Aluminum Exposure from Parenteral Nutrition: Early Bile Canaliculus Changes of the Hepatocyte
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Work
2.2. Transmission Electron Microscopy
2.3. Bile Acid Assay
2.4. Statistical Analysis
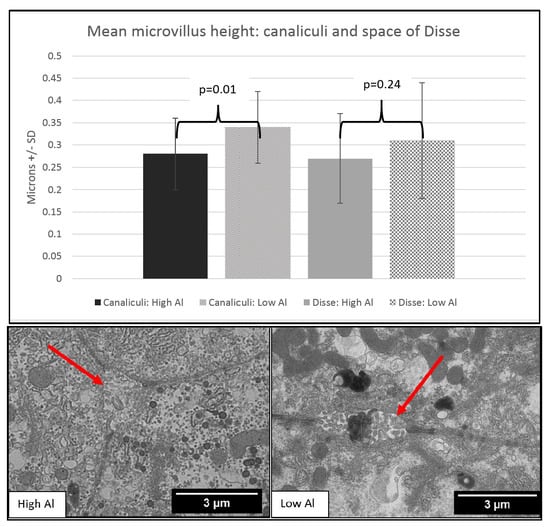
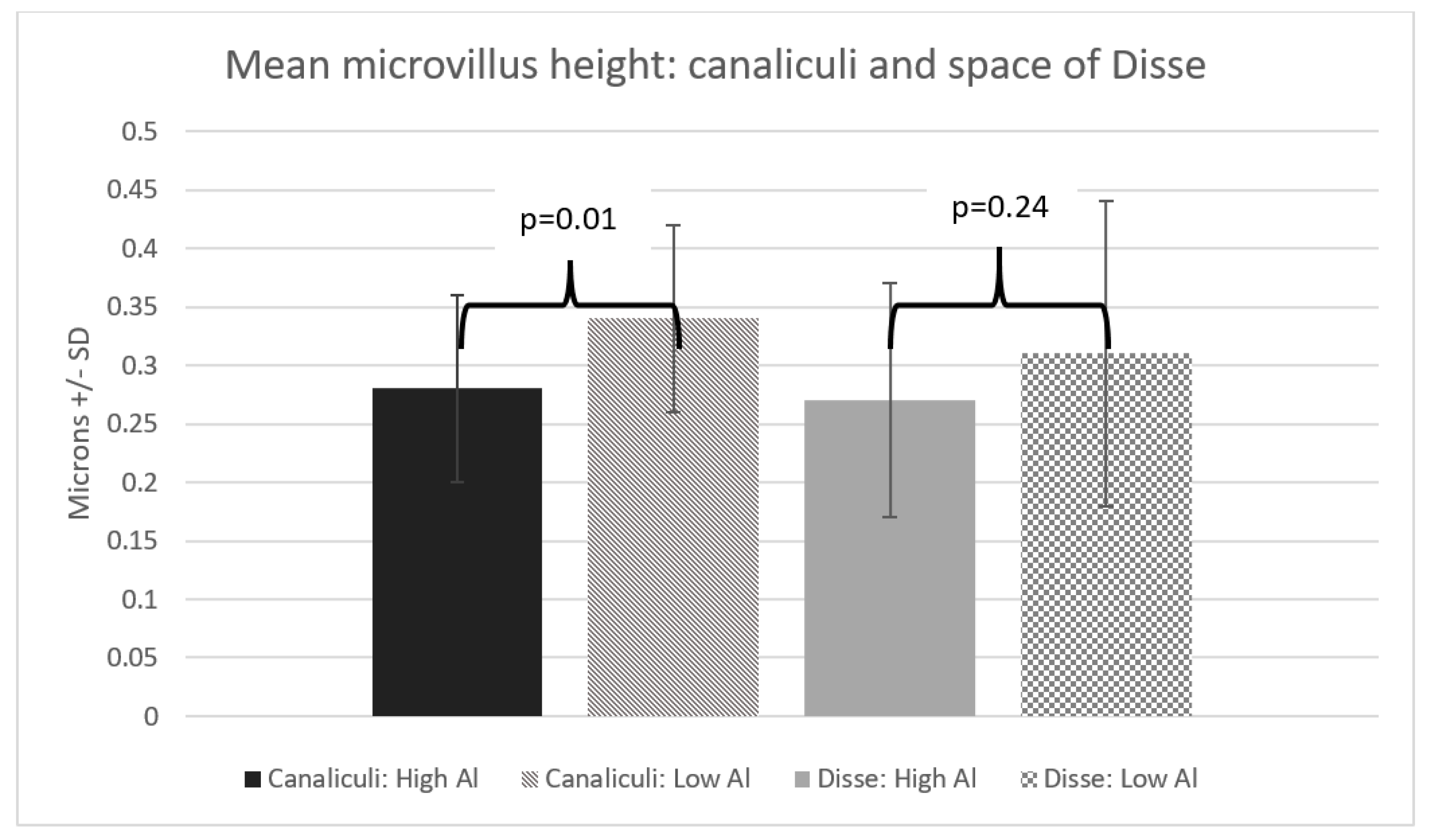
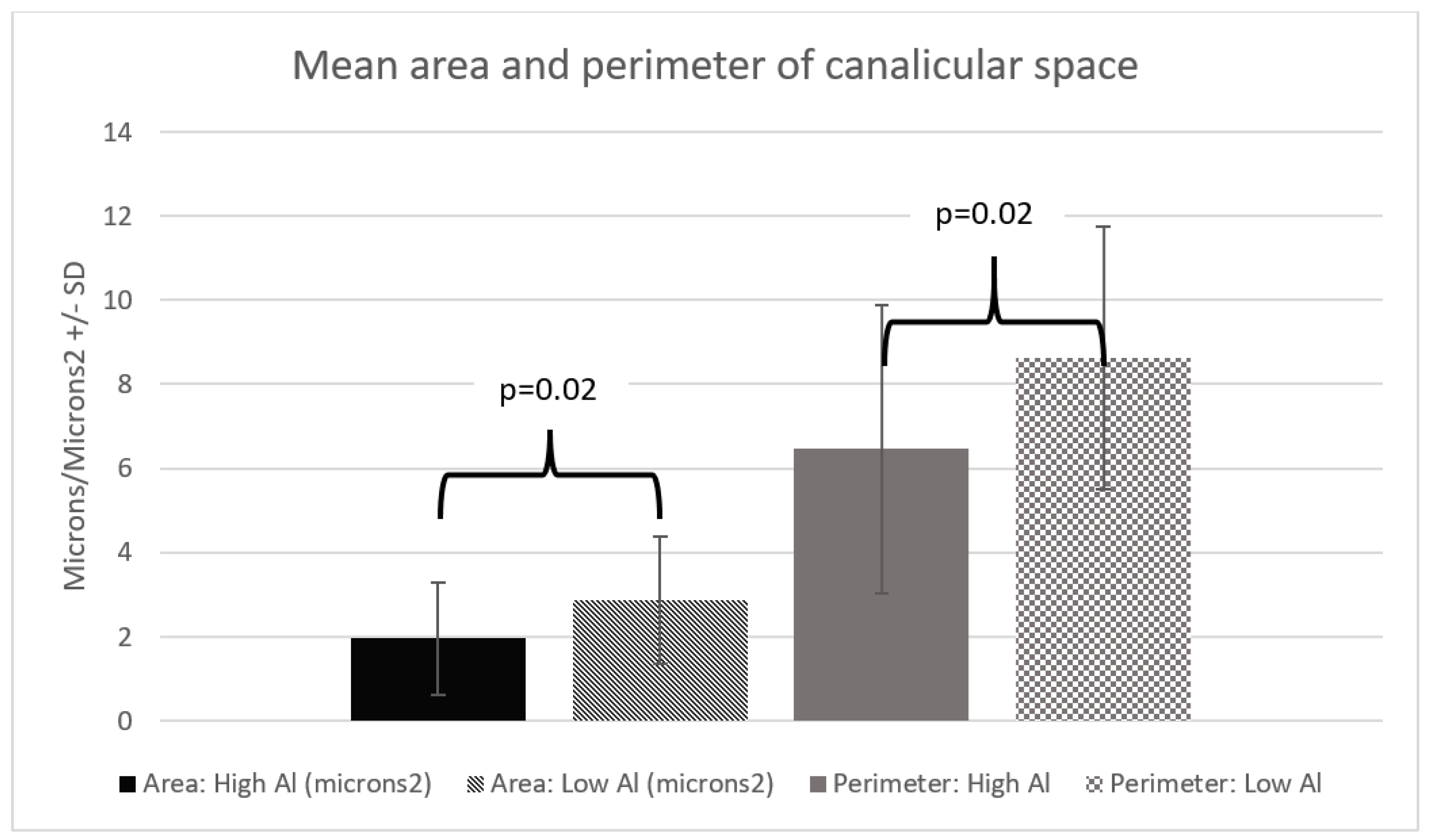
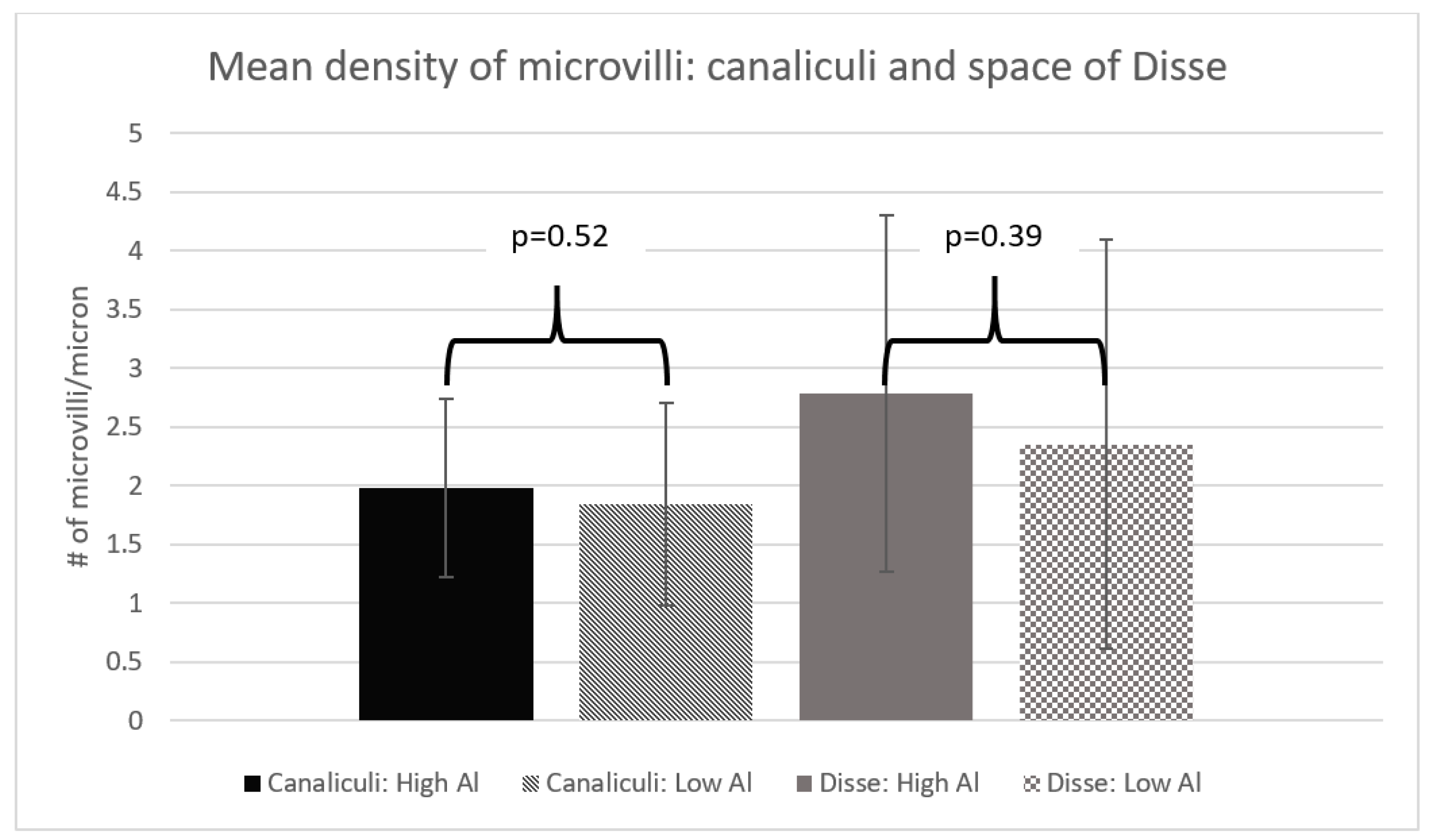
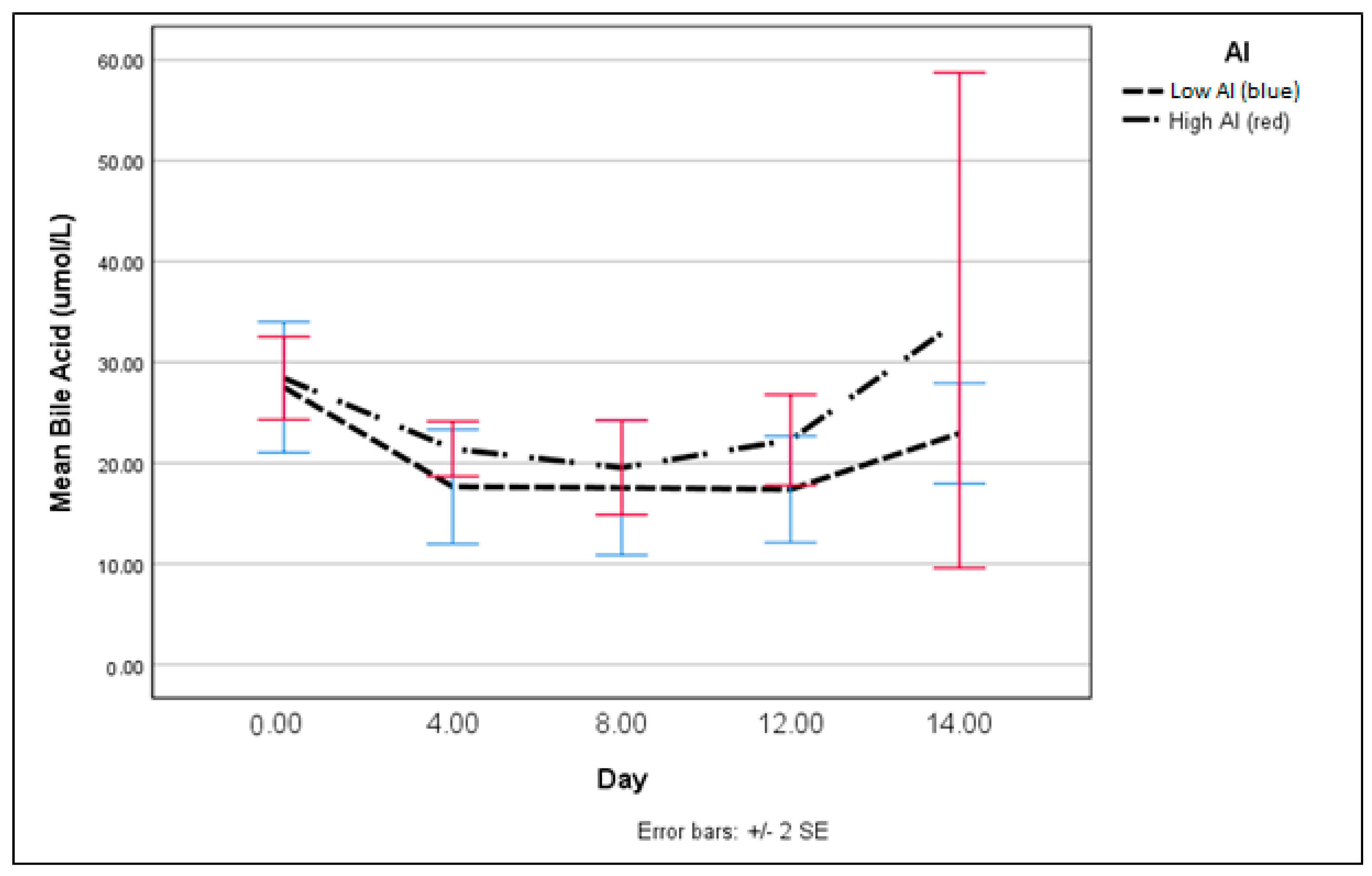
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Poole, R.L.; Schiff, L.; Hintz, S.R.; Wong, A.; Mackenzie, N.; Kerner, J.A., Jr. Aluminum content of parenteral nutrition in neonates: Measured versus calculated levels. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 208–211. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, S.R.; Bohrer, D.; Garcia, S.C.; do Nascimento, P.C.; Noremberg, S. Aluminum content in intravenous solutions for administration to neonates: Role of product preparation and administration methods. J. Parenter. Enter. Nutr. 2010, 34, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Bohrer, D.; Oliveira, S.M.; Garcia, S.C.; Nascimento, P.C.; Carvalho, L.M. Aluminum loading in preterm neonates revisited. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Koo, W.W.; Kaplan, L.A.; Bendon, R.; Succop, P.; Tsang, R.C.; Horn, J.; Steichen, J.J. Response to aluminum in parenteral nutrition during infancy. J. Pediatr. 1986, 109, 877–883. [Google Scholar] [CrossRef]
- Courtney-Martin, G.; Kosar, C.; Campbell, A.; Avitzur, Y.; Wales, P.W.; Steinberg, K.; Harrison, D.; Chambers, K. Plasma aluminum concentrations in pediatric patients receiving long-term parenteral nutrition. JPEN J. Parenter. Enter. Nutr. 2015, 39, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.A.; Roma, M.G.; Bernal, C.A.; Alvarez Mde, L.; Carrillo, M.C. Biliary secretory function in rats chronically intoxicated with aluminum. Toxicol. Sci. 2004, 79, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Klein, G.L.; Heyman, M.B.; Lee, T.C.; Miller, N.L.; Marathe, G.; Gourley, W.K.; Alfrey, A.C. Aluminum-associated hepatobiliary dysfunction in rats: Relationships to dosage and duration of exposure. Pediatr. Res. 1988, 23, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Bertholf, R.L.; Herman, M.M.; Savory, J.; Carpenter, R.M.; Sturgill, B.C.; Katsetos, C.D.; Vandenberg, S.R.; Wills, M.R. A long-term intravenous model of aluminum maltol toxicity in rabbits: Tissue distribution, hepatic, renal, and neuronal cytoskeletal changes associated with systemic exposure. Toxicol. Appl. Pharmacol. 1989, 98, 58–74. [Google Scholar] [CrossRef]
- Demircan, M.; Ergun, O.; Coker, C.; Yilmaz, F.; Avanoglu, S.; Ozok, G. Aluminum in total parenteral nutrition solutions produces portal inflammation in rats. J. Pediatr. Gastroenterol. Nutr. 1998, 26, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Percy, M.E.; Kruck, T.P.; Pogue, A.I.; Lukiw, W.J. Towards the prevention of potential aluminum toxic effects and an effective treatment for alzheimer’s disease. J. Inorg. Biochem. 2011, 105, 1505–1512. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.A.; Alvarez Mdel, L.; Pisani, G.B.; Bernal, C.A.; Roma, M.G.; Carrillo, M.C. Involvement of oxidative stress in the impairment in biliary secretory function induced by intraperitoneal administration of aluminum to rats. Biol. Trace Elem. Res. 2007, 116, 329–348. [Google Scholar] [CrossRef] [PubMed]
- Gura, K.M.; Lee, S.; Valim, C.; Zhou, J.; Kim, S.; Modi, B.P.; Arsenault, D.A.; Strijbosch, R.A.; Lopes, S.; Duggan, C.; et al. Safety and efficacy of a fish-oil-based fat emulsion in the treatment of parenteral nutrition-associated liver disease. Pediatrics 2008, 121, e678–e686. [Google Scholar] [CrossRef] [PubMed]
- Cober, M.P.; Teitelbaum, D.H. Prevention of parenteral nutrition-associated liver disease: Lipid minimization. Curr. Opin. Organ Transplant. 2010, 15, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Cowan, E.; Nandivada, P.; Puder, M. Fish oil-based lipid emulsion in the treatment of parenteral nutrition-associated liver disease. Curr. Opin. Pediatr. 2013, 25, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Nghiem-Rao, T.H.; Tunc, I.; Mavis, A.M.; Cao, Y.; Polzin, E.M.; Firary, M.F.; Wang, X.; Simpson, P.M.; Patel, S.B. Kinetics of phytosterol metabolism in neonates receiving parenteral nutrition. Pediatr. Res. 2015, 78, 181–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, A.R.; Arnold, C.J.; Miller, G.G.; Zello, G.A. Infant parenteral nutrition remains a significant source for aluminum toxicity. JPEN J. Parenter. Enter. Nutr. 2017, 41, 1228–1233. [Google Scholar] [CrossRef] [PubMed]
- Chapman, K.P.; Courtney-Martin, G.; Moore, A.M.; Ball, R.O.; Pencharz, P.B. Threonine requirement of parenterally fed postsurgical human neonates. Am. J. Clin. Nutr. 2009, 89, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Cheville, N.F.; Stasko, J. Techniques in electron microscopy of animal tissue. Vet. Pathol. 2014, 51, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. Nih image to imagej: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Adler, M.; Chung, K.W.; Schaffner, F. Pericanalicular hepatocytic and bile ductular microfilaments in cholestasis in man. Am. J. Pathol. 1980, 98, 603–616. [Google Scholar] [PubMed]
- Sergi, C.; Abdualmjid, R.; Abuetabh, Y. Canine liver transplantation model and the intermediate filaments of the cytoskeleton of the hepatocytes. J. Biomed. Biotechnol. 2012, 2012, 131324. [Google Scholar] [CrossRef] [PubMed]
- Sergi, C.; Gross, W.; Mory, M.; Schaefer, M.; Gebhard, M.M. Biliary-type cytokeratin pattern in a canine isolated perfused liver transplantation model. J. Surg. Res. 2008, 146, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Arrese, M.; Ananthanarayanan, M. The bile salt export pump: Molecular properties, function and regulation. Pflug. Arch. 2004, 449, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Alemmari, A.; Miller, G.G.; Bertolo, R.F.; Dinesh, C.; Brunton, J.A.; Arnold, C.J.; Zello, G.A. Reduced aluminum contamination decreases parenteral nutrition associated liver injury. J. Pediatr. Surg. 2012, 47, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Alexandrov, P.N.; Zhao, Y.; Pogue, A.I.; Tarr, M.A.; Kruck, T.P.; Percy, M.E.; Cui, J.G.; Lukiw, W.J. Synergistic effects of iron and aluminum on stress-related gene expression in primary human neural cells. J. Alzheimers Dis. 2005, 8, 117–127, discussion 209–115. [Google Scholar] [CrossRef] [PubMed]
- Lemire, J.; Appanna, V.D. Aluminum toxicity and astrocyte dysfunction: A metabolic link to neurological disorders. J. Inorg. Biochem. 2011, 105, 1513–1517. [Google Scholar] [CrossRef] [PubMed]
- Mailloux, R.J.; Lemire, J.; Appanna, V.D. Hepatic response to aluminum toxicity: Dyslipidemia and liver diseases. Exp. Cell Res. 2011, 317, 2231–2238. [Google Scholar] [CrossRef] [PubMed]
- Solenski, N.J.; diPierro, C.G.; Trimmer, P.A.; Kwan, A.L.; Helm, G.A. Ultrastructural changes of neuronal mitochondria after transient and permanent cerebral ischemia. Stroke 2002, 33, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; White, F.C.; Bloor, C.M. Effect of acute exercise stress in cardiac hypertrophy: I. Correlation of regional blood flow and qualitative ultrastructural changes. Yale J. Biol. Med. 1980, 53, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, D.; Sakumi, K.; Ohno, M.; Sakai, Y.; Furuichi, M.; Iwai, S.; Nakabeppu, Y. An oxidized purine nucleoside triphosphatase, mth1, suppresses cell death caused by oxidative stress. J. Biol. Chem. 2003, 278, 37965–37973. [Google Scholar] [CrossRef] [PubMed]
- Zischka, H.; Lichtmannegger, J.; Schmitt, S.; Jagemann, N.; Schulz, S.; Wartini, D.; Jennen, L.; Rust, C.; Larochette, N.; Galluzzi, L.; et al. Liver mitochondrial membrane crosslinking and destruction in a rat model of wilson disease. J. Clin. Investig. 2011, 121, 1508–1518. [Google Scholar] [CrossRef] [PubMed]
- Lough, J.; Wiglesworth, F.W. Wilson disease. Comparative ultrastructure in a sibship of nine. Arch. Pathol. Lab. Med. 1976, 100, 653–659. [Google Scholar] [PubMed]
- Cai, W.; Wu, J.; Hong, L.; Xu, Y.; Tang, Q.; Shi, C. Oxidative injury and hepatocyte apoptosis in total parenteral nutrition-associated liver dysfunction. J. Pediatr. Surg. 2006, 41, 1663–1668. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Wang, X.; Wu, J.; Cai, W. Mitochondria-initiated apoptosis triggered by oxidative injury play a role in total parenteral nutrition-associated liver dysfunction in infant rabbit model. J. Pediatr. Surg. 2009, 44, 1712–1718. [Google Scholar] [CrossRef] [PubMed]
- Tannuri, U. Oxidative injury and hepatocyte apoptosis in total parenteral nutrition-associated liver dysfunction. J. Pediatr. Surg. 2007, 42, 596, author reply 596–597. [Google Scholar] [CrossRef] [PubMed]
- Bahitham, W.; Mohammed, S.; Chan, A.; Bamforth, F.; Chiu, B.; Sergi, C. Mitochondrial DNA related cardiomyopathies. J. Pathol. 2012, 226, S10. [Google Scholar]
- Hsu, Y.H.; Yogasundaram, H.; Parajuli, N.; Valtuille, L.; Sergi, C.; Oudit, G.Y. MELAS syndrome and cardiomyopathy: Linking mitochondrial function to heart failure pathogenesis. Heart Fail. Rev. 2016, 21, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Ariza, J.; Gonzalez-Reyes, J.A.; Jodar, L.; Diaz-Ruiz, A.; de Cabo, R.; Villalba, J.M. Mitochondrial permeabilization without caspase activation mediates the increase of basal apoptosis in cells lacking nrf2. Free Radic. Biol. Med. 2016, 95, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS sources in physiological and pathological conditions. Oxid. Med. Cell. Longev. 2016, 2016, 1245049. [Google Scholar] [CrossRef] [PubMed]
- Penna, C.; Perrelli, M.G.; Pagliaro, P. Mitochondrial pathways, permeability transition pore, and redox signaling in cardioprotection: Therapeutic implications. Antioxid. Redox Signal. 2013, 18, 556–599. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Prather, E.R.; Garrison, D.E.; Zuo, L. Interplay between ROS and antioxidants during ischemia-reperfusion injuries in cardiac and skeletal muscle. Int. J. Mol. Sci. 2018, 19, 417. [Google Scholar] [CrossRef] [PubMed]
- Verri, M.; Pastoris, O.; Dossena, M.; Aquilani, R.; Guerriero, F.; Cuzzoni, G.; Venturini, L.; Ricevuti, G.; Bongiorno, A.I. Mitochondrial alterations, oxidative stress and neuroinflammation in alzheimer’s disease. Int. J. Immunopathol. Pharmacol. 2012, 25, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Shu, Z.J.; Li, J.S.; Zhou, Z.S.; Shi, Q.L.; Zhang, T.H. Histopathologic study of cholestasis induced by total parenteral nutrition or intraperitoneal sepsis in rats. JPEN J. Parenter. Enter. Nutr. 1991, 15, 630–636. [Google Scholar]
- Hua, Z.; Sergi, C.; Nation, P.N.; Wizzard, P.R.; Ball, R.O.; Pencharz, P.B.; Turner, J.M.; Wales, P.W. Hepatic ultrastructure in a neonatal piglet model of intestinal failure-associated liver disease (ifald). J. Electron Micros. 2012, 61, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Vlaardingerbroek, H.; Ng, K.; Stoll, B.; Benight, N.; Chacko, S.; Kluijtmans, L.A.; Kulik, W.; Squires, E.J.; Olutoye, O.; Schady, D.; et al. New generation lipid emulsions prevent pnald in chronic parenterally fed preterm pigs. J. Lipid Res. 2014, 55, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Burrin, D.G.; Ng, K.; Stoll, B.; Saenz De Pipaon, M. Impact of new-generation lipid emulsions on cellular mechanisms of parenteral nutrition-associated liver disease. Adv. Nutr. 2014, 5, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Premkumar, M.H.; Carter, B.A.; Hawthorne, K.M.; King, K.; Abrams, S.A. High rates of resolution of cholestasis in parenteral nutrition-associated liver disease with fish oil-based lipid emulsion monotherapy. J. Pediatr. 2013, 162, 793–798.e1. [Google Scholar] [CrossRef] [PubMed]
- Sergi, C.; Gross, W.; Mory, M.; Schaefer, M.; Gebhard, M. Biliary indocyanine green excretion in an isolated perfused liver transplantation model after ischemia-reperfusion. In Journal of Pathology; Wiley-Blackwell: Hoboken, NJ, USA, 2005; p. 30. [Google Scholar]
- Baracos, V.E. Animal models of amino acid metabolism: A focus on the intestine. J. Nutr. 2004, 134, 1656S–1659S, discussion 1664S–1666S, 1667S–1672S. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.M.; Wales, P.W.; Nation, P.N.; Wizzard, P.; Pendlebury, C.; Sergi, C.; Ball, R.O.; Pencharz, P.B. Novel neonatal piglet models of surgical short bowel syndrome with intestinal failure. J. Pediatr. Gastroenterol. Nutr. 2011, 52, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.R.; Ullrey, D.E. The pig as a model for human nutrition. Annu. Rev. Nutr. 1987, 7, 361–382. [Google Scholar] [CrossRef] [PubMed]
- Bohrer, D.; do Nascimento, P.C.; Binotto, R.; Becker, E. Influence of the glass packing on the contamination of pharmaceutical products by aluminium. Part iii: Interaction container-chemicals during the heating for sterilisation. J. Trace Elem. Med. Biol. 2003, 17, 107–115. [Google Scholar] [CrossRef]
- Bohrer, D.; do Nascimento, P.C.; Binotto, R.; Pomblum, S.C. Influence of the glass packing on the contamination of pharmaceutical products by aluminium. Part i: Salts, glucose, heparin and albumin. J. Trace Elem. Med. Biol. 2001, 15, 95–101. [Google Scholar] [CrossRef]
- Bohrer, D.; do Nascimento, P.C.; Binotto, R.; Carlesso, R. Influence of the glass packing on the contamination of pharmaceutical products by aluminium. Part ii: Amino acids for parenteral nutrition. J. Trace Elem. Med. Biol. 2001, 15, 103–108. [Google Scholar] [CrossRef]
- Canada, T.W. Aluminum exposure through parenteral nutrition formulations: Mathematical versus clinical relevance. Am. J. Health Syst. Pharm. 2005, 62, 315–318. [Google Scholar] [PubMed]
- Bishop, N.J.; Morley, R.; Day, J.P.; Lucas, A. Aluminum neurotoxicity in preterm infants receiving intravenous-feeding solutions. N. Engl. J. Med. 1997, 336, 1557–1561. [Google Scholar] [CrossRef] [PubMed]
- MHRA. Calcium gluconate injection in small-volume glass containers: New contraindications due to aluminum exposure risk. In MHRA Drug Safety Update (UK); Medicines and Healthcare Products Regulatory Agency, GOV.UK: London, UK, 2010; Volume 4, pp. 1–13. [Google Scholar]

| High Al (N = 8) | Standard Al (N = 7) | Reference (N = 4) | Difference (p Value) | |
|---|---|---|---|---|
| Mean starting age (days + SD) | 8.38 (±2.92) | 7.71 (±2.43) | 10.25 (±1.50) | p = 0.20 |
| Mean starting weight (kg+ SD) | 1.58 (±0.14) | 1.57 (±0.15) | 1.32 (±0.24) | p = 0.06 |
| Females: Males | 5:3 | 3:4 | 3:1 | p = 0.60 |
| High Al | Standard Al | Reference | Difference (p Value) | |
|---|---|---|---|---|
| Mean number of high density lesions in mitochondria per hepatocyte (±SD) | 1.58 (±0.88) | 1.43 (±0.68) | 0.42 (±0.52) | p < 0.001 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hall, A.R.; Le, H.; Arnold, C.; Brunton, J.; Bertolo, R.; Miller, G.G.; Zello, G.A.; Sergi, C. Aluminum Exposure from Parenteral Nutrition: Early Bile Canaliculus Changes of the Hepatocyte. Nutrients 2018, 10, 723. https://doi.org/10.3390/nu10060723
Hall AR, Le H, Arnold C, Brunton J, Bertolo R, Miller GG, Zello GA, Sergi C. Aluminum Exposure from Parenteral Nutrition: Early Bile Canaliculus Changes of the Hepatocyte. Nutrients. 2018; 10(6):723. https://doi.org/10.3390/nu10060723
Chicago/Turabian StyleHall, Amanda R., Ha Le, Chris Arnold, Janet Brunton, Robert Bertolo, Grant G. Miller, Gordon A. Zello, and Consolato Sergi. 2018. "Aluminum Exposure from Parenteral Nutrition: Early Bile Canaliculus Changes of the Hepatocyte" Nutrients 10, no. 6: 723. https://doi.org/10.3390/nu10060723