ milk, and infant formula in the Boston area. J. Clin. Endocrinol. Metab. 2004, 89, 3421–3424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Institute of Medicine Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academies Press: Washington, DC, USA, 2001. [Google Scholar]
- Abt, E.; Spungen, J.; Pouillot, R.; Gamalo-Siebers, M.; Wirtz, M. Update on dietary intake of perchlorate and iodine from U.S. food and drug administration’s total diet study: 2008–2012. J. Expo. Sci. Environ. Epidemiol. 2018, 28, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Juan, W.; Trumbo, P.R.; Spungen, J.H.; Dwyer, J.T.; Carriquiry, A.L.; Zimmerman, T.P.; Swanson, C.A.; Murphy, S.P. Comparison of 2 methods for estimating the prevalences of inadequate and excessive iodine intakes. Am. J. Clin. Nutr. 2016, 104 (Suppl. S3), 888s–897s. [Google Scholar] [CrossRef] [PubMed]
- Borucki Castro, S.I.; Berthiaume, R.; Laffey, P.; Fouquet, A.; Beraldin, F.; Robichaud, A.; Lacasse, P. Iodine concentration in milk sampled from Canadian farms. J. Food Prot. 2010, 73, 1658–1663. [Google Scholar] [CrossRef] [PubMed]
- Castro, S.I.; Berthiaume, R.; Robichaud, A.; Lacasse, P. Effects of iodine intake and teat-dipping practices on milk iodine concentrations in dairy cows. J. Dairy Sci. 2012, 95, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Weiss, W.P.; Wyatt, D.J.; Kleinschmit, D.H.; Socha, M.T. Effect of including canola meal and supplemental iodine in diets of dairy cows on short-term changes in iodine concentrations in milk. J. Dairy Sci. 2015, 98, 4841–4849. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Shin, D.; Cho, M.S.; Song, W.O. Food Group Intakes as Determinants of Iodine Status among US Adult Population. Nutrients 2016, 8, 325. [Google Scholar] [CrossRef] [PubMed]
- Markel, H. “When it rains it pours”: Endemic goiter, iodized salt, and David Murray Cowie, MD. Am. J. Public Health 1987, 77, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Stagnaro-Green, A.; MacKay, D.; Wong, A.W.; Pearce, E.N. Iodine Contents in Prenatal Vitamins in the United States. Thyroid 2017, 27, 1101–1102. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, P.K.; Liu, Y.; Dyke, J.V. Iodine nutrition: Iodine content of iodized salt in the United States. Environ. Sci. Technol. 2008, 42, 1315–1323. [Google Scholar] [CrossRef] [PubMed]
- Charlton, K.; Skeaff, S. Iodine fortification: Why, when, what, how, and who? Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 618–624. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Urinary Iodine Concentrations for Determining Iodine Status Deficiency in Populations. In Vitamin and Mineral Nutrition Information Systems; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Caldwell, K.L.; Jones, R.; Hollowell, J.G. Urinary iodine concentration: United States National Health and Nutrition Examination Survey 2001–2002. Thyroid 2005, 15, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Iodine: Fact Sheet for Health Professionals. Available online: https://ods.od.nih.gov/factsheets/Iodine-HealthProfessional/ (accessed on 1 January 2017).
- Caldwell, K.L.; Pan, Y.; Mortensen, M.E.; Makhmudov, A.; Merrill, L.; Moye, J. Iodine status in pregnant women in the National Children’s Study and in U.S. women (15–44 years), National Health and Nutrition Examination Survey 2005–2010. Thyroid 2013, 23, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Cho, M.S.; Shin, D.; Song, W.O. Changes in iodine status among US adults, 2001–2012. Int. J. Food Sci. Nutr. 2016, 67, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, K.M.; Perrine, C.G.; Pearce, E.N.; Caldwell, K.L. Monitoring the iodine status of pregnant women in the United States. Thyroid 2013, 23, 520–521. [Google Scholar] [CrossRef] [PubMed]
- Perrine, C.G.; Herrick, K.A.; Serdula, M.K.; Sullivan, K.M. Some subgroups of reproductive age women in the United States may be at risk for iodine deficiency. J. Nutr. 2010, 140, 1489–1494. [Google Scholar] [CrossRef] [PubMed]
- Perrine, C.G.; Sullivan, K.M.; Flores, R.; Caldwell, K.L.; Grummer-Strawn, L.M. Intakes of dairy products and dietary supplements are positively associated with iodine status among U.S. children. J. Nutr. 2013, 143, 1155–1160. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.L.; Dohrmann, S.M.; Burt, V.L.; Mohadjer, L.K. National Health and Nutrition Examination Survey: Sample Design, 2011–2014; Vital Health Stat 2(162); National Center for Health Statistics: Neisville, MD, USA, 2014.
- National Health and Nutrition Examination Survey: NHANES Response Rates and CPS Totals. Available online: http://www.cdc.gov/nchs/nhanes/response_rates_CPS.htm (accessed on 1 January 2017).
- Laboratory Procedure Manual Iodine and Mercury in Urine by Inductively Coupled Plasma Dynamic Reaction Cell Mass Spectrometry (ICP-DRC-MS), NHANES 2011–2012. Available online: https://wwwn.cdc.gov/nchs/data/nhanes/2011-2012/labmethods/uiouhg_g_met_iodine_mercury.pdf (accessed on 1 January 2017).
- Laboratory Procedure Manual for Iodine and Mercury in Urine by ICP-DRC-MS, NHANES 2013–2014. Available online: https://wwwn.cdc.gov/nchs/data/nhanes/2013-2014/labmethods/UHG_UIO_URIO_H_MET.pdf (accessed on 1 January 2017).
- PerkinElmer. Available online: http://www.perkinelmer.com/ (accessed on 5 July 2018).
- World Health Organization; International Council for Control of Iodine Deficiency Disorders; United Nations Children’s Fund. Assessment of Iodine Deficiency Disorders and Monitoring Their Elimination: A Guide for Programme Managers, 3rd ed.; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Moshfegh, A.J.; Rhodes, D.G.; Baer, D.J.; Murayi, T.; Clemens, J.C.; Rumpler, W.V.; Paul, D.R.; Sebastian, R.S.; Kuczynski, K.J.; Ingwersen, L.A.; et al. The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. Am. J. Clin. Nutr. 2008, 88, 324–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dietary Interview–Total Nutrient Intakes 2011–2012. Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2011-2012/DR1TOT_G.htm (accessed on 1 January 2017).
- USDA Food and Nutrient Database for Dietary Studies 2011–2012. Food Surveys Research Group Home Page. Available online: https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/fndds-download-databases/ (accessed on 1 January 2017).
- USDA Food and Nutrient Database for Dietary Studies 2013–2014. Food Surveys Research Group Home Page. Available online: https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/fndds-download-databases/ (accessed on 1 January 2017).
- Doerge, D.R.; Sheehan, D.M. Goitrogenic and estrogenic activity of soy isoflavones. Environ. Health Perspect. 2002, 110 (Suppl. S3), 349–353. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/rdc/ (accessed on 5 July 2018).[Green Version]
- Johnson, C.L.; Paulose-Ram, R.; Ogden, C.L.; Carroll, M.D.; Kruszon-Moran, D.; Dohrmann, S.M.; Curtin, L.R. National Health and Nutrition Examination Survey: Analytic Guidelines, 1999–2010; U.S. Department of Health & Human Services: Hyattsville, MD, USA, 2013; pp. 1–24.
- NHANES 2011–2012 Data Documentation Dietary Interview–Total Nutrient Intakes First Day (DR1TOT_G). Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2011-2012/DR1TOT_G.htm (accessed on 1 January 2017).
- NHANES 2013–2014 Data Documentation Dietary Interview–Total Nutrient Intakes First Day (DR1TOT_H). Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2013-2014/DR1TOT_H.htm (accessed on 1 January 2017).
- Adjusting Sample Weights for Linkage-Eligibility Using SUDAAN. Available online: http://www.cdc.gov/nchs/data/datalinkage/adjusting_sample_weights_for_linkage_eligibility_using_sudaan.pdf (accessed on 1 January 2017).
- Willett, W. Nutritional Epidemiology, 2nd ed.; Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
- Arsenault, M.A.; Lee, K.W.; Song, W.O. Dairy Products and Iodine-Containing Supplements Use are Positively Associated with Iodine Status in Childbearing Age Women in the United States. FASEB J. 2017, 31, 446–447. [Google Scholar]
- Batres-Marquez, S.P.; Jensen, H.H.; Upton, J. Rice consumption in the United States: Recent evidence from food consumption surveys. J. Am. Diet. Assoc. 2009, 109, 1719–1727. [Google Scholar] [CrossRef] [PubMed]
- Pennington, J.A.T.; Schoen, S.A.; Salmon, G.D.; Young, B.; Johnson, R.D.; Marts, R.W. Composition of Core Foods of the U.S. Food Supply, 1982–1991: III. Copper, Manganese, Selenium, and Iodine. J. Food Compos. Anal. 1995, 8, 171–217. [Google Scholar] [CrossRef]
- Ma, W.; He, X.; Braverman, L. Iodine Content in Milk Alternatives. Thyroid 2016, 26, 1308–1310. [Google Scholar] [CrossRef] [PubMed]
- Teas, J.; Braverman, L.E.; Kurzer, M.S.; Pino, S.; Hurley, T.G.; Hebert, J.R. Seaweed and soy: Companion foods in Asian cuisine and their effects on thyroid function in American women. J. Med. Food. 2007, 10, 90–100. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization; United Nations Children’s Fund; The International Council for the Control of Iodine Deficiency Disorders. Indicators for Assessment of Iodine Deficiency Disorders and Their Control Programmes; Report of a Joint WHO/UNICEF/ICCIDD Consultation, 3–5 November 1992; World Health Organization: Geneva, Switzerland, 1993. [Google Scholar]
- CFR—Code of Federal Regulations Title 21: Food and Drugs. Sections 184.1634 and 184.1265 2011. Available online: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=184 (accessed on 1 January 2017).
- Maalouf, J.; Barron, J.; Gunn, J.P.; Yuan, K.; Perrine, C.G.; Cogswell, M.E. Iodized salt sales in the United States. Nutrients 2015, 7, 1691–1695. [Google Scholar] [CrossRef] [PubMed]
- Harnack, L.J.; Cogswell, M.E.; Shikany, J.M.; Gardner, C.D.; Gillespie, C.; Loria, C.M.; Zhou, X.; Yuan, K.; Steffen, L.M. Sources of Sodium in US Adults From 3 Geographic Regions. Circulation 2017, 135, 1775–1783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boon, C.S.; Taylor, C.L.; Henney, J.E. (Eds.) Strategies to Reduce Sodium Intake in the United States; The National Academies Press: Washington, DC, USA, 2010. [Google Scholar]
- Council on Environmental Health. Iodine deficiency, pollutant chemicals, and the thyroid: New information on an old problem. Pediatrics 2014, 133, 1163–1166. [Google Scholar]
- Leung, A.M.; Pearce, E.N.; Braverman, L.E. Iodine content of prenatal multivitamins in the United States. N. Engl. J. Med. 2009, 360, 939–940. [Google Scholar] [CrossRef] [PubMed]
- Hynes, K.L.; Otahal, P.; Hay, I.; Burgess, J.R. Mild iodine deficiency during pregnancy is associated with reduced educational outcomes in the offspring: 9-year follow-up of the gestational iodine cohort. J. Clin. Endocrinol. Metab. 2013, 98, 1954–1962. [Google Scholar] [CrossRef] [PubMed]
- Bath, S.C.; Steer, C.D.; Golding, J.; Emmett, P.; Rayman, M.P. Effect of inadequate iodine status in UK pregnant women on cognitive outcomes in their children: Results from the Avon Longitudinal Study of Parents and Children (ALSPAC). Lancet 2013, 382, 331–337. [Google Scholar] [CrossRef]
- Abel, M.H.; Caspersen, I.H.; Meltzer, H.M.; Haugen, M.; Brandlistuen, R.E.; Aase, H.; Alexander, J.; Torheim, L.E.; Brantsaeter, A.L. Suboptimal Maternal Iodine Intake Is Associated with Impaired Child Neurodevelopment at 3 Years of Age in the Norwegian Mother and Child Cohort Study. J. Nutr. 2017, 147, 1314–1324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, S.; Karmisholt, J.; Pedersen, K.M.; Laurberg, P. Reliability of studies of iodine intake and recommendations for number of samples in groups and in individuals. Br. J. Nutr. 2008, 99, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Konig, F.; Andersson, M.; Hotz, K.; Aeberli, I.; Zimmermann, M.B. Ten repeat collections for urinary iodine from spot samples or 24-hour samples are needed to reliably estimate individual iodine status in women. J. Nutr. 2011, 141, 2049–2054. [Google Scholar] [CrossRef] [PubMed]
- Corey, L.M.; Bell, G.P.; Pleus, R.C. Exposure of the US Population to Nitrate, Thiocyanate, Perchlorate, and Iodine Based on NHANES 2005–2014. Bull. Environ. Contam. Toxicol. 2017, 99, 83–88. [Google Scholar] [CrossRef] [PubMed]
| n | mUIC (µg/L) | 95% CI | |
|---|---|---|---|
| Overall | 4613 | 133 | (128, 141) |
| Age 1, years | |||
| 6–11 | 698 | 190 | (161, 211) |
| 12–19 | 781 | 139 | (126, 162) |
| 20–29 | 569 | 120 | (106, 138) |
| 30–39 | 554 | 109 | (99, 128) |
| 40–49 | 522 | 107 | (97, 124) |
| 50–59 | 532 | 128 | (111, 145) |
| 60–69 | 504 | 138 | (114, 164) |
| 70+ | 453 | 169 | (154, 197) |
| Sex | |||
| Male | 2344 | 147 | (136, 161) |
| Female | 2269 | 122 a | (112, 129) |
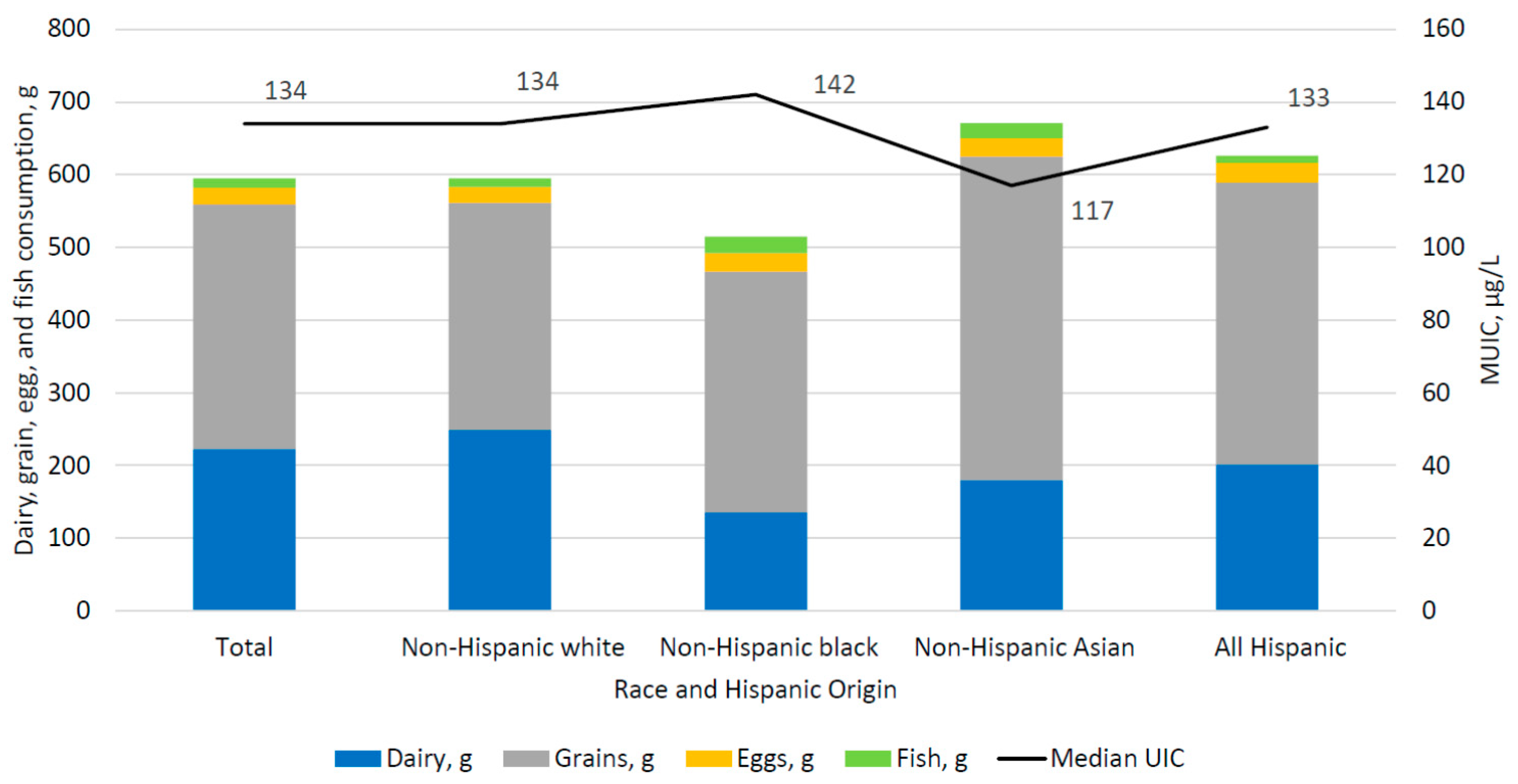
| Race/Hispanic origin 2 | |||
| Non-Hispanic white | 1671 | 134 | (125, 144) |
| Non-Hispanic black | 1112 | 142 | (127, 158) |
| Non-Hispanic Asian | 517 | 117 b | (104, 134) |
| All Hispanic | 1137 | 133 | (123, 143) |
| Supplement containing iodine yesterday | |||
| Yes | 586 | 174 | (152, 193) |
| No | 4027 | 127 a | (118, 133) |
| Salt used at the Table 3 | |||
| Never | 1495 | 142 | (130, 158) |
| Rare | 1593 | 136 | (128, 145) |
| Occasional | 919 | 123 | (106, 143) |
| Very Often | 544 | 128 | (119, 138) |
| Salt used in food preparation 4 | |||
| Never | 291 | 172 | (140, 210) |
| Rare | 788 | 138 a | (127, 156) |
| Occasional | 1553 | 128 a | (120, 137) |
| Very Often | 1897 | 128 a | (117, 140) |
| n | mUIC (µg/L) | 95% CI | |
|---|---|---|---|
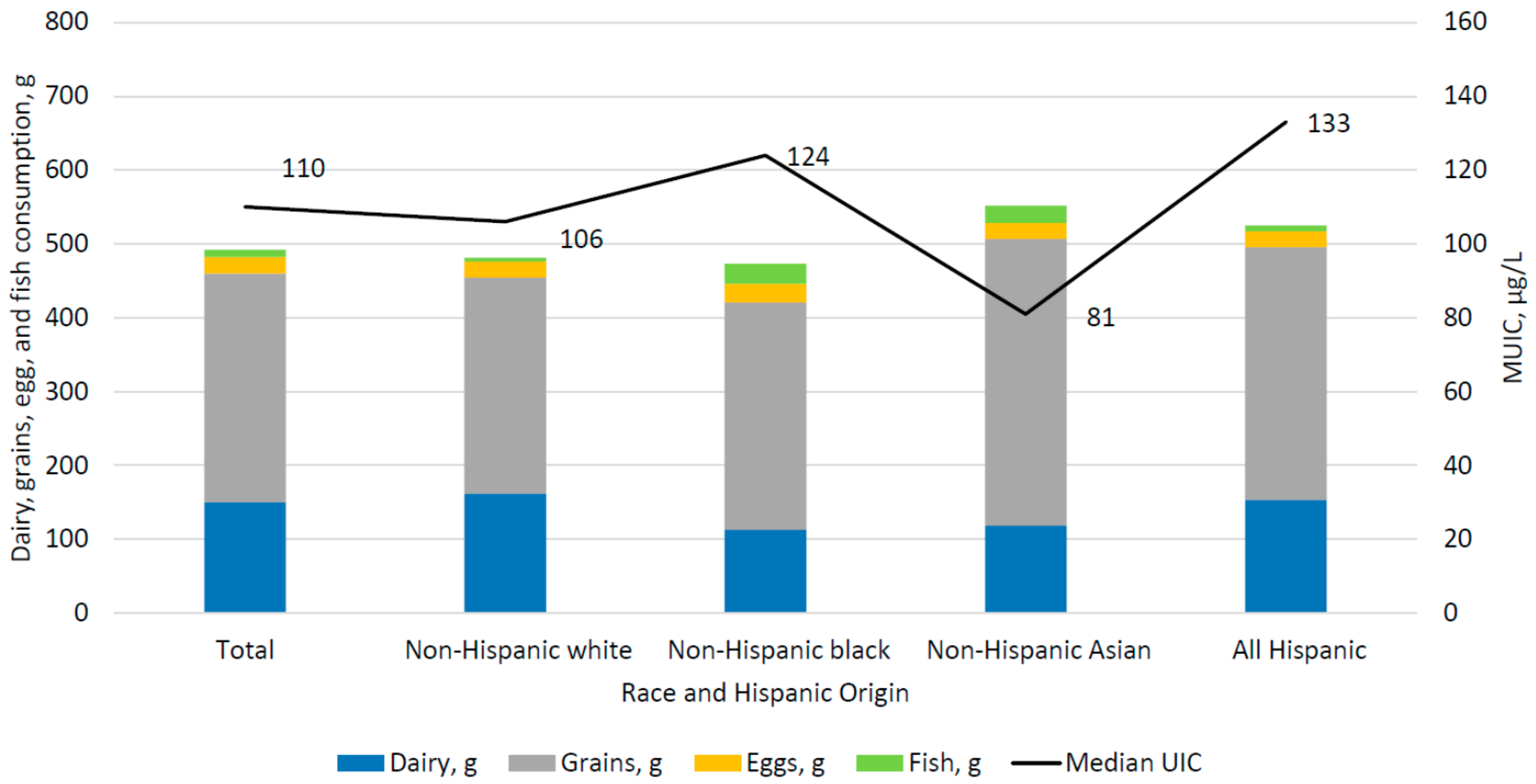
| All | 901 | 110 | (99, 124) |
| Women of childbearing age 1, 15–44 years | |||
| 15–19 | 241 | 128 | (107, 170) |
| 20–29 | 268 | 119 | (98, 137) |
| 30–39 | 255 | 107 | (88, 134) |
| 40–44 | 137 | 91 | (75, 107) |
| Race/Hispanic origin 2 | |||
| Non-Hispanic white | 310 | 106 | (92, 121) |
| Non-Hispanic black | 186 | 124 | (95, 159) |
| Non-Hispanic Asian | 113 * | 81 | (54, 118) |
| All Hispanic | 245 | 133 a,c | (107, 163) |
| Supplement containing iodine yesterday | |||
| Yes | 54 * | 147 | (69, 297) |
| No | 847 | 107 | (98, 122) |
| Salt used at the Table 3 | |||
| Never | 224 | 109 | (85, 138) |
| Rare | 341 | 122 | (106, 144) |
| Occasional | 215 | 82 b | (64, 119) |
| Very Often | 114 * | 108 | (87, 128) |
| Salt used in food preparation 4 | |||
| Never | 39 * | 133 | (66, 208) |
| Rare | 135 | 117 | (106, 125) |
| Occasional | 308 | 100 b | (85, 131) |
| Very Often | 415 | 107 b | (97, 130) |
| Dairy (g) | Grains (g) | Eggs (g) | Fish (g) | Soy Products 1 (g) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | |
| All | 4613 | 223 | (205, 240) | 336 | (324, 347) | 23 | (21, 26) | 13 | (9, 18) | 8 | (5, 10) |
| Race/Hispanic origin 2 | |||||||||||
| Non-Hispanic white | 1671 | 249 | (228, 271) | 312 | (296, 328) | 22 | (19, 26) | 12 | (7, 17) | 7 | (4, 11) |
| Non-Hispanic black | 1112 | 136 a | (119, 152) | 331 | (308, 354) | 25 | (21, 30) | 23 a | (13, 34) | 3 a | (1, 7) |
| Non-Hispanic Asian | 517 | 180 a,b | (148, 213) | 445 a,b | (417, 473) | 25 | (18, 32) | 21 a | (14, 28) | 26 a,b | (18, 33) |
| All Hispanic | 1137 | 202 a,b | (183, 221) | 387 a,b,c | (360, 415) | 27 | (23, 32) | 10 b,c | (6, 14) | 6 a,c | (2, 11) |
| Dairy (g) | Grains (g) | Eggs (g) | Fish (g) | Soy Products 1 (g) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | |
| All | 901 | 151 | (133, 169) | 310 | (285, 336) | 22 | (17, 26) | 10 | (5, 14) | 8 | (4, 12) |
| Race/Hispanic origin 2 | |||||||||||
| Non-Hispanic white | 310 | 162 | (136, 187) | 293 | (253, 333) | 21 | (13, 29) | 5 | (2, 9) | 8 | (2, 14) |
| Non-Hispanic black | 186 | 113 a | (83, 142) | 308 | (269, 347) | 25 | (17, 34) | 27 a | (10, 44) | 1 a | (0, 3) |
| Non-Hispanic Asian | 113 | 119 | (79, 159) | 388 a | (314, 461) | 21 | (14, 28) | 24 | (5, 43) | 18 b | (4, 32) |
| All Hispanic | 245 | 154 | (123, 186) | 343 | (295, 391) | 21 | (12, 30) | 8 b | (3, 13) | 9 | (0.1,16) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrick, K.A.; Perrine, C.G.; Aoki, Y.; Caldwell, K.L. Iodine Status and Consumption of Key Iodine Sources in the U.S. Population with Special Attention to Reproductive Age Women. Nutrients 2018, 10, 874. https://doi.org/10.3390/nu10070874
Herrick KA, Perrine CG, Aoki Y, Caldwell KL. Iodine Status and Consumption of Key Iodine Sources in the U.S. Population with Special Attention to Reproductive Age Women. Nutrients. 2018; 10(7):874. https://doi.org/10.3390/nu10070874
Chicago/Turabian StyleHerrick, Kirsten A., Cria G. Perrine, Yutaka Aoki, and Kathleen L. Caldwell. 2018. "Iodine Status and Consumption of Key Iodine Sources in the U.S. Population with Special Attention to Reproductive Age Women" Nutrients 10, no. 7: 874. https://doi.org/10.3390/nu10070874





