Effects of Synbiotics among Constipated Adults in Serdang, Selangor, Malaysia—A Randomised, Double-Blind, Placebo-Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Ethical Considerations
2.3. Safety Considerations
2.4. Study Agents
2.5. Eligibility
2.6. Efficacy Measurements
2.7. Sample Size
2.8. Statistical Analyses
3. Results
3.1. Participants’ Flow
3.2. Baseline Characteristics
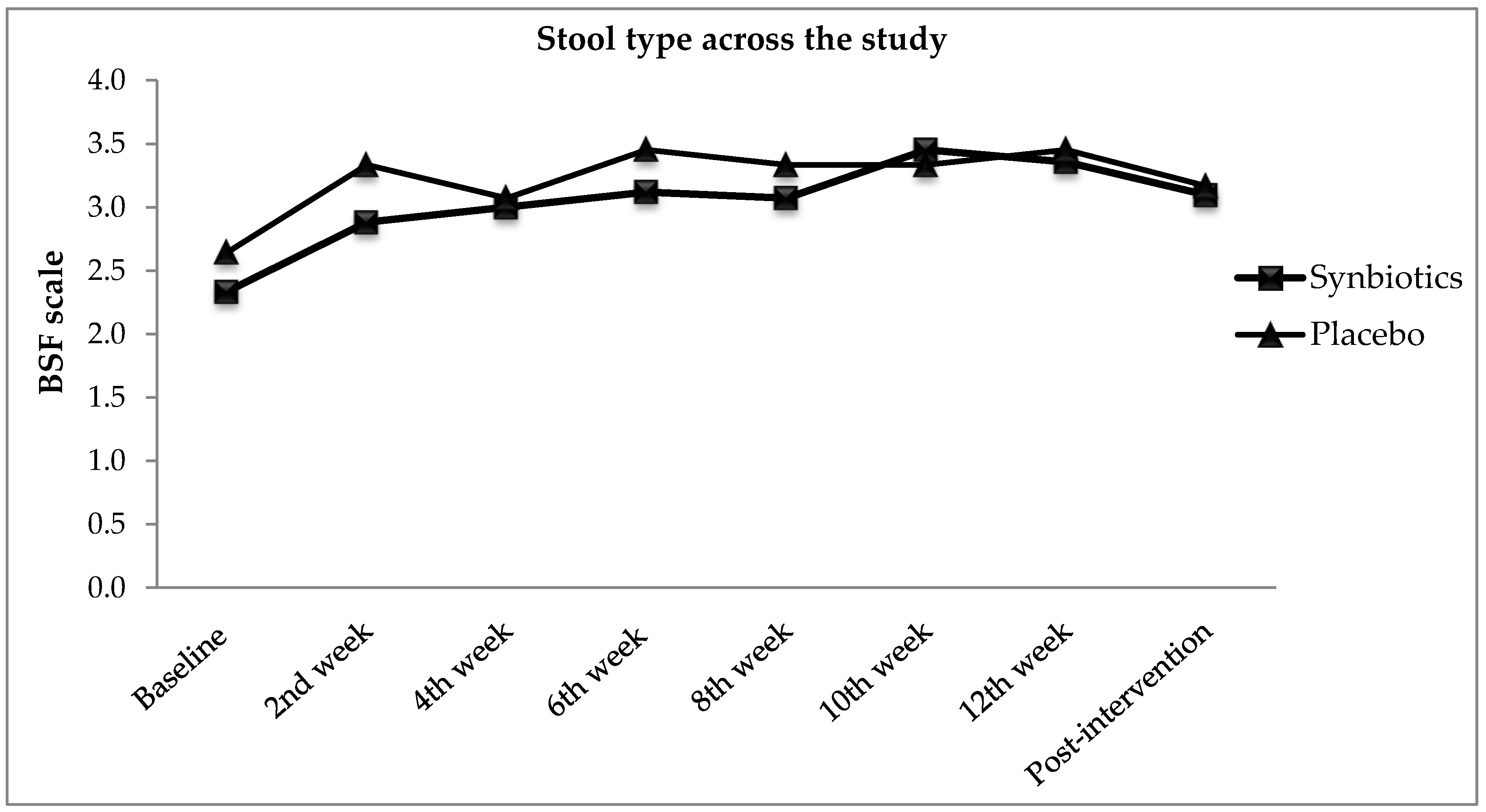
3.3. Functional Constipation Symptoms
3.4. Quality of Life
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bharucha, A.E.; Pemberton, J.H.; Locke, G.R., III. American gastroenterological association technical review on constipation. Gastroenterology 2013, 144, 218–238. [Google Scholar] [CrossRef] [PubMed]
- WGO. Constipation: A Global Perspective; 12/85; WGO: Forest City, IA, USA, 2010. [Google Scholar]
- Ministry of Health Malaysia. Health Facts 2012; Ministry of Health Malaysia: Kuala Lumpur, Malaysia, 2012.
- Ministry of Health Malaysia. Health Facts 2010; Ministry of Health Malaysia: Kuala Lumpur, Malaysia, 2011.
- Ministry of Health Malaysia. Health Facts 2011; Ministry of Health Malaysia: Kuala Lumpur, Malaysia, 2011.
- Ministry of Health Malaysia. Health Facts 2013; Ministry of Health Malaysia: Kuala Lumpur, Malaysia, 2013.
- Ministry of Health Malaysia. Health Facts 2014; Ministry of Health Malaysia: Kuala Lumpur, Malaysia, 2014.
- Ministry of Health Malaysia. Annual Report Ministry of Health 2011; Ministry of Health Malaysia: Kuala Lumpur, Malaysia, 2011.
- Arnaud, M. Mild dehydration: A risk factor of constipation? Eur. J. Clin. Nutr. 2003, 57, S88–S95. [Google Scholar] [CrossRef] [PubMed]
- Kaboli, S.A.; Pourhoseingholi, M.A.; Moghimi-Dehkordi, B.; Safaee, A.; Habibi, M.; Pourhoseingholi, A.; Vahedi, M. Factors associated with functional constipation in iranian adults: A population-based study. In Gastroenterology and Hepatology from Bed to Bench; Shahid Beheshti University: Tehran, Iran, 2010; Volume 3. [Google Scholar]
- Johannesson, E.; Simrén, M.; Strid, H.; Bajor, A.; Sadik, R. Physical activity improves symptoms in irritable bowel syndrome: A randomized controlled trial. Am. J. Gastroenterol. 2011, 106, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Mugie, S.M.; Benninga, M.A.; Di Lorenzo, C. Epidemiology of constipation in children and adults: A systematic review. Best Pract. Res. Clin. Gastroenterol. 2011, 25, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Nakaji, S.; Tokunaga, S.; Sakamoto, J.; Todate, M.; Shimoyama, T.; Umeda, T.; Sugawara, K. Relationship between lifestyle factors and defecation in a japanese population. Eur. J. Nutr. 2002, 41, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Drossman, D.A. The Functional Gastrointestinal Disorders and the Rome II Process. Gut 1999, 45 (Suppl. II), II1–II5. [Google Scholar] [CrossRef] [PubMed]
- Mertz, H. Stress and the Gut; Med. UNC: Chapel Hill, NC, USA, 2013; pp. 1–5. [Google Scholar]
- Suares, N.C.; Ford, A.C. Prevalence of, and risk factors for, chronic idiopathic constipation in the community: Systematic review and meta-analysis. Am. J. Gastroenterol. 2011, 106, 1582–1591. [Google Scholar] [CrossRef] [PubMed]
- FAO; WHO. Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria; Amerian Córdoba Park Hotel: Córdoba, Argentina, 2001; pp. 1–34. [Google Scholar]
- Reid, G.; Sanders, M.; Gaskins, H.R.; Gibson, G.R.; Mercenier, A.; Rastall, R.; Roberfroid, M.; Rowland, I.; Cherbut, C.; Klaenhammer, T.R. New scientific paradigms for probiotics and prebiotics. J. Clin. Gastroenterol. 2003, 37, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Picard, C.; Fioramonti, J.; Francois, A.; Robinson, T.; Neant, F.; Matuchansky, C. Review article: Bifidobacteria as probiotic agents—Physiological effects and clinical benefits. Aliment. Pharmacol. Ther. 2005, 22, 495–512. [Google Scholar] [CrossRef] [PubMed]
- Roberfroid, M.B. Prebiotics and probiotics: Are they functional foods? Am. J. Clin. Nutr. 2000, 71, 1682s–1687s. [Google Scholar] [CrossRef] [PubMed]
- FDA, U.F.D.A. Agency Response Letter Gras Notice No. GRN 000049; FDA: Washington, DC, USA, 2002.
- Jungersen, M.; Wind, A.; Johansen, E.; Christensen, J.E.; Stuer-Lauridsen, B.; Eskesen, D. The science behind the probiotic strain Bifidobacterium animalis subsp. Lactis BB-12®. Microorganisms 2014, 2, 92–110. [Google Scholar] [CrossRef] [PubMed]
- Vernazza, C.L.; Gibson, G.R.; Rastall, R.A. Carbohydrate preference, acid tolerance and bile tolerance in five strains of Bifidobacterium. J. Appl. Microbiol. 2006, 100, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Juntunen, M.; Kirjavainen, P.; Ouwehand, A.; Salminen, S.; Isolauri, E. Adherence of probiotic bacteria to human intestinal mucus in healthy infants and during rotavirus infection. Clin. Diagn. Lab. Immunol. 2001, 8, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Kirjavainen, P.V.; Ouwehand, A.C.; Isolauri, E.; Salminen, S.J. The ability of probiotic bacteria to bind to human intestinal mucus. FEMS Microbiol. Lett. 1998, 167, 185–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saavedra, J.M.; Bauman, N.A.; Perman, J.; Yolken, R.; Oung, I. Feeding of Bifidobacterium bifidum and Streptococcus thermophilus to infants in hospital for prevention of diarrhoea and shedding of rotavirus. Lancet 1994, 344, 1046–1049. [Google Scholar] [CrossRef]
- Fukushima, Y.; Kawata, Y.; Hara, H.; Terada, A.; Mitsuoka, T. Effect of a probiotic formula on intestinal immunoglobulin a production in healthy children. Int. J. Food Microbiol. 1998, 42, 39–44. [Google Scholar] [CrossRef]
- He, F.; Ouwehand, A.C.; Hashimoto, H.; Isolauri, E.; Benno, Y.; Salminen, S. Adhesion of Bifidobacterium spp. To human intestinal mucus. Microbiol. Immunol. 2001, 45, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Rinkinen, M.; Westermarck, E.; Salminen, S.; Ouwehand, A.C. Absence of host specificity for in vitro adhesion of probiotic lactic acid bacteria to intestinal mucus. Vet. Microbiol. 2003, 97, 55–61. [Google Scholar] [CrossRef]
- Pitkälä, K.H.; Strandberg, T.; Finne-Soveri, U.; Ouwehand, A. Fermented cereal with specific bifidobacteria normalizes bowel movements in elderly nursing home residents. A randomized, controlled trial. J. Nutr. Health Aging 2007, 11, 305–311. [Google Scholar] [PubMed]
- Matsumoto, M.; Imai, T.; Hironaka, T.; Kume, H.; Watanabe, M.; Benno, Y. Effect of yogurt with Bifidobacterium lactis LKM 512 in improving fecal microflora and defecation of healthy volunteers. J. Intest. Microbiol. 2000, 14, 97–102. [Google Scholar]
- Uchida, K.; Akashi, K.; Kusunoki, I.; Ikeda, T.; Katano, N.; Motoshima, H.; Benno, Y. Effect of fermented milk containing Bifidobacterium lactis BB-12® on stool frequency, defecation, fecal microbiota and safety of excessive ingestion in healthy female students. J. Nutr. Food Sci. 2005, 8, 39–51. [Google Scholar]
- Nishida, S.; Gotou, M.; Akutsu, S.; Ono, M.; Hitomi, Y.; Nakamura, T.; Iino, H. Effect of yogurt containing Bifidobacterium lactis BB-12 on improvement of defecation and fecal microflora of healthy female adults. Milk Sci. 2004, 53, 71–80. [Google Scholar]
- Murakami, T.; Miyahara, H.; Yukisata, S. Safety and effect of yoghurt containing Bifidobacterium lactis BB-12 on improvement of defecation and fecal microflora in healthy volunteers. J. Nutr. Food 2006, 9, 15–26. [Google Scholar]
- Schiffrin, E.J.; Brassart, D.; Servin, A.L.; Rochat, F.; Donnet-Hughes, A. Immune modulation of blood leukocytes in humans by lactic acid bacteria: Criteria for strain selection. Am. J. Clin. Nutr. 1997, 66, 515S–520S. [Google Scholar] [CrossRef] [PubMed]
- Del Piano, M.; Montino, F.; Carmagnola, S.; Anderloni, A.; Orsello, M.; Garello, E.; Sforza, F.; Ballare, M. The use of probiotics in the treatment of constipation in the elderly. Cibus 2005, 1, 23–30. [Google Scholar]
- Del Piano, M.; Carmagnola, S.; Anderloni, A.; Andorno, S.; Ballarè, M.; Balzarini, M.; Montino, F.; Orsello, M.; Pagliarulo, M.; Sartori, M. The use of probiotics in healthy volunteers with evacuation disorders and hard stools: A double-blind, randomized, placebo-controlled study. J. Clin. Gastroenterol. 2010, 44, S30–S34. [Google Scholar] [CrossRef] [PubMed]
- Nicola, S.; Mogna, L.; Allesina, S.; Barba, M.; Deidda, F.; Lorenzini, P.; Raiteri, E.; Strozzi, G.P.; Mogna, G. Interaction between probiotics and human immune cells. Focus Diet. Fibres Pre/Probiot. 2010, 21, 9–12. [Google Scholar]
- Zago, M.; Carminati, D.; Giraffa, G. Lactobacillus Plantarum: Functional Characteristics for Probiotic Applications; Nova Science Publishers Hauppauge: New York, NY, USA, 2012. [Google Scholar]
- Mogna, G.; Strozzi, G.P.; Mogna, L. Bacteriocin-Producing Lactobacillus and the Use Thereof in Food and Pharmaceutical Compositions. U.S. Patent 9,185,927 B2, 17 November 2015. [Google Scholar]
- Cenci, G.; Rossi, J.; Trotta, F.; Caldini, G. Lactic acid bacteria isolated from dairy products inhibit genotoxic effect of 4-nitroquinoline-1-oxide in sos-chromotest. Syst. Appl. Microbiol. 2002, 25, 483–490. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products; Nutrition and Allergies (NDA). Scientific opinion on the substantiation of health claims related to non-characterised micro-organisms (id 2936, 2937, 2938, 2941, 2944, 2965, 2968, 2969, 3035, 3047, 3056, 3059, further assessment) pursuant to article 13(1) of regulation (ec) no 1924/2006. EFSA J. 2012, 10, 2854. [Google Scholar]
- Gibson, G.; Roberfroid, M. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [PubMed]
- Jinno, S.; Toshimitsu, T.; Nakamura, Y.; Kubota, T.; Igoshi, Y.; Ozawa, N.; Suzuki, S.; Nakano, T.; Morita, Y.; Arima, T. Maternal prebiotic ingestion increased the number of fecal bifidobacteria in pregnant women but not in their neonates aged one month. Nutrients 2017, 9, 196. [Google Scholar] [CrossRef] [PubMed]
- Buddington, R.K.; Kapadia, C.; Neumer, F.; Theis, S. Oligofructose provides laxation for irregularity associated with low fiber intake. Nutrients 2017, 9, 1372. [Google Scholar] [CrossRef] [PubMed]
- Coussement, P. Inulin and oligofructose: Safe intakes and legal status. J. Nutr. 1999, 129, 1412S–1417S. [Google Scholar] [CrossRef] [PubMed]
- Coussement, P. Inulin and Oligofructose as Dietary Fiber: Analytical, Nutritional and Legal Aspects; Marcel Dekker: New York, NY, USA, 1999; pp. 203–212. [Google Scholar]
- Scott, K.P.; Martin, J.C.; Duncan, S.H.; Flint, H.J. Prebiotic stimulation of human colonic butyrate-producing bacteria and bifidobacteria, in vitro. FEMS Microbiol. Ecol. 2014, 87, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Cencic, A.; Chingwaru, W. The role of functional foods, nutraceuticals, and food supplements in intestinal health. Nutrients 2010, 2, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Pineiro, M.; Asp, N.-G.; Reid, G.; Macfarlane, S.; Morelli, L.; Brunser, O.; Tuohy, K. Fao technical meeting on prebiotics. J. Clin. Gastroenterol. 2008, 42, S156–S159. [Google Scholar] [CrossRef] [PubMed]
- Morelli, L.; Zonenschain, D.; Callegari, M.L.; Grossi, E.; Maisano, F.; Fusillo, M. Assessment of a new synbiotic preparation in healthy volunteers: Survival, persistence of probiotic strains and its effect on the indigenous flora. Nutr. J. 2003, 2, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, C.; Ge, X.; Zhang, X.; Tian, H.; Wang, H.; Gu, L.; Gong, J.; Zhu, W.; Li, N. Efficacy of synbiotics in patients with slow transit constipation: A prospective randomized trial. Nutrients 2016, 8, 605. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.; Heaton, K. Stool form scale as a useful guide to intestinal transit time. Scand. J. Gastroenterol. 1997, 32, 920–924. [Google Scholar] [CrossRef] [PubMed]
- Fateh, R.; Iravani, S.; Frootan, M.; Rasouli, M.R.; Saadat, S. Synbiotic preparation in men suffering from functional constipation: A randomised controlled trial. Swiss Med. Wkly. 2011, 141, w13239. [Google Scholar] [CrossRef] [PubMed]
- Pallant, J. Spss Survival Manual; McGraw-Hill Education: Berkshire, UK, 2013. [Google Scholar]
- Bonnema, A.L.; Kolberg, L.W.; Thomas, W.; Slavin, J.L. Gastrointestinal tolerance of chicory inulin products. J. Am. Diet. Assoc. 2010, 110, 865–868. [Google Scholar] [CrossRef] [PubMed]
- Bouhnik, Y.; Raskine, L.; Champion, K.; Andrieux, C.; Penven, S.; Jacobs, H.; Simoneau, G. Prolonged administration of low-dose inulin stimulates the growth of bifidobacteria in humans. Nutr. Res. 2007, 27, 187–193. [Google Scholar] [CrossRef]
- Rao, V.A. The prebiotic properties of oligofructose at low intake levels. Nutr. Res. 2001, 21, 843–848. [Google Scholar] [CrossRef]
- Lim, Y.J.; Rosita, J.; Chieng, J.Y.; Hazizi, A.S. The prevalence and symptoms characteristic of functional constipation using rome III diagnostic criteria among tertiary education students. PLoS ONE 2016, 11, e0167243. [Google Scholar] [CrossRef] [PubMed]
- Bazzocchi, G.; Giovannini, T.; Giussani, C.; Brigidi, P.; Turroni, S. Effect of a new synbiotic supplement on symptoms, stool consistency, intestinal transit time and gut microbiota in patients with severe functional constipation: A pilot randomized double-blind, controlled trial. Tech. Coloproctol. 2014, 18, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Krammer, H.-J.; von Seggern, H.; Schaumburg, J.; Neumer, F. Effect of Lactobacillus casei shirota on colonic transit time in patients with chronic constipation. Coloproctology 2011, 33, 109–113. [Google Scholar] [CrossRef]
- Waller, P.A.; Gopal, P.K.; Leyer, G.J.; Ouwehand, A.C.; Reifer, C.; Stewart, M.E.; Miller, L.E. Dose-response effect of Bifidobacterium lactis HN019 on whole gut transit time and functional gastrointestinal symptoms in adults. Scand. J. Gastroenterol. 2011, 46, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Mazlyn, M.M.; Nagarajah, L.H.L.; Fatimah, A.; Norimah, A.K.; Goh, K.L. Effects of a probiotic fermented milk on functional constipation: A randomized, double-blind, placebo-controlled study. J. Gastroenterol. Hepatol. 2013, 28, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Mazlyn, M.M.; Nagarajah, L.H.L.; Fatimah, A.; Norimah, A.K.; Goh, K.L. Stool patterns of malaysian adults with functional constipation: Association with diet and physical activity. Malays. J. Nutr. 2013, 19, 53–64. [Google Scholar] [PubMed]
- Wald, A.; Scarpignato, C.; Kamm, M.; Mueller-Lissner, S.; Helfrich, I.; Schuijt, C.; Bubeck, J.; Limoni, C.; Petrini, O. The burden of constipation on quality of life: Results of a multinational survey. Aliment. Pharmacol. Ther. 2007, 26, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Wald, A.; Sigurdsson, L. Quality of life in children and adults with constipation. Best Pract. Res. Clin. Gastroenterol. 2011, 25, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Jayasimhan, S.; Yap, N.-Y.; Roest, Y.; Rajandram, R.; Chin, K.-F. Efficacy of microbial cell preparation in improving chronic constipation: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2013, 32, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Tabbers, M.; de Milliano, I.; Roseboom, M.; Benninga, M. Is Bifidobacterium breve effective in the treatment of childhood constipation? Results from a pilot study. Nutr. J. 2011, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Chapman, C.; Gibson, G.R.; Rowland, I. Health benefits of probiotics: Are mixtures more effective than single strains? Eur. J. Nutr. 2011, 50, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, T.C.; Costa-Ribeiro, H., Jr.; Almeida, P.S.; Pontes, M.V.; Leite, M.E.; Filadelfo, L.R.; Khoury, J.C.; Bean, J.A.; Mitmesser, S.H.; Vanderhoof, J.A. Stool pattern changes in toddlers consuming a follow-on formula supplemented with polydextrose and galactooligosaccharides. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 288–290. [Google Scholar] [CrossRef] [PubMed]
- Bongers, M.E.; Benninga, M.A.; Maurice-Stam, H.; Grootenhuis, M.A. Health-related quality of life in young adults with symptoms of constipation continuing from childhood into adulthood. Health Qual. Life Outcomes 2009, 7, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dennison, C.; Prasad, M.; Lloyd, A.; Bhattacharyya, S.K.; Dhawan, R.; Coyne, K. The health-related quality of life and economic burden of constipation. Pharmacoeconomics 2005, 23, 461–476. [Google Scholar] [CrossRef] [PubMed]
- Higgins, P.D.; Johanson, J.F. Epidemiology of constipation in north america: A systematic review. Am. J. Gastroenterol. 2004, 99, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Tuteja, A.K.; Biskupiak, J.E. Chronic Constipation: Overview and Treatment Options. 2007. Available online: https://www.ptcommunity.com/journal/article/archives/2007/2/91/chronic-constipation-overview-and-treatment-options-0 (accessed on 25 June 2018).




| Characteristics | Synbiotics (n = 43) | Placebo (n = 42) | p-Value |
|---|---|---|---|
| Sex * | |||
| Male | 7 (16.3) | 5 (11.9) | 0.533 |
| Female | 36 (83.7) | 37 (88.1) | |
| Age (years) φ | 29.5 ± 8.34 | 27.5 ± 6.5 | 0.368 |
| Ethnicity * | |||
| Malay | 36 (83.7) | 38 (90.5) | 0.332 |
| Non-Malay | 7 (16.3) | 4 (9.5) | |
| Weight (kg) φ | 59.4 ± 11.7 | 58.7 ± 12.6 | 0.911 |
| Height (m) φ | 1.59 ± 0.09 | 1.59 ± 0.09 | 0.738 |
| BMI (kg/m2) φ | 23.6 ± 4.1 | 23.6 ± 4.1 | 0.578 |
| Waist circumference (cm) φ | 80.0 ± 9.5 | 80.0 ± 9.5 | 0.977 |
| Characteristics | Synbiotics (n = 42) | Placebo (n = 42) | p-Value |
|---|---|---|---|
| Functional constipation symptoms | |||
| Defecation frequency φ | |||
| Baseline week-1 | 2.7 ± 1.1 | 3.1 ± 1.0 | 0.148 |
| Baseline week-2 | 2.9 ± 1.2 | 3.2 ± 1.3 | 0.268 |
| BSF scale φ | 2.3 ± 0.9 | 2.6 ± 1.3 | 0.352 |
| PAC-SYM score φ | 1.40 ± 0.72 | 17.93 ± 7.76 | 0.525 |
| Quality of life | |||
| PAC-QOL score φ | 1.53 ± 0.67 | 35.8 ± 16.9 | 0.736 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, Y.J.; Jamaluddin, R.; Hazizi, A.S.; Chieng, J.Y. Effects of Synbiotics among Constipated Adults in Serdang, Selangor, Malaysia—A Randomised, Double-Blind, Placebo-Controlled Trial. Nutrients 2018, 10, 824. https://doi.org/10.3390/nu10070824
Lim YJ, Jamaluddin R, Hazizi AS, Chieng JY. Effects of Synbiotics among Constipated Adults in Serdang, Selangor, Malaysia—A Randomised, Double-Blind, Placebo-Controlled Trial. Nutrients. 2018; 10(7):824. https://doi.org/10.3390/nu10070824
Chicago/Turabian StyleLim, Ying Jye, Rosita Jamaluddin, Abu Saad Hazizi, and Jin Yu Chieng. 2018. "Effects of Synbiotics among Constipated Adults in Serdang, Selangor, Malaysia—A Randomised, Double-Blind, Placebo-Controlled Trial" Nutrients 10, no. 7: 824. https://doi.org/10.3390/nu10070824





