Neuroprotective Effects of Soy Isoflavones on Scopolamine-Induced Amnesia in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Vehicle
2.3. Experimental Animals
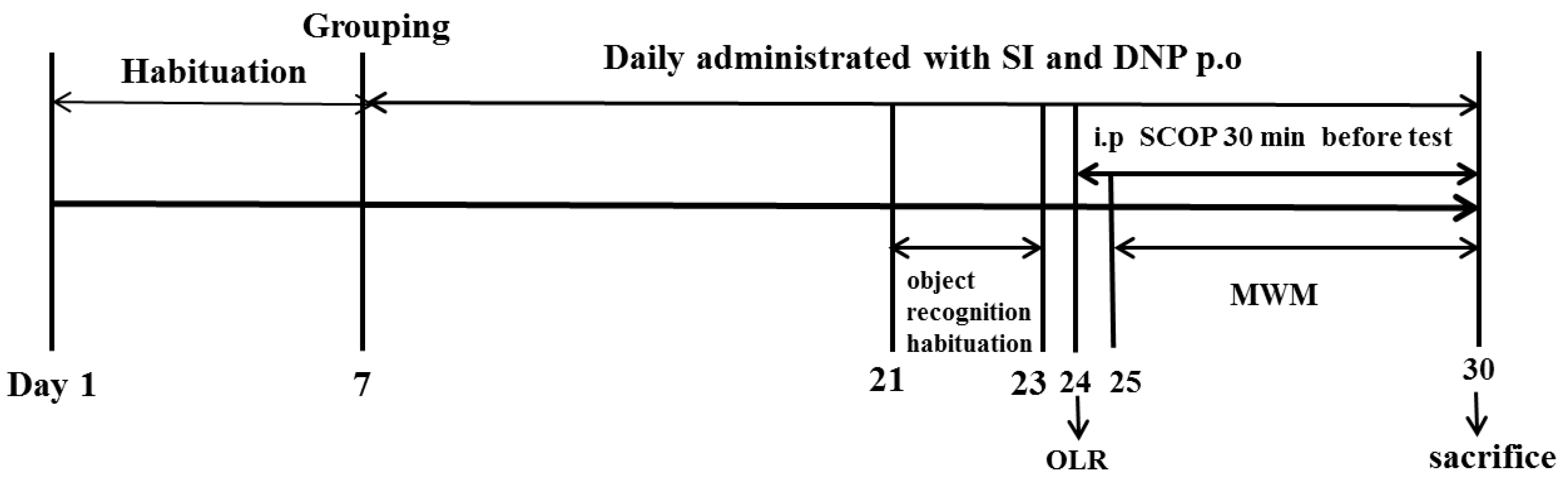
2.4. Design of Animal Experiment
2.5. Object Location Recognition (OLR) Test
2.6. Morris Water Maze (MWM) Task
2.7. Brain Sample Preparation
2.8. Biochemical Parameter Assay of the Hippocampus
2.9. Western Blotting Analysis
2.10. Statistical Analysis
3. Results
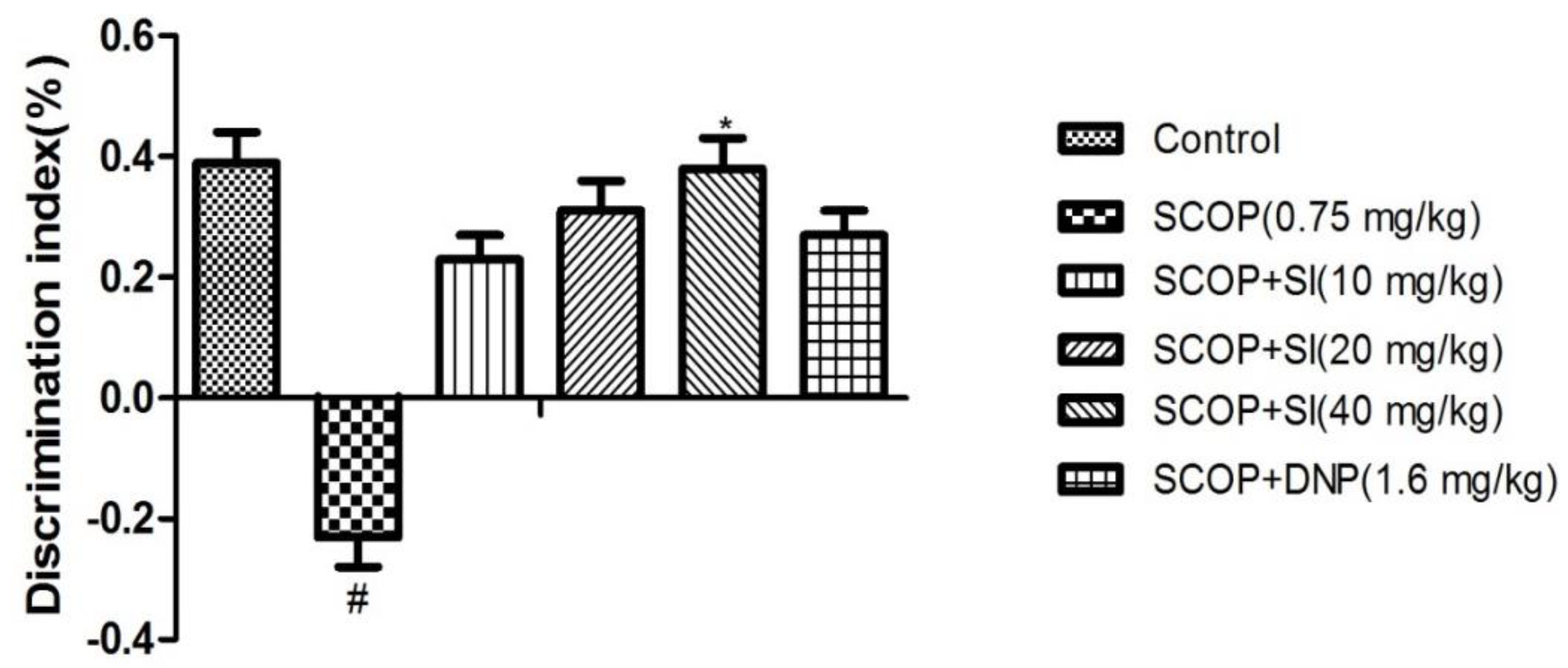
3.1. Effects of SI on Short-Term, Spatial Learning and Memory Deficits in the OLR Test
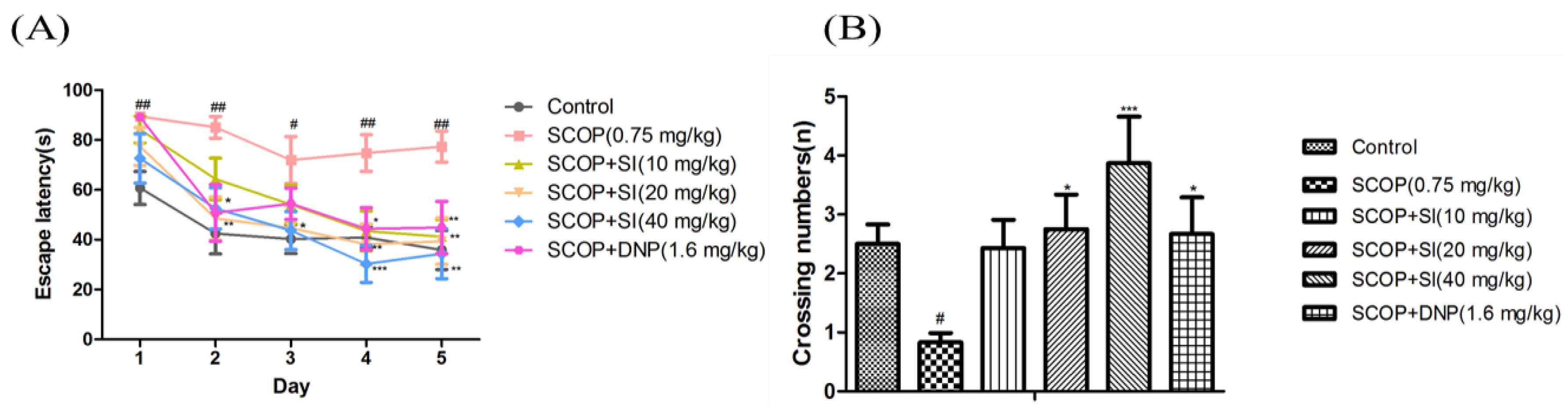
3.2. Effects of SI on Long-Term, Spatial Reference Memory in the MWM Task
3.3. Effects of SI on the Function of Cholinergic System in the Hippocampus of SCOP-Treated Mice
3.4. Effects of SI on Oxidative Stress Biomarkers in the Hippocampus of SCOP-Treated Mice
3.5. Effects of SI on the Expression Levels of Memory-Related Molecules in the Hippocampus of SCOP-Treated Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lee, S.H.; Park, H.J.; Jeon, S.J.; Kim, E.J.; Lee, H.E.; Ryu, J.H.; Kwon, Y.; Zhang, J.; Jung, I.H.; Ryu, J.H. Cognitive Ameliorating effect of Acanthopanax koreanum against scopolamine-induced memory impairment in mice. Phytother. Res. 2017, 31, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Lima, H.S.; Kima, B.Y.; Kim, Y.J.; Jeong, S.J. Phytochemical allylguaiacol exerts a neuroprotective effect on hippocampal cells and ameliorates scopolamine-induced memory impairment in mice. Behav. Brain Res. 2018, 339, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Spires-Jones, T.L.; Hyman, B.T. The intersection of amyloid beta and tau at synapses in Alzheimer’s disease. Neuron 2014, 82, 756–771. [Google Scholar] [CrossRef] [PubMed]
- Hyman, B.T.; Phelps, C.H.; Beach, T.G.; Bigio, E.H.; Cairns, N.J.; Montine, T.J.; Dickson, D.W.; Duyckaerts, C.; Frosch, M.P.; Masliah, E.; et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimer’s Dement. 2012, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tappayuthpijarn, P.; Itharat, A.; Makchuchit, S. Acetylcholinesterase inhibitory activity of Thai traditional nootropic remedy and its herbal ingredients. J. Med. Assoc. Thail. 2011, 94 (Suppl. 7), S183–S189. [Google Scholar]
- Mendiola-Precoma, J.; Berumen, L.C.; Padilla, K.; Garcia-Alcocer, G. Therapies for prevention and treatment of Alzheimer’s disease. BioMed Res. Int. 2016, 2016, 2589276. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Lee, A.H.; Binns, C.W.; Huang, R.; Hu, D.; Shao, H. Soy consumption reduces risk of ischemic stroke: A case-control study in Southern China. Neuroepidemiology 2009, 33, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, S.; Burris, R.L.; Stewart, B.W.; Wilkerson, J.E.; Badger, T.M. Dietary soy protein isolate ameliorates atherosclerotic lesions in apolipoprotein Edeficient mice potentially by inhibiting monocyte chemoattractant protein-1 expression. J. Nutr. 2008, 138, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Ramasamy, K.; Jaafar, S.M.; Majeed, A.B.; Mani, V. Total isoflavones from soybean and tempeh reversed scopolamine-induced amnesia, improved cholinergic activities and reduced neuroinflammation in brain. Food Chem. Toxicol. 2014, 65, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Q.; Xin, T.R.; Liang, J.J.; Wang, W.M.; Zhang, Y.Y. Memory Performance, Brain Excitatory Amino Acid and Acetylcholinesterase Activity of Chronically Aluminum Exposed Mice in Response to Soy Isoflavones Treatment. Phytother. Res. 2010, 24, 1451–1456. [Google Scholar] [CrossRef] [PubMed]
- Gleason, C.E.; Fischer, B.L.; Dowling, N.M.; Setchell, D.R.; Atwood, C.S.; Carlsson, C.M.; Asthana, S. Cognitive effects of soy isoflavones in patients with Alzheimer’s disease. J. Alzheimer’s Dis. 2015, 47, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Duffy, R.; Wiseman, H.; File, S.E. Improved cognitive function in postmenopausal women after 12 weeks of consumption of a soya extract containing isoflavones. Pharmacol. Biochem. Behav. 2003, 75, 721–729. [Google Scholar] [CrossRef]
- Bagheri, M.; Joghataei, M.T.; Mohseni, S.; Roghani, M. Genistein ameliorates learning and memory deficits in amyloid beta(1–40) rat model of Alzheimer’s disease. Neurobiol. Learn. Mem. 2011, 95, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.W.; Xiang, L.; Yu, H.L.; Yuan, L.H.; Guo, A.M.; Xiao, Y.X.; Li, L.; Xiao, R. Neuroprotection of soyabean isoflavone co-administration with folic acid against beta-amyloid 1–40-induced neurotoxicity in rats. Br. J. Nutr. 2009, 102, 502–505. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.L.; Grimes, J.M.; Larco, D.O.; Cruthirds, D.F.; Joanna, W.; Wooten, L.; Keil, M.; Weiser, M.J.; Landauer, M.R.; Handa, R.J.; et al. The interaction of dietary isoflavones and estradiol replacement on behavior and brain-derived neurotrophic factor in the ovariectomized rat. Neurosci. Lett. 2017, 640, 53–59. [Google Scholar] [CrossRef] [PubMed]
- MacLusky, N.J.; Thomas, G.; Leranth, C. Low dietary soy isoflavonoids increase hippocampal spine synapse density in ovariectomized rats. Brain Res. 2017, 1657, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Wang, K.Z.; Lu, C.; Dong, L.M.; Yan, M.; Liu, X.M. Protective effect of lavender oil on scopolamine induced cognitive deficits in mice and H2O2 induced cytotoxicity in PC12 cells. J. Ethnopharmacol. 2016, 193, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Dong, L.M.; Lv, J.V.; Wang, Y.; Fan, B.; Wang, F.Z.; Liu, X.M. 20(S)-protopanaxadiol (PPD) alleviates scopolamine-induced memory impairment via regulation of cholinergic and antioxidant systems, and expression of Egr-1, c-Fos and c-Jun in mice. Chem.-Biol. Interact. 2018, 279, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Ennaceur, A.; Michalikova, S.; Chazot, P.L. Do rats really express neophobia towards novel objects? Experimental evidence from exposure to novelty and to an object recognition task in an open space and an enclosed space. Behav. Brain Res. 2009, 197, 417–434. [Google Scholar] [CrossRef] [PubMed]
- Saida, H.; Saiqa, T.; Tahira, P. Scopolamine-induced greater alterations in neurochemical profile and increased oxidative stress demonstrated a better model of dementia: A comparative study. Brain Res. Bull. 2016, 127, 234–247. [Google Scholar]
- Wang, Q.; Sun, L.H.; William, J.; Liu, X.M.; Dang, H.X.; Xu, C.J. Comparison of ginsenosides Rg1 and Rb1 for their effects on improving scopolamine induced learning and memory impairment in mice. Phytother. Res. 2010, 24, 1748–1754. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Feather-Stone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [Green Version]
- Mihara, M.; Uchiyama, M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978, 86, 271–278. [Google Scholar] [PubMed]
- Lu, C.; Shi, Z.; Dong, L.M.; Lv, J.W.; Liu, X.M. Exploring the effect of ginsenoside Rh1 in a sleep deprivation-induced mouse memory impairment model. Phytother. Res. 2017, 31, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Lv, J.W.; Dong, L.M.; Jiang, N.; Wang, Y.; Wang, Q.; Liu, X.M. Neuroprotective effects of 20(S)-protopanaxatriol (PPT) on scopolamine-induced cognitive deficits in mice. Phytother. Res. 2018, 32, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer’s Association. Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2014, 10, 47–92. [Google Scholar]
- Omar, S.H.; Scott, C.J.; Hamlin, A.S.; Obied, H.K. The protective role of plant biophenols in mechanisms of Alzheimer’s disease. J. Nutr. Biochem. 2017, 47, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.O.; Wightman, E.L. Herbal extracts and phytochemicals: Plant secondary metabolites and the enhancement of human brain function. Adv. Nutr. 2011, 2, 32–50. [Google Scholar] [CrossRef] [PubMed]
- Malar, D.S.; Devi, K.P. Dietary polyphenols for treatment of Alzheimer’s disease–future research and development. Curr. Pharm. Biotechnol. 2014, 15, 330–342. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.Z.; Wang, H.F.; Wang, D.H.; Fang, F.; Lai, J.X.; Tsao, R. Isoflavone, γ-aminobutyric acid contents and antioxidant activities are significantly increased during germination of three Chinese soybean cultivars. J. Funct. Foods 2015, 14, 596–604. [Google Scholar] [CrossRef]
- Ennaceur, A.; Michalikova, S.; Bradford, A.; Ahmed, S. Detailed analysis of the behavior of lister and wistar rats in anxiety, object recognition and object location tasks. Behav. Brain Res. 2005, 159, 247–266. [Google Scholar] [CrossRef] [PubMed]
- Ennaceur, A.; Delacour, J. A new one-trial test for neurobiological studies of memory in rats. Behav. Brain Res. 1988, 31, 47–59. [Google Scholar] [CrossRef]
- Doguc, D.K.; Delibas, N.; Vural, H.; Altuntas, I.; Sutcu, R.; Sonmez, Y. Effects of chronic scopolamine administration on spatial working memory and hippocampal receptors related to learning. Behav. Pharmacol. 2012, 23, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Weitzner, D.S.; Engler-Chiurazzi, E.B.; Kotilinek, L.A.; Ashe, K.H.; Reed, M.N. Morris water maze test: Optimization for mouse strain and testing environment. J. Vis. Exp. 2015, 100, 52706. [Google Scholar] [CrossRef] [PubMed]
- Park, H.R.; Lee, H.; Park, H.; Cho, W.K.; Ma, J.Y. Fermented Sipjeondaebo-tang alleviates memory deficits and loss of hippocampal neurogenesis in scopolamine-induced amnesia in mice. Sci. Rep. 2016, 6, 22405. [Google Scholar] [CrossRef] [PubMed]
- Blake, M.G.; Krawczyk, M.C.; Baratti, C.M.; Boccia, M.M. Neuropharmacology of memory consolidation and reconsolidation: Insights on central cholinergic mechanisms. J. Physiol. Paris 2014, 108, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Berté, T.E.; Dalmagro, A.P.; Zimath, P.L.; Gonçalves, A.E.; Meyre-Silva, C.; Bürger, C.; Weber, C.J.; dos Santos, D.A.; Cechinel-Filho, V.; de Souza, M.M. Taraxerol as a possible therapeutic agent on memory impairments and Alzheimer’s disease: Effects against scopolamine and streptozotocin-induced cognitive dysfunctions. Steroids 2018, 132, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.Y.; Kim, H.; Park, H.J.; Jeon, S.J.; Park, H.J.; Ryu, J.H. The ethanolic extract of the Eclipta prostrata, L. ameliorates the cognitive impairment in mice induced by scopolamine. J. Ethnopharmacol. 2016, 190, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Kim, B.W.; Song, S.Y.; Kim, J.S.; Kim, I.S.; Kwon, Y.S.; Koppula, S.; Choi, D.K. Cognitive enhancing effects of alpha asarone in amnesic mice by influencing cholinergic and antioxidant defense mechanisms. Biosci. Biotechnol. Biochem. 2012, 76, 1518–1522. [Google Scholar] [CrossRef] [PubMed]
- Song, S.H.; Choi, S.M.; Kim, J.E.; Sung, J.E.; Lee, H.A.; Choi, Y.H.; Bae, C.J.; Choi, Y.W.; Hwang, D.Y. Isocubebenol alleviates scopolamine induced cognitive impairment by repressing acetylcholinesterase activity. Neurosci. Lett. 2017, 638, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.H.; Dar, K.B.; Anees, S.; Zargar, M.A.; Masood, A.; Ganie, S.A. Oxidative stress, mitochondrial dysfunction and neurodegenerative diseases; A mechanistic insight. Biomed. Pharmacother. 2015, 74, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Melo, J.B.; Agostinho, P.; Oliveira, C.R. Involvement of oxidative stress in the enhancement of acetylcholinesterase activity induced by amyloid beta-peptide. Neurosci. Res. 2003, 45, 117–127. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Yang, W.; Bosland, M.C. Soy isoflavones and prostate cancer: A review of molecular mechanisms. J. Steroid Biochem. Mol. Biol. 2014, 140, 116–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishikawa, S.; Saito, Y.; Yanagawa, Y.; Otani, S.; Hiraide, S.; Shimamura, K.I.; Matsumoto, M.; Togashi, H. Early postnatal stress alters extracellular signal-regulated kinase signaling in the corticolimbic system modulating emotional circuitry in adult rats. Eur. J. Neurosci. 2012, 35, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Reichardt, L.F. Neurotrophin-regulated signalling pathways. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006, 361, 1545–1564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bozon, B.; Kelly, A.; Josselyn, S.A.; Silva, A.J.; Davis, S.; Laroche, S. MAPK, CREB and zif268 are all required for the consolidation of recognition memory. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2003, 358, 805–814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, Y.H.; Kwon, S.H.; Lee, S.Y.; Jang, C.G. Liquiritigenin ameliorates memory and cognitive impairment through cholinergic and BDNF pathways in the mouse hippocampus. Arch. Pharmacal Res. 2017, 40, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
- Joh, E.H.; Lee, I.A.; Kim, D.H. Kalopanaxsaponins A and B isolated from Kalopanax pictus ameliorate memory deficits in mice. Phytother. Res. 2012, 26, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Schreihofer, D.A.; Anthony, O.G. Genistein: Mechanisms of action for a pleiotropic neuroprotective agent in stroke. Nutr. Neurosci. 2017, 24, 1–17. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, C.; Wang, Y.; Wang, D.; Zhang, L.; Lv, J.; Jiang, N.; Fan, B.; Liu, X.; Wang, F. Neuroprotective Effects of Soy Isoflavones on Scopolamine-Induced Amnesia in Mice. Nutrients 2018, 10, 853. https://doi.org/10.3390/nu10070853
Lu C, Wang Y, Wang D, Zhang L, Lv J, Jiang N, Fan B, Liu X, Wang F. Neuroprotective Effects of Soy Isoflavones on Scopolamine-Induced Amnesia in Mice. Nutrients. 2018; 10(7):853. https://doi.org/10.3390/nu10070853
Chicago/Turabian StyleLu, Cong, Yan Wang, Donghui Wang, Lijing Zhang, Jingwei Lv, Ning Jiang, Bei Fan, Xinmin Liu, and Fengzhong Wang. 2018. "Neuroprotective Effects of Soy Isoflavones on Scopolamine-Induced Amnesia in Mice" Nutrients 10, no. 7: 853. https://doi.org/10.3390/nu10070853



