Adiposity and Serum Selenium in U.S. Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Body Mass Index, Percent Body Fat and Waist Circumference
2.3. Serum Selenium
2.4. Other Variables
2.5. Statistical Analysis
3. Results
3.1. Characteristics of Study Population
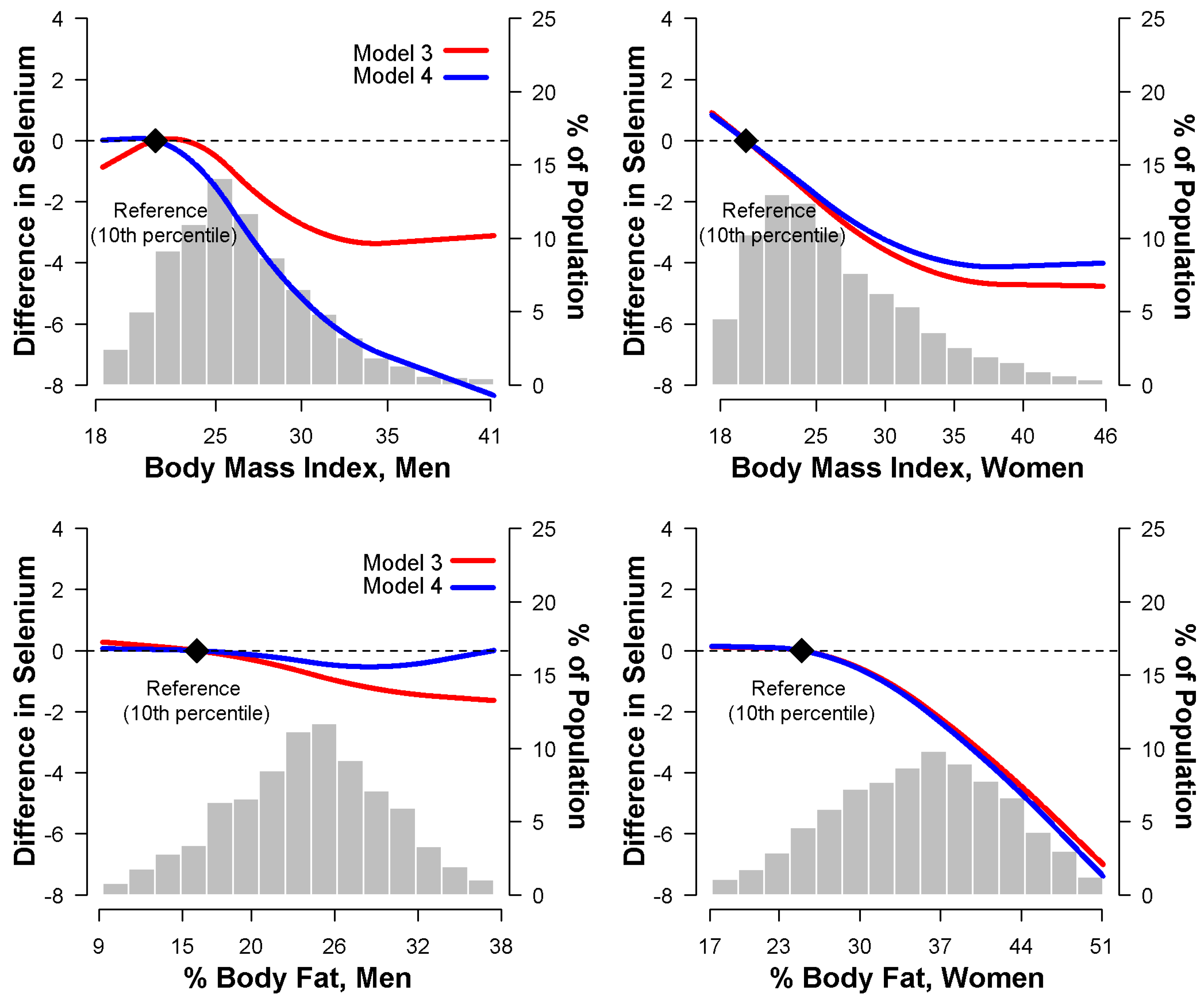
3.2. Relationship between Body Mass Index and Serum Selenium
3.3. Relationship between Percent Body Fat and Serum Selenium
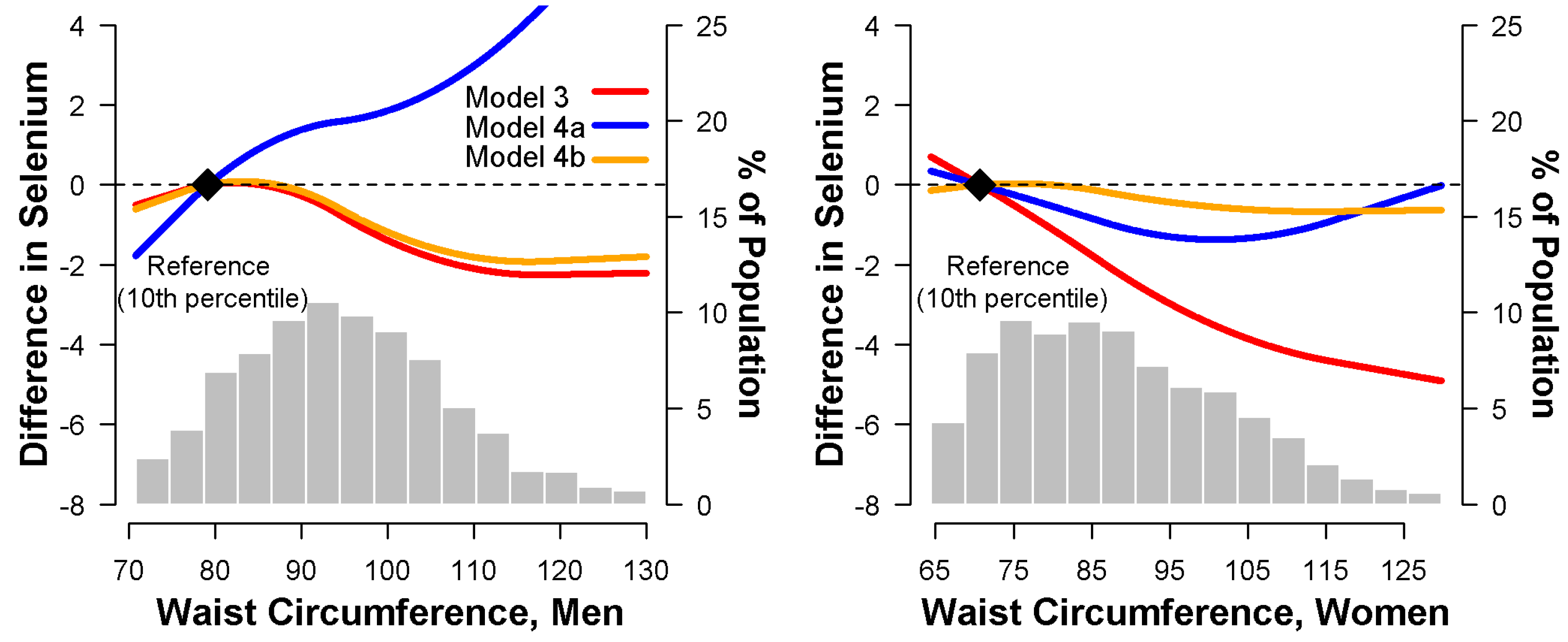
3.4. Relationship between Waist Circumference and Serum Selenium
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Kelli, H.M.; Corrigan, F.E., 3rd; Heinl, R.E.; Dhindsa, D.S.; Hammadah, M.; Samman-Tahhan, A.; Sandesara, P.; O’Neal, W.T.; Al Mheid, I.; Ko, Y.A.; et al. Relation of Changes in Body Fat Distribution to Oxidative Stress. Am. J. Cardiol. 2017, 120, 2289–2293. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Sanchez, A.; Madrigal-Santillan, E.; Bautista, M.; Esquivel-Soto, J.; Morales-Gonzalez, A.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sanchez-Rivera, G.; Valadez-Vega, C.; Morales-Gonzalez, J.A. Inflammation, oxidative stress, and obesity. Int. J. Mol. Sci. 2011, 12, 3117–3132. [Google Scholar] [CrossRef] [PubMed]
- Keaney, J.F., Jr.; Larson, M.G.; Vasan, R.S.; Wilson, P.W.; Lipinska, I.; Corey, D.; Massaro, J.M.; Sutherland, P.; Vita, J.A.; Benjamin, E.J. Obesity and systemic oxidative stress: Clinical correlates of oxidative stress in the Framingham Study. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Galili, O.; Versari, D.; Sattler, K.J.; Olson, M.L.; Mannheim, D.; McConnell, J.P.; Chade, A.R.; Lerman, L.O.; Lerman, A. Early experimental obesity is associated with coronary endothelial dysfunction and oxidative stress. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H904–H911. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amirkhizi, F.; Siassi, F.; Djalali, M.; Shahraki, S.H. Impaired enzymatic antioxidant defense in erythrocytes of women with general and abdominal obesity. Obes. Res. Clin. Pract. 2014, 8, e26–e34. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Yang, T.B.; Wei, J.; Lei, G.H.; Zeng, C. Association between serum selenium level and type 2 diabetes mellitus: A non-linear dose-response meta-analysis of observational studies. Nutr. J. 2016, 15, 48. [Google Scholar] [CrossRef] [PubMed]
- Bleys, J.; Navas-Acien, A.; Guallar, E. Serum selenium and diabetes in U.S. adults. Diabetes Care 2007, 30, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Christensen, K.; Werner, M.; Malecki, K. Serum selenium and lipid levels: Associations observed in the National Health and Nutrition Examination Survey (NHANES) 2011–2012. Environ. Res. 2015, 140, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Laclaustra, M.; Stranges, S.; Navas-Acien, A.; Ordovas, J.M.; Guallar, E. Serum selenium and serum lipids in US adults: National Health and Nutrition Examination Survey (NHANES) 2003–2004. Atherosclerosis 2010, 210, 643–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bleys, J.; Navas-Acien, A.; Stranges, S.; Menke, A.; Miller, E.R., 3rd; Guallar, E. Serum selenium and serum lipids in US adults. Am. J. Clin. Nutr. 2008, 88, 416–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laclaustra, M.; Navas-Acien, A.; Stranges, S.; Ordovas, J.M.; Guallar, E. Serum selenium concentrations and hypertension in the US Population. Circ. Cardiovasc. Qual. Outcomes 2009, 2, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Kimmons, J.E.; Blanck, H.M.; Tohill, B.C.; Zhang, J.; Khan, L.K. Associations between body mass index and the prevalence of low micronutrient levels among US adults. Med. Gen. Med. 2006, 8, 59. [Google Scholar]
- Arnaud, J.; Bertrais, S.; Roussel, A.M.; Arnault, N.; Ruffieux, D.; Favier, A.; Berthelin, S.; Estaquio, C.; Galan, P.; Czernichow, S.; et al. Serum selenium determinants in French adults: The SU.VI.M.AX study. Br. J. Nutr. 2006, 95, 313–320. [Google Scholar] [CrossRef] [PubMed]
- National Center for Health Statistics. National Health and Nutrition Examination Survey Ш: Body Measurements (Anthropometry); Westat, Inc.: Rockville, MD, USA, 1988.
- Quinones, J.L.; Thapar, U.; Yu, K.; Fang, Q.; Sobol, R.W.; Demple, B. Enzyme mechanism-based, oxidative DNA-protein cross-links formed with DNA polymerase beta in vivo. Proc. Natl. Acad. Sci. USA 2015, 112, 8602–8607. [Google Scholar] [CrossRef] [PubMed]
- Kuczmarski, R.J. Bioelectrical impedance analysis measurements as part of a national nutrition survey. Am. J. Clin. Nutr. 1996, 64, 453S–458S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chumlea, W.C.; Guo, S.S.; Kuczmarski, R.J.; Flegal, K.M.; Johnson, C.L.; Heymsfield, S.B.; Lukaski, H.C.; Friedl, K.; Hubbard, V.S. Body composition estimates from NHANES III bioelectrical impedance data. Int. J. Obes. Relat. Metab. Disord. 2002, 26, 1596–1609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, S.S.; Chumlea, W.C.; Heymsfield, S.B.; Lukaski, H.C.; Schoeller, D.; Friedl, K.; Kuczmarski, R.J.; Flegal, K.M.; Johnson, C.L.; Hubbard, V.S. Development of bioelectrical impedance analysis prediction equations for body composition with the use of a multicomponent model for use in epidemiologic surveys. Am. J. Clin. Nutr. 2003, 77, 331–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, S.A.; Hardison, N.W.; Veillon, C. Comparison of isotope dilution mass spectrometry and graphite furnace atomic absorption spectrometry with Zeeman background correction for determination of plasma selenium. Anal. Chem. 1986, 58, 1272–1273. [Google Scholar] [CrossRef] [PubMed]
- Paschal, D.C.; Kimberly, M.M. Automated direct determination of selenium in serum by electrothermal atomic absorption spectroscopy. At. Spectrosc. 1986, 7, 75–78. [Google Scholar]
- Gunter, E.W.; Lewis, B.G.; Koncikowski, S.M. Laboratory procedures used for the Third National Health and Nutrition Examination Survey; U.S. Department of Health and Human Services: Atlanta, GA, USA, 1996. [Google Scholar]
- Ainsworth, B.E.; Haskell, W.L.; Leon, A.S.; Jacobs, D.R., Jr.; Montoye, H.J.; Sallis, J.F.; Paffenbarger, R.S., Jr. Compendium of physical activities: Classification of energy costs of human physical activities. Med. Sci. Sports Exerc. 1993, 25, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Alasfar, F.; Ben-Nakhi, M.; Khoursheed, M.; Kehinde, E.O.; Alsaleh, M. Selenium is significantly depleted among morbidly obese female patients seeking bariatric surgery. Obes. Surg. 2011, 21, 1710–1713. [Google Scholar] [CrossRef] [PubMed]
- Blazewicz, A.; Klatka, M.; Astel, A.; Korona-Glowniak, I.; Dolliver, W.; Szwerc, W.; Kocjan, R. Serum and urinary selenium levels in obese children: A cross-sectional study. J. Trace Elem. Med. Biol. 2015, 29, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Ghayour-Mobarhan, M.; Taylor, A.; New, S.A.; Lamb, D.J.; Ferns, G.A. Determinants of serum copper, zinc and selenium in healthy subjects. Ann. Clin. Biochem. 2005, 42, 364–375. [Google Scholar] [CrossRef] [PubMed]
- Spina, A.; Guallar, E.; Rayman, M.P.; Tigbe, W.; Kandala, N.B.; Stranges, S. Anthropometric indices and selenium status in British adults: The U.K. National Diet and Nutrition Survey. Free. Radic. Biol. Med. 2013, 65, 1315–1321. [Google Scholar] [CrossRef] [PubMed]
- Suadicani, P.; Hein, H.O.; Gyntelberg, F. Serum selenium concentration and risk of ischaemic heart disease in a prospective cohort study of 3000 males. Atherosclerosis 1992, 96, 33–42. [Google Scholar] [CrossRef]
- Van Nhien, N.; Yabutani, T.; Khan, N.C.; Khanh le, N.B.; Ninh, N.X.; Chung le, T.K.; Motonaka, J.; Nakaya, Y. Association of low serum selenium with anemia among adolescent girls living in rural Vietnam. Nutrition 2009, 25, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.; Katz, E.G.; Huxley, R.R. Associations between gender, age and waist circumference. Eur. J. Clin. Nutr. 2010, 64, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Charles, L.E.; Burchfiel, C.M.; Violanti, J.M.; Fekedulegn, D.; Slaven, J.E.; Browne, R.W.; Hartley, T.A.; Andrew, M.E. Adiposity measures and oxidative stress among police officers. Obesity 2008, 16, 2489–2497. [Google Scholar] [CrossRef] [PubMed]
- Satia, J.A.; King, I.B.; Morris, J.S.; Stratton, K.; White, E. Toenail and plasma levels as biomarkers of selenium exposure. Ann. Epidemiol. 2006, 16, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Nichol, C.; Herdman, J.; Sattar, N.; O’Dwyer, P.J.; St, J.O.R.D.; Littlejohn, D.; Fell, G. Changes in the concentrations of plasma selenium and selenoproteins after minor elective surgery: Further evidence for a negative acute phase response? Clin. Chem. 1998, 44, 1764–1766. [Google Scholar] [PubMed]
- Duncan, A.; Talwar, D.; McMillan, D.C.; Stefanowicz, F.; O’Reilly, D.S. Quantitative data on the magnitude of the systemic inflammatory response and its effect on micronutrient status based on plasma measurements. Am. J. Clin. Nutr. 2012, 95, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Hesse-Bahr, K.; Dreher, I.; Kohrle, J. The influence of the cytokines Il-1beta and INFgamma on the expression of selenoproteins in the human hepatocarcinoma cell line HepG2. Biofactors 2000, 11, 83–85. [Google Scholar] [CrossRef] [PubMed]
- Renko, K.; Hofmann, P.J.; Stoedter, M.; Hollenbach, B.; Behrends, T.; Kohrle, J.; Schweizer, U.; Schomburg, L. Down-regulation of the hepatic selenoprotein biosynthesis machinery impairs selenium metabolism during the acute phase response in mice. FASEB J. 2009, 23, 1758–1765. [Google Scholar] [CrossRef] [PubMed]
- Litwak, S.A.; Pang, L.; Galic, S.; Igoillo-Esteve, M.; Stanley, W.J.; Turatsinze, J.V.; Loh, K.; Thomas, H.E.; Sharma, A.; Trepo, E.; et al. JNK Activation of BIM Promotes Hepatic Oxidative Stress, Steatosis, and Insulin Resistance in Obesity. Diabetes 2017, 66, 2973–2986. [Google Scholar] [CrossRef] [PubMed]
- Reho, J.J.; Rahmouni, K. Oxidative and inflammatory signals in obesity-associated vascular abnormalities. Clin. Sci. 2017, 131, 1689–1700. [Google Scholar] [CrossRef] [PubMed]
- Stranges, S.; Marshall, J.R.; Natarajan, R.; Donahue, R.P.; Trevisan, M.; Combs, G.F.; Cappuccio, F.P.; Ceriello, A.; Reid, M.E. Effects of long-term selenium supplementation on the incidence of type 2 diabetes: A randomized trial. Ann. Intern. Med. 2007, 147, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Flegal, K.M.; Shepherd, J.A.; Looker, A.C.; Graubard, B.I.; Borrud, L.G.; Ogden, C.L.; Harris, T.B.; Everhart, J.E.; Schenker, N. Comparisons of percentage body fat, body mass index, waist circumference, and waist-stature ratio in adults. Am. J. Clin. Nutr. 2009, 89, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Di Renzo, L.; Galvano, F.; Orlandi, C.; Bianchi, A.; Di Giacomo, C.; La Fauci, L.; Acquaviva, R.; De Lorenzo, A. Oxidative stress in normal-weight obese syndrome. Obesity 2010, 18, 2125–2130. [Google Scholar] [CrossRef] [PubMed]
- Frossing, S.; Nylander, M.C.; Chabanova, E.; Kistorp, C.; Skouby, S.O.; Faber, J. Quantification of visceral adipose tissue in polycystic ovary syndrome: Dual-energy X-ray absorptiometry versus magnetic resonance imaging. Acta Radiol. 2018, 59, 13–17. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Overall (n = 13,289) | Men (n = 6440) | Women (n = 6849) | p |
|---|---|---|---|---|
| Age, year | 44.2 (0.4) | 43.0 (0.4) | 45.3 (0.5) | 0.75 |
| White, % | 77.2 (1.3) | 77.3 (1.4) | 77.2 (1.3) | 0.88 |
| Education (≥12 years), % | 76.2 (1.1) | 75.2 (1.2) | 77.3 (1.1) | 0.03 |
| Current smoker, % | 34.0 (0.9) | 40.2 (1.0) | 27.8 (0.9) | <0.001 |
| Postmenopausal, % | — | — | 39.6 (1.4) | |
| Sedentary lifestyle, % | 34.8 (0.9) | 27.0 (1.2) | 42.4 (1.4) | <0.001 |
| Systolic blood pressure, mmHg | 122.2 (0.4) | 124.3 (0.4) | 120.0 (0.5) | < 0.001 |
| Antihypertensive medication, % | 11.6 (0.5) | 9.7 (0.7) | 13.5 (0.6) | < 0.001 |
| Diabetes, % | 5.3 (0.3) | 5.6 (0.4) | 4.9 (0.4) | 0.18 |
| Serum cotinine, ng/mL | 78.5 (2.6) | 96.1 (3.3) | 61.1 (2.6) | < 0.001 |
| C-reactive protein (≥1 mg/dL), % | 7.0 (0.4) | 4.4 (0.4) | 9.6 (0.6) | < 0.001 |
| Serum total cholesterol, mmol/L | 5.3 (0.02) | 5.2 (0.03) | 5.3 (0.02) | 0.001 |
| Serum triglycerides, mmol/L | 1.6 (0.02) | 1.7 (0.03) | 1.4 (0.02) | < 0.001 |
| Serum HDL-cholesterol, mmol/L | 1.3 (0.01) | 1.2 (0.01) | 1.4 (0.01) | < 0.001 |
| Body mass index, kg/m2 | 26.5 (0.1) | 26.6 (0.1) | 26.4 (0.2) | < 0.001 |
| Percent body fat, % | 29.6 (0.2) | 24.0 (0.2) | 35.1 (0.3) | < 0.001 |
| Waist circumference, cm | 91.8 (0.3) | 95.4 (0.3) | 88.5 (0.4) | < 0.001 |
| Serum selenium, ng/mL | 125.8 (0.9) | 127.2 (1.0) | 124.5 (0.9) | < 0.001 |
| Quartiles of BMI (kg/m2) | p for Trend | ||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ||
| Men | |||||
| BMI, kg/m2 | ≤23.5 | 23.6–25.9 | 26.0–29.0 | ≥29.1 | |
| Number (%) | 1636 (25.4) | 1466 (22.8) | 1671 (26.0) | 1667 (25.9) | |
| Serum selenium, ng/mL * | 128.8 | 127.2 | 127.2 | 125.6 | |
| Model 1 † | 0.0 (reference) | −1.6 (−3.2, 0.2) | −0.8 (−3.2, 0.8) | −2.4 (−4.7, −0.8) | 0.01 |
| Model 2 ‡ | 0.0 (reference) | −1.6 (−3.2, −0.3) | −1.6 (−3.2, −0.1) | −3.2 (−4.7, −1.6) | 0.003 |
| Model 3 ** | 0.0 (reference) | −2.4 (−4.0, −0.8) | −2.4 (−4.0, −0.3) | −4.0 (−5.5, −1.6) | <0.001 |
| Model 4 †† | 0.0 (reference) | −3.2 (−4.7, −0.8) | −3.2 (−4.7, −0.8) | −5.5 (−8.7, −1.6) | 0.005 |
| Women | |||||
| BMI, kg/m2 | ≤21.9 | 22.0–25.1 | 25.2–29.9 | ≥30.0 | |
| Number (%) | 1317 (19.2) | 1506 (22.0) | 1985 (29.0) | 2041 (29.8) | |
| Serum selenium, ng/mL * | 125.6 | 125.6 | 124.0 | 123.2 | |
| Model 1 † | 0.0 (reference) | −0.2 (−1.6, 1.6) | −1.6 (−3.2, −0.1) | −2.4 (−4.0, −0.8) | 0.006 |
| Model 2 ‡ | 0.0 (reference) | −0.8 (−2.4, 1.6) | −1.6 (−3.2, −0.2) | −2.4 (−4.0, −0.8) | 0.003 |
| Model 3 ** | 0.0 (reference) | −1.6 (−3.2, 0.3) | −3.2 (−4.7, −1.6) | −4.0 (−5.5, −1.6) | <0.001 |
| Model 4 †† | 0.0 (reference) | −0.8 (−3.2, 0.8) | −2.4 (−4.0, −0.8) | −1.6 (−4.0, 0.2) | 0.11 |
| Quartile of %BF | p for Trend | ||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ||
| Men | |||||
| %BF | ≤20.1 | 20.1–24.2 | 24.2–27.9 | 27.9–49.8 | |
| Number (%) | 1369 (21.3) | 1510 (23.5) | 1571 (24.4) | 1990 (30.9) | |
| Serum selenium, ng/mL * | 127.6 | 127.5 | 127.4 | 126.2 | |
| Model 1 † | 0.0 (reference) | −0.1 (−1.6, 1.4) | −0.2 (−2.5, 2.1) | −1.1 (−3.6, 1.4) | 0.41 |
| Model 2 ‡ | 0.0 (reference) | −0.5 (−2.0, 1.0) | −0.7 (−2.9, 1.5) | −1.3 (−3.8, 1.1) | 0.31 |
| Model 3 ** | 0.0 (reference) | −0.9 (−2.5, 0.7) | −1.3 (−3.5, 1.0) | −1.7 (−4.2, 0.7) | 0.18 |
| Model 4 †† | 0.0 (reference) | −0.7 (−2.5, 1.0) | −0.9 (−3.8, 2.0) | −1.0 (−5.2, 3.1) | 0.61 |
| Women | |||||
| %BF | ≤ 29.7 | 29.7–35.4 | 35.4–40.7 | 40.7–59.4 | |
| Number (%) | 1196 (17.5) | 1633 (23.8) | 1916 (28.0) | 2104 (30.7) | |
| Serum selenium, ng/mL * | 125.4 | 126.1 | 124.7 | 121.9 | |
| Model 1 † | 0.0 (reference) | 1.0 (−0.6, 2.6) | −0.4 (−2.2, 1.4) | −2.8 (−4.7, −0.9) | 0.002 |
| Model 2 ‡ | 0.0 (reference) | 0.7 (−0.9, 2.3) | −0.6 (−2.4, 1.2) | −3.1 (−5.0, −1.2) | 0.001 |
| Model 3 ** | 0.0 (reference) | −0.1 (−1.8, 1.6) | −2.2 (−4.4, 0.02) | −4.5 (−7.0, −1.9) | 0.001 |
| Model 4 †† | 0.0 (reference) | −0.05 (−1.9, 1.8) | −2.0 (−4.7, 0.7) | −4.1 (−7.8, −0.4) | 0.03 |
| Quartile of WC (cm) | p for Trend | ||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ||
| Men | |||||
| WC, cm | ≤86.2 | 86.3–94.4 | 94.5–102.9 | 103.0–168.8 | |
| Number (%) | 1616 (25.1) | 1534 (23.8) | 1602 (24.9) | 1688 (26.2) | |
| Serum selenium, ng/mL * | 127.3 | 128.1 | 126.8 | 126.5 | |
| Model 1 † | 0.0 (reference) | 0.3 (−1.6, 2.3) | −1.0 (−3.2, 1.1) | −1.2 (−3.2, 0.7) | 0.14 |
| Model 2 ‡ | 0.0 (reference) | −0.1 (−2.0, 1.9) | −1.3 (−3.4, 0.8) | −1.5 (−3.4, 0.4) | 0.08 |
| Model 3 ** | 0.0 (reference) | −0.6 (−2.9, 1.6) | −1.7 (−4.2, 0.7) | −1.9 (−3.8, −0.1) | 0.03 |
| Model 4a †† | 0.0 (reference) | 0.1 (−2.0, 2.2) | −0.4 (−2.9, 2.2) | 0.7 (−2.1, 3.4) | 0.80 |
| Model 4b ▲ | 0.0 (reference) | −0.5 (−3.3, 2.3) | −1.5 (−4.9, 1.8) | −1.6 (−5.1, 1.8) | 0.29 |
| Women | |||||
| WC, cm | ≤76.6 | 76.8–86.4 | 86.5–98.4 | 98.6–157.8 | |
| Number (%) | 1269 (18.5) | 1522 (22.2) | 2019 (29.5) | 2039 (29.8) | |
| Serum selenium, ng/mL * | 125.5 | 125.2 | 124.2 | 123.2 | |
| Model 1 † | 0.0 (reference) | −0.4 (−2.2, 1.4) | −1.4 (−3.1, 0.4) | −2.3 (−4.0, −0.6) | 0.005 |
| Model 2 ‡ | 0.0 (reference) | −0.5 (−2.2, 1.3) | −1.3 (−3.1, 0.5) | −2.3 (−3.9, −0.6) | 0.005 |
| Model 3 ** | 0.0 (reference) | −1.0 (−2.8, 0.7) | −2.6 (−4.5, −0.6) | −3.9 (−5.8, −2.0) | <0.001 |
| Model 4a †† | 0.0 (reference) | −0.5 (−2.3, 1.3) | −1.5 (−3.7, 0.6) | −1.8 (−4.3, 0.7) | 0.11 |
| Model 4b ▲ | 0.0 (reference) | 0.1 (−1.9, 2.1) | −0.6 (−3.3, 2.2) | −0.9 (−4.5, 2.7) | 0.53 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, Q.; Lin, R.; Nong, Q. Adiposity and Serum Selenium in U.S. Adults. Nutrients 2018, 10, 727. https://doi.org/10.3390/nu10060727
Zhong Q, Lin R, Nong Q. Adiposity and Serum Selenium in U.S. Adults. Nutrients. 2018; 10(6):727. https://doi.org/10.3390/nu10060727
Chicago/Turabian StyleZhong, Qiuan, Ruoxi Lin, and Qingjiao Nong. 2018. "Adiposity and Serum Selenium in U.S. Adults" Nutrients 10, no. 6: 727. https://doi.org/10.3390/nu10060727





