Effects of Dietary Daidzein Supplementation on Reproductive Performance, Serum Hormones, and Reproductive-Related Genes in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal and Experimental Diets
2.2. Sample Collection
2.3. Measurements and Analytical Methods
2.3.1. Reproductive Performance
2.3.2. Serum Reproductive Hormones
2.3.3. Serum Immunoglobulins
2.3.4. Serum Metabolites
2.3.5. Antioxidant Indices of Maternal Rats’ Sera, Uteri, Ovaries, and Fetal Longissimus Dorsi Muscles
2.3.6. RNA Extraction and Quantitative Real-Time PCR of Maternal Rats’ Uteri, Ovaries, and Fetal Longissimus Dorsi Muscles
2.4. Statistical Analysis
3. Results
3.1. Reproductive Performance as Affected by Daidzein
3.2. Effects of Daidzein on Sera Hormone Levels in Maternal Rats
3.3. Effects of Daidzein on Serum Immunoglobulin Levels in Maternal Rats
3.4. Effects of Daidzein on Sera Metabolite Levels in Maternal Rats
3.5. Effects of Daidzein on Antioxidant Indices in Maternal Rats’ Uteri, Ovaries, and Fetal Longissimus Dorsi Muscles
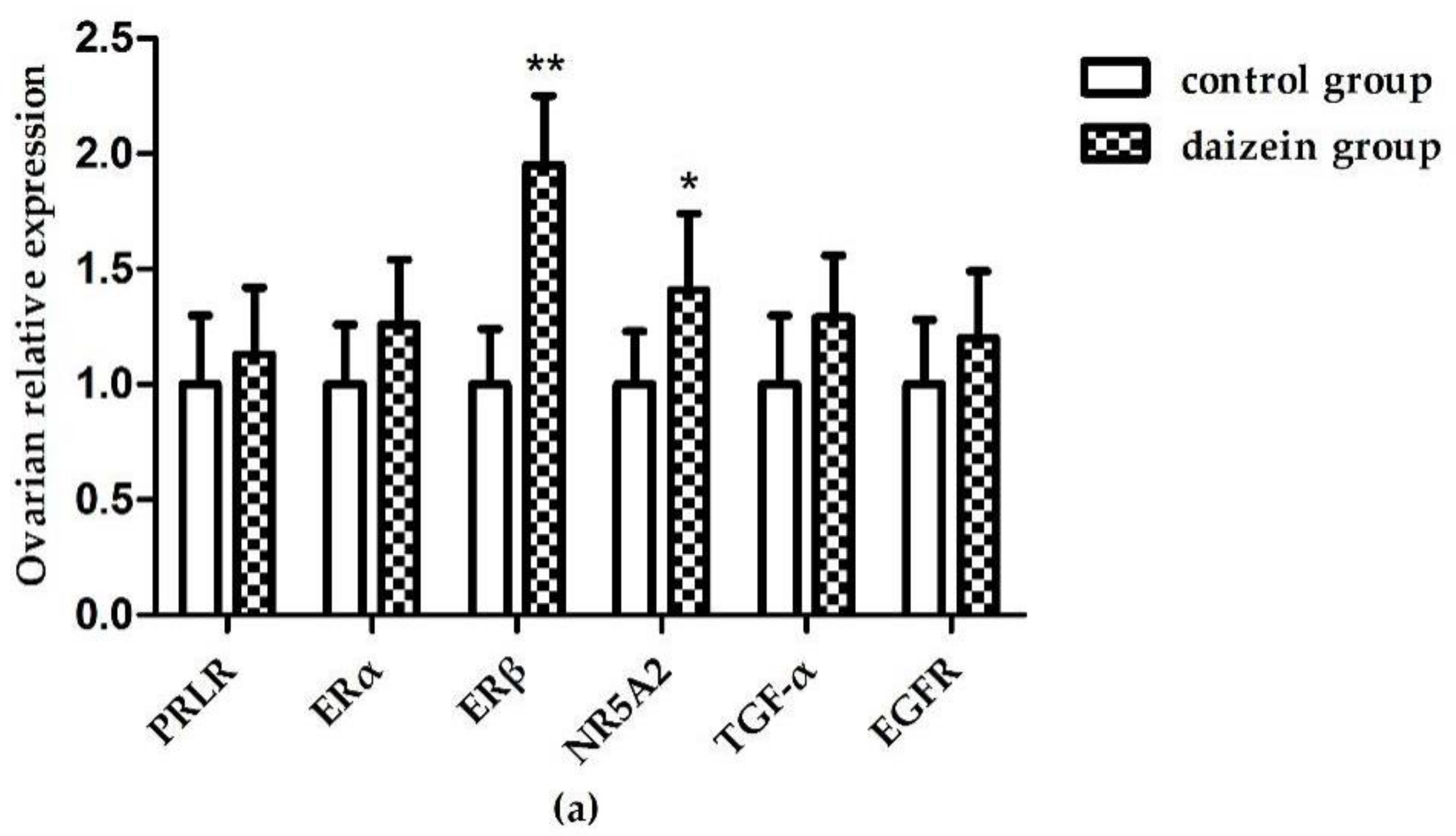
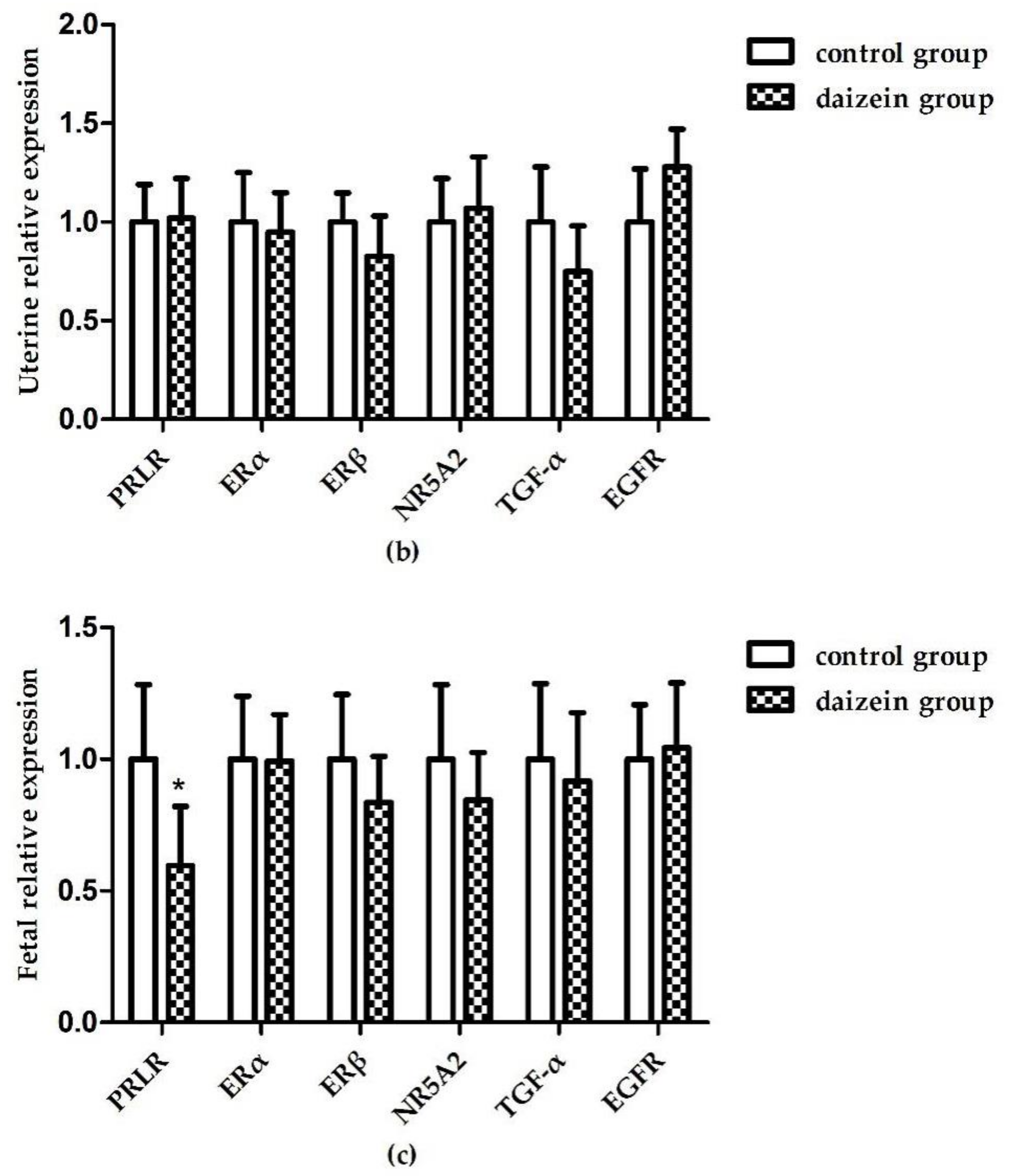
3.6. Relative Expression Levels of Related Genes in Maternal Rats Uteri, Ovaries, and Fetal Longissimus Dorsi Muscles
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kuiper, G.G.; Lemmen, J.G.; Carlsson, B.; Corton, J.C.; Safe, S.H.; Vandersaag, P.T.; Vander, B.B.; Gustafsson, J.A. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology 1998, 139, 4252–4263. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.; Cassidy, A. Dietary isoflavones: Biological effects and relevance to human health. J. Nutr. 1999, 129, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, X.; Han, Z.; Liu, Z.; Liu, W. Effects of daidzein on body weight gain, serum IGF-I level and cellular immune function in intact male piglets. Asian Aust. J. Anim. 2002, 15, 1066–1070. [Google Scholar]
- Li, G.; Zheng, Y.; Chen, W.; Chen, J.; Han, Z. Effect of daidzein fed to pregnant sows on milk production and the levels of hormones in colostrum. J. Nanjing Agric. Univ. 1999, 22, 831–839. [Google Scholar]
- Rehfeldt, C.; Adamovic, I.; Kuhn, G. Effects of dietary daidzein supplementation of pregnant sows on carcass and meat quality and skeletal muscle cellularity of the progeny. Meat Sci. 2007, 75, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Gu, H.; Shi, S.; Tong, H. Effects of high-dose daidzein on laying performance, egg quality and antioxidation in laying hens. J. Poult. Sci. 2013, 50, 237–241. [Google Scholar] [CrossRef]
- Zhao, X.; Shao, T.; Wang, Y.Q.; Lu, X.L.; Luo, J.B.; Zhou, W.D. The phytoestrogen daidzein may affect reproductive performance of Zhedong White geese by regulating gene mRNA levels in the HPG axis. Br. Poult. Sci. 2013, 54, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Han, Z.; Chen, J.; Zhang, C. Daidzein diet promotes mammary gland development and lactation in pregnant rat. Acta Zool. Sin. 1995, 4, 332–338. [Google Scholar]
- Tousen, Y.; Umeki, M.; Ishimi, Y.; Ikegami, S. Different effects of the soy isoflavones, genistein and daidzein, on pregnant and lactating rats and their offspring. J. Nutr. Diet. 2006, 64, 161–172. [Google Scholar] [CrossRef]
- Lamartiniere, C.A.; Wang, J.; Smith-Johnson, M.; Eltoum, I.E. Daidzein: Bioavailability, potential for reproductive toxicity, and breast cancer chemoprevention in female rats. Toxicol. Sci. 2002, 65, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Lafuente, A.; Guillamon, E.; Villares, A.; Rostagno, M.A.; Martinez, J.A. Flavonoids as anti-inflammatory agents: Implications in cancer and cardiovascular disease. Inflamm. Res. 2009, 58, 537–552. [Google Scholar] [CrossRef] [PubMed]
- Terashima, M.; Kakuno, Y.; Kitano, N.; Matsuoka, C.; Murase, M.; Togo, N.; Watanabe, R.; Matsumura, S. Antioxidant activity of flavonoids evaluated with myoglobin method. Plant Cell Rep. 2012, 31, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.Q.; Kuhn, G.; Wegner, J.; Nürnberg, G.; Chen, J.; Ender, K. Feeding daidzein to late pregnant sows influences the estrogen receptor beta and type 1 insulin-like growth factor receptor mRNA expression in newborn piglets. J. Endocrinol. 2001, 170, 129–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Payne, R.L.; Bidner, T.D.; Southern, L.L.; Geaghan, J.P. Effects of dietary soy isoflavones on growth, carcass traits, and meat quality in growing-finishing pigs. J. Anim. Sci. 2001, 79, 1230–1239. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D. Phytoestrogens: The biochemistry, physiology, and implications for human health of soy isoflavones. Am. J. Clin. Nutr. 1998, 68, 1333–1346. [Google Scholar] [CrossRef] [PubMed]
- D’Ercole, A.J.; Ye, P.; Calikoglu, A.S.; Gutierrez-Ospina, G. The role of the insulin-like growth factors in the central nervous system. Mol. Neurobiol. 1996, 13, 227–255. [Google Scholar] [CrossRef] [PubMed]
- Florini, J.R.; Ewton, D.Z.; Coolican, S.A. Growth hormone and the insulin-like growth factor system in myogenesis. Endocr. Rev. 1996, 17, 481–517. [Google Scholar] [PubMed]
- Klotz, D.M.; Hewitt, S.C.; Korach, K.S.; Diaugustine, R.P. Activation of a uterine insulin-like growth factor 1 signaling pathway by clinical and environmental estrogens: Requirement of estrogen receptor-alpha. Endocrinology 2000, 141, 3430–3439. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.V.; Jackson, J.G.; Gooch, J.L.; Hilsenbeck, S.G.; Coronado-Heinsohn, E.; Osborne, C.K.; Yee, D. Enhancement of insulin-like growth factor signaling in human breast cancer: estrogen regulation of insulin receptor substrate-1 expression in vitro and in vivo. Mol Endocrinol 1999, 13, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.H.; Chen, Z.D.; Zhou, S.; Song, X.Z.; Ouyang, K.H.; Pan, K.; Xu, L.J.; Liu, C.J.; Qu, M.R. Effects of daidzein on performance, serum metabolites, nutrient digestibility, and fecal bacterial community in bull calves. Anim. Feed Sci. Technol. 2017, 225, 87–96. [Google Scholar] [CrossRef]
- Arck, P.; Hansen, P.J.; Mulac Jericevic, B.; Piccinni, M.P.; Szekeres-Bartho, J. Progesterone during pregnancy: Endocrine-immune cross talk in mammalian species and therole of stress. Am. J. Reprod. Immunol. 2007, 58, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, E.D.; Aberdeen, G.W.; Pepe, G.J. The role of estrogen in the maintenance of primate pregnancy. Am. J. Obstet. Gynecol. 2000, 182, 432–438. [Google Scholar] [CrossRef]
- Topper, Y.J. Multiple hormone interactions in the development of mammary gland in vitro. Recent Prog. Horm. Res. 1970, 26, 287–308. [Google Scholar] [PubMed]
- Moudgal, N.R. Luteal function during the periimplantation period and requirement for estrogen for implantation and pregnancy maintenance in the non-human primate. J. Biosci. 1984, 6, 93–96. [Google Scholar] [CrossRef]
- Medigović, I.M.; Živanović, J.B.; Ajdžanović, V.Z.; Nikolić-Kokić, A.L.; Stanković, S.D.; Trifunović, S.L.; Milošević, V.L.; Nestorović, N.M. Effects of soy phytoestrogens on pituitary-ovarian function in middle-aged female rats. Endocrine 2015, 50, 764–776. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Han, Z.; Chen, W. Effects of daidzein on muscle growth and some endogenous hormone levels in rats. Zool. Res. 1995, 16, 23–29. [Google Scholar]
- Zhang, R.; Li, Y.; Wang, W. Enhancement of immune function in mice fed high doses of soy daidzein. Nutr. Cancer 1997, 29, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.Y.; He, S.J.; Liu, S.Q.; Tang, Y.G.; Jin, E.H.; Chen, H.L.; Li, S.H.; Zhong, L.T. Daidzein enhances immune function in late lactation cows under heat stress. Anim. Sci. J. 2014, 85, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Rimbach, G.; De Pascual-Teresa, S.; Ewins, B.A.; Matsugo, S.; Uchida, Y.; Minihane, A.M.; Turner, R.; VafeiAdou, K.; Weinberg, P.D. Antioxidant and free radical scavenging activity of isoflavone metabolites. Xenobiotica 2003, 33, 913–925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimoda, K.; Hamada, H.; Hamada, H. Synthesis of xylooligosaccharides of daidzein and their anti-oxidant and anti-allergic activities. Int. J. Mol. Sci. 2011, 12, 5616–5625. [Google Scholar] [CrossRef] [PubMed]
- Bachelot, A.; Binart, N. Reproductive role of prolactin Reproduction (Cambridge, England). Reproduction 2007, 133, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Royster, M.; Driscoll, P.; Kelly, P.A.; Freemark, M. The prolactin receptor in the fetal rat: Cellular localization of messenger ribonucleic acid, immunoreactive protein, and ligand-binding activity and induction of expression in late gestation. Endocrinology 1995, 136, 3892–3900. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, S.J.; Linzer, D.I. Prolactin receptor expression in the developing mouse embryo. Mol. Reprod. Dev. 1997, 48, 45–52. [Google Scholar] [CrossRef]
- Nynca, A.; Jablonska, O.; Slomczynska, M.; Petroff, B.K.; Ciereszko, R.E. Effects of phytoestrogen daizein and estradiol on steroidogenesis and expression of estrogenreceptors in porcine luteinized granulosa cells from large follicles. J. Physiol. Pharmacol. 2009, 60, 95–105. [Google Scholar] [PubMed]
- Takashima-Sasaki, K.; Komiyama, M.; Adachi, T.; Sakurai, K.; Kato, H.; Iguchi, T.; Mori, C. Effect of exposure to high isoflavone-containing diets on prenatal and postnatal offspring mice. Biosci. Biotechnol. Biochem. 2006, 70, 2874–2882. [Google Scholar] [CrossRef] [PubMed]
- Labelle-Dumais, C.; Jacob-Wagner, M.; Paré, J.F.; Bélanger, L.; Dufort, D. Nuclear receptor NR5A2 is required for proper primitive streak morphogenesis. Dev. Dyn. 2006, 235, 3359–3369. [Google Scholar] [CrossRef] [PubMed]
- Kaluarachchi, D.C.; Momany, A.M.; Busch, T.D.; Gimenez, L.G.; Saleme, C.; Cosentino, V.; Christensen, K.; Dagle, J.M.; Ryckman, K.K.; Murray, C. Polymorphisms in NR5A2, gene encoding liver receptor homolog-1 are associated with preterm birth. Pediatr. Res. 2016, 79, 776–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertolin, K.; Gossen, J.; Schoonjans, K.; Murphy, B.D. The orphan nuclear receptor Nr5a2 is essential for luteinization in the female mouse ovary. Endocrinology 2014, 155, 1931–1943. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Large, M.J.; Duggavathi, R. Liver receptor homolog-1 is essential for pregnancy. Nat. Med. 2013, 19, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Pare´, J.F.; Malenfant, D.; Courtemanche, C.; Jacob-Wagner, M.; Roy, S.; Allard, D.; Be´langer, L. The fetoprotein transcription factor (FTF) gene is essential to embryogenesis and cholesterol homeostasis and is regulated by a DR4 element. J. Biol. Chem. 2004, 279, 21206–21216. [Google Scholar] [CrossRef] [PubMed]
| Genes | Primer Sequences | Size/bp | Accession Number | Annealing Temperature/°C |
|---|---|---|---|---|
| PRLR | F: CTACTTCTGACTGTGAGGACTTGCTG R: GGCTTAACACCTTGACCTGGATACTC | 112 | NM_001034111 | 59 |
| ERα | F: TCTGGAGTGTGCCTGGTTGGAG R: GCGGAATCGACTTGACGTAGCC | 175 | NM_012689 | 61 |
| ERβ | F: TCACGTCAGGCACATCAGTAACAAG R: CATCTCCAGCAGCAGGTCATACAC | 94 | NM_012754 | 60 |
| NR5A2 | F: GTCTCAGGTGATCCAAGCGATGC R: AGTCTGTCGGAGGCAAGGCTAC | 110 | NM_021742 | 61 |
| Tgf-α | F: CCCTCCTGAAAGGAAGGACTG R: AGACACCTTTCCTTGGTTGGG | 111 | NM_012671 | 58 |
| EGFR | F: AATCCTTGATGAAGCCTACGTGATGG R: TGGACAGTGGAGGTCAGACAGATG | 87 | NM_031507 | 60 |
| β-actin | F: TGTCACCAACTGGGACGATA R: GGGGTGTTGAAGGTCTCAAA | 165 | NM_031144.3 | 60 |
| Parameters | Control Group | Daidzein Group | p-Value |
|---|---|---|---|
| Maternal initial pregnancy body weight (g) | 274.50 ± 10.90 | 275.53 ± 9.75 | 0.866 |
| Maternal postpartum body weight (g) | 343.03 ± 36.08 | 340.42 ± 20.53 | 0.881 |
| Maternal body weight gain (g) | 68.53 ± 26.59 | 64.89 ± 12.94 | 0.769 |
| Total newborns (n) | 12.17 ± 3.87 | 16.50 ± 3.73 | 0.076 |
| Live newborns (n) | 11.83 ± 3.54 | 16.17 ± 3.82 | 0.069 |
| Individual newborn weight (g) | 6.37 ± 0.67 | 6.20 ± 0.83 | 0.769 |
| Individual newborn length (mm) | 42.71 ± 4.32 | 43.71 ± 4.36 | 0.143 |
| Total litter weight (g) | 75.35 ± 17.49 | 101.34 ± 14.04 | 0.018 |
| Total viable newborn weight (g) | 75.33 ± 17.46 | 101.32 ± 14.05 | 0.018 |
| Total inviable newborn weight (n) | 0.33 ± 0.52 | 0.33 ± 0.52 | 1.000 |
| Uterine weight (g) | 3.65 ± 0.84 | 4.28 ± 0.82 | 0.222 |
| Ovarian weight (g) | 0.16 ± 0.02 | 0.16 ± 0.03 | 0.891 |
| Uterus index | 10.64 ± 2.26 | 12.56 ± 2.30 | 0.175 |
| Ovary index | 0.46 ± 0.04 | 0.46 ± 0.07 | 0.963 |
| Newborn survival rate (%) | 97.85 ± 3.34 | 97.69 ± 3.83 | 0.942 |
| Food intake (g/day) | 18.98 ± 1.29 | 20.18 ± 1.02 | 0.104 |
| Parameters | Control Group | Daidzein Group | p-Value |
|---|---|---|---|
| Estrogen (pg/mL) | 106.23 ± 14.60 | 136.79 ± 14.59 | <0.01 |
| Progesterone (ng/mL) | 10.04 ± 1.47 | 11.37 ± 0.53 | <0.01 |
| Insulin-like growth factor-1 (ng/mL) | 1093.23 ± 187.33 | 1444.64 ± 182.20 | <0.01 |
| Parameters | Control Group | Daidzein Group | p-Value |
|---|---|---|---|
| IgA (μg/mL) | 159.96 ± 15.47 | 184.38 ± 18.72 | 0.034 |
| IgG (μg/mL) | 370.03 ± 44.99 | 498.55 ± 50.76 | <0.01 |
| Parameters | Control Group | Daidzein Group | p-Value |
|---|---|---|---|
| Glucose (mmol/L) | 7.65 ± 1.44 | 8.63 ± 2.10 | 0.369 |
| Urea nitrogen BUN (mmol/L) | 10.91 ± 2.28 | 11.69 ± 2.63 | 0.597 |
| Total protein (g/L) | 35.84 ± 6.81 | 32.1725 ± 8.56 | 0.431 |
| Albumin protein (g/L) | 21.87 ± 4.60 | 19.62 ± 3.36 | 0.357 |
| ALT (U/L) | 22.99 ± 9.49 | 24.65 ± 9.12 | 0.765 |
| AST (U/L) | 30.47 ± 3.47 | 31.16 ± 4.48 | 0.774 |
| Triglycerides (mmol/L) | 1.46 ± 0.67 | 1.64 ± 1.05 | 0.720 |
| Total cholesterol (mmol/L) | 3.31 ± 0.52 | 3.26 ± 0.36 | 0.864 |
| HDL-C (mmol/L) | 1.78 ± 0.37 | 1.71 ± 0.21 | 0.693 |
| LDL-C (mmol/L) | 0.74 ± 0.31 | 0.96 ± 0.50 | 0.396 |
| T-AOC (U/mL) | 6.32 ± 0.82 | 8.17 ± 1.68 | 0.036 |
| SOD (U/mL) | 161.08 ± 8.23 | 175.26 ± 6.11 | <0.01 |
| CAT (U/mL) | 12.08 ± 5.02 | 15.10 ± 5.77 | 0.357 |
| GSH-Px (U/mL) | 1403.91 ± 107.04 | 1463.30 ± 87.47 | 0.317 |
| MDA (nmol/mL) | 10.96 ± 2.16 | 8.72 ± 1.67 | 0.073 |
| Parameters | Control Group | Daidzein Group | p-Value |
|---|---|---|---|
| Uterus | |||
| T-AOC (U/mg protein) | 1.39 ± 0.46 | 1.79 ± 0.60 | 0.224 |
| SOD (U/mg protein) | 25.92 ± 4.93 | 33.12 ± 8.20 | 0.095 |
| CAT (U/mg protein) | 66.50 ± 12.40 | 71.62 ± 10.74 | 0.462 |
| GSH-Px (U/mg protein) | 1564.87 ± 191.16 | 1691.88 ± 238.83 | 0.333 |
| MDA (nmol/mg protein) | 1.39 ± 0.46 | 1.79 ± 0.60 | 0.224 |
| Ovaries | |||
| T-AOC (U/mg protein) | 1.76 ± 0.47 | 1.84 ± 0.28 | 0.746 |
| SOD (U/mg protein) | 25.60 ± 4.51 | 31.47 ± 3.64 | 0.032 |
| CAT (U/mg protein) | 119.80 ± 21.78 | 122.22 ± 11.36 | 0.462 |
| GSH-Px (U/mg protein) | 1336.93 ± 118.80 | 1268.70 ± 100.74 | 0.814 |
| MDA (nmol/mg protein) | 5.85 ± 3.30 | 5.15 ± 2.64 | 0.693 |
| Fetal longissimus dorsi muscle | |||
| T-AOC (U/mg protein) | 0.40 ± 0.12 | 0.60 ± 0.13 | 0.022 |
| SOD (U/mg protein) | 23.72 ± 4.58 | 24.20 ± 4.53 | 0.860 |
| CAT (U/mg protein) | 139.48 ± 26.86 | 146.73 ± 20.76 | 0.613 |
| GSH-Px (U/mg protein) | 953.87 ± 183.85 | 1005.29 ± 159.79 | 0.616 |
| MDA (nmol/mg protein) | 6.00 ± 2.00 | 5.53 ± 1.43 | 0.651 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Chen, D.; Yu, B.; Mao, X.; Huang, Z.; Yu, J.; Luo, J.; Zheng, P.; Luo, Y.; He, J. Effects of Dietary Daidzein Supplementation on Reproductive Performance, Serum Hormones, and Reproductive-Related Genes in Rats. Nutrients 2018, 10, 766. https://doi.org/10.3390/nu10060766
Zhang Q, Chen D, Yu B, Mao X, Huang Z, Yu J, Luo J, Zheng P, Luo Y, He J. Effects of Dietary Daidzein Supplementation on Reproductive Performance, Serum Hormones, and Reproductive-Related Genes in Rats. Nutrients. 2018; 10(6):766. https://doi.org/10.3390/nu10060766
Chicago/Turabian StyleZhang, Qiqi, Daiwen Chen, Bing Yu, Xiangbing Mao, Zhiqing Huang, Jie Yu, Junqiu Luo, Ping Zheng, Yuheng Luo, and Jun He. 2018. "Effects of Dietary Daidzein Supplementation on Reproductive Performance, Serum Hormones, and Reproductive-Related Genes in Rats" Nutrients 10, no. 6: 766. https://doi.org/10.3390/nu10060766




