Associations of Dairy Intake with Arterial Stiffness in Brazilian Adults: The Brazilian Longitudinal Study of Adult Health (ELSA-Brasil)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Dietary Assessment and Dairy Measurement
2.2. Pulse Wave Velocity and Blood Pressure
2.3. Covariates
2.4. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pan American Health Organization (PAHO/WHO). Deaths Due to Noncommunicable Diseases in Countries of the Americas. Available online: http://www.paho.org/hq/index.php?option=com_content&view=article&id=10169&Itemid=41167&lang=en (accessed on 9 August 2017).
- GBD 2016 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1345–1422. [Google Scholar] [CrossRef]
- Alexander, D.D.; Bylsma, L.C.; Vargas, A.J.; Cohen, S.S.; Doucette, A.; Mohamed, M.; Irvin, S.R.; Miller, P.E.; Watson, H.; Fryzek, J.P. Dairy consumption and CVD: A systematic review and meta-analysis. Br. J. Nutr. 2016, 115, 737–750. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.-Q.; Xu, J.-Y.; Han, S.-F.; Zhang, Z.-L.; Zhao, Y.-Y.; Szeto, I.M. Dairy consumption and risk of cardiovascular disease: An updated meta-analysis of prospective cohort studies. Asia Pac. J. Clin. Nutr. 2015, 24, 90–100. [Google Scholar] [PubMed]
- Wang, H.; Fox, C.S.; Troy, L.M.; Mckeown, N.M.; Jacques, P.F. Longitudinal association of dairy consumption with the changes in blood pressure and the risk of incident hypertension: The Framingham Heart Study. Br. J. Nutr. 2015, 114, 1887–1899. [Google Scholar] [CrossRef] [PubMed]
- Zong, G.; Sun, Q.; Yu, D.; Zhu, J.; Sun, L.; Ye, X.; Li, H.; Jin, Q.; Zheng, H.; Hu, F.B.; et al. Dairy consumption, type 2 diabetes, and changes in cardiometabolic traits: A prospective cohort study of middle-aged and older Chinese in Beijing and Shanghai. Diabetes Care 2014, 37, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Azadbakht, L.; Mirmiran, P.; Esmaillzadeh, A.; Azizi, T.; Azizi, F. Beneficial effects of a Dietary Approaches to Stop Hypertension eating plan on features of the metabolic syndrome. Diabetes Care 2005, 28, 2823–2831. [Google Scholar] [CrossRef] [PubMed]
- Saneei, P.; Salehi-Abargouei, A.; Esmaillzadeh, A.; Azadbakht, L. Influence of Dietary Approaches to Stop Hypertension (DASH) diet on blood pressure: A systematic review and meta-analysis on randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. NMCD 2014, 24, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Crichton, G.E.; Elias, M.F.; Dore, G.A.; Abhayaratna, W.P.; Robbins, M.A. Relations between dairy food intake and arterial stiffness: Pulse wave velocity and pulse pressure. Hypertension 2012, 59, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, K.M.; Lovegrove, J.A.; Cockcroft, J.R.; Elwood, P.C.; Pickering, J.E.; Givens, D.I. Does dairy food intake predict arterial stiffness and blood pressure in men?: Evidence from the Caerphilly Prospective Study. Hypertension 2013, 61, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-F. Therapeutic modification of arterial stiffness: An update and comprehensive review. World J. Cardiol. 2015, 7, 742. [Google Scholar] [CrossRef] [PubMed]
- Mikael, L.R.; de Paiva, A.M.G.; Gomes, M.M.; Sousa, A.L.L.; Jardim, P.C.B.V.; de Vitorino, P.V.O.; Euzébio, M.B.; de Sousa, W.M.; Barroso, W.K.S. Vascular Aging and Arterial Stiffness. Arq. Bras. Cardiol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Recio-Rodriguez, J.I.; Gomez-Marcos, M.A.; Patino-Alonso, M.-C.; Sanchez, A.; Agudo-Conde, C.; Maderuelo-Fernandez, J.A.; Garcia-Ortiz, L. Association between fat amount of dairy products with pulse wave velocity and carotid intima-media thickness in adults. Nutr. J. 2014, 13. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.S.; Keogh, J.B.; Meikle, P.J.; Garg, M.L.; Clifton, P.M. Dietary predictors of arterial stiffness in a cohort with type 1 and type 2 diabetes. Atherosclerosis 2015, 238, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Pase, M.P.; Grima, N.A.; Sarris, J. The effects of dietary and nutrient interventions on arterial stiffness: A systematic review. Am. J. Clin. Nutr. 2011, 93, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Machin, D.R.; Park, W.; Alkatan, M.; Mouton, M.; Tanaka, H. Effects of non-fat dairy products added to the routine diet on vascular function: A randomized controlled crossover trial. Nutr. Metab. Cardiovasc. Dis. NMCD 2015, 25, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.; Clifton, P.; Lister, N.; Keogh, J. Effect of Improving Dietary Quality on Arterial Stiffness in Subjects with Type 1 and Type 2 Diabetes: A 12 Months Randomised Controlled Trial. Nutrients 2016, 8, 382. [Google Scholar] [CrossRef] [PubMed]
- Molina, M.C.B.; Benseñor, I.M.; de Cardoso, L.O.; Velasquez-Melendez, G.; Drehmer, M.; Pereira, T.S.S.; de Faria, C.P.; Melere, C.; Manato, L.; Gomes, A.L.C.; et al. Reprodutibilidade e validade relativa do Questionário de Frequência Alimentar do ELSA-Brasil. Cadernos de Saúde Pública 2013, 29, 379–389. [Google Scholar] [CrossRef]
- Aquino, E.M.; Araujo, M.J.; Almeida, M.D.C.C.; Conceicao, P.; Andrade, C.R.D.; Cade, N.V.; Carvalho, M.S.; Figueiredo, R.C.D.; da Fonseca, M.D.J.M.; Giatti, L.; et al. Revista de Saúde Pública, 2013; 47, 10–18. [CrossRef]
- Sichieri, R.; Everhart, J.E. Validity of a Brazilian food frequency questionnaire against dietary recalls and estimated energy intake. Nutr. Res. 1998, 18, 1649–1659. [Google Scholar] [CrossRef]
- Ministério da Saúde. Guia Alimentar Para a População Brasileira, 2nd ed.; Ministério da Saúde: Brasília, Brazil, 2014; ISBN 978-85-334-2176-9.
- Mill, J.G.; Pinto, K.; Griep, R.H.; Goulart, A.; Foppa, M.; Lotufo, P.A.; Maestri, M.K.; Ribeiro, A.L.; Andreao, R.V.; Dantas, E.M.; et al. Afericoes e exames clinicos realizados nos participantes do ELSA-Brasil. Revista de Saúde Pública 2013, 47, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Smulyan, H.; Marchais, S.J.; Pannier, B.; Guerin, A.P.; Safar, M.E.; London, G.M. Influence of body height on pulsatile arterial hemodynamic data. J. Am. Coll. Cardiol. 1998, 31, 1103–1109. [Google Scholar] [CrossRef]
- Soedamah-Muthu, S.S.; Verberne, L.D.M.; Ding, E.L.; Engberink, M.F.; Geleijnse, J.M. Dairy consumption and incidence of hypertension: A dose-response meta-analysis of prospective cohort studies. Hypertension 2012, 60, 1131–1137. [Google Scholar] [CrossRef] [PubMed]
- Benatar, J.R.; Sidhu, K.; Stewart, R.A.H. Effects of high and low fat dairy food on cardio-metabolic risk factors: A meta-analysis of randomized studies. PLoS ONE 2013, 8, e76480. [Google Scholar] [CrossRef] [PubMed]
- Protogerou, A.; Safar, M. Dissociation between Central Augmentation Index and Carotid–Femoral Pulse-Wave Velocity: When and Why? Am. J. Hypertens. 2007, 20, 648–649. [Google Scholar] [CrossRef] [PubMed]
- Fekete, Á.A.; Givens, D.I.; Lovegrove, J.A. The impact of milk proteins and peptides on blood pressure and vascular function: A review of evidence from human intervention studies. Nutr. Res. Rev. 2013, 26, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Lovegrove, J.A.; Hobbs, D.A. New perspectives on dairy and cardiovascular health. Proc. Nutr. Soc. 2016, 75, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Maes, W.; Van Camp, J.; Vermeirssen, V.; Hemeryck, M.; Ketelslegers, J.M.; Schrezenmeir, J.; Van Oostveldt, P.; Huyghebaert, A. Influence of the lactokinin Ala-Leu-Pro-Met-His-Ile-Arg (ALPMHIR) on the release of endothelin-1 by endothelial cells. Regul. Pept. 2004, 118, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Jauhiainen, T.; Rönnback, M.; Vapaatalo, H.; Wuolle, K.; Kautiainen, H.; Groop, P.; Korpela, R. Long-term intervention with Lactobacillus helveticus fermented milk reduces augmentation index in hypertensive subjects. Eur. J. Clin. Nutr. 2010, 64, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Ellis, V. The chronic effects of whey proteins on blood pressure, vascular function, and inflammatory markers in overweight individuals. Obes. Silver Spring 2010, 18, 1354–1359. [Google Scholar] [CrossRef] [PubMed]
- Rautiainen, S.; Wang, L.; Manson, J.E.; Sesso, H.D. The Role of Calcium in the Prevention of Cardiovascular Disease—A Review of Observational Studies and Randomized Clinical Trials. Curr. Atheroscler. Rep. 2013, 15. [Google Scholar] [CrossRef] [PubMed]
- Houston, M.C. The Importance of Potassium in Managing Hypertension. Curr. Hypertens. Rep. 2011, 13, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Houston, M. The Role of Magnesium in Hypertension and Cardiovascular Disease: Magnesium, Hypertension, and Cardiovascular Disease. J. Clin. Hypertens. 2011, 13, 843–847. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Astrup, A.; Lovegrove, J.A.; Gijsbers, L.; Givens, D.I.; Soedamah-Muthu, S.S. Milk and dairy consumption and risk of cardiovascular diseases and all-cause mortality: Dose–response meta-analysis of prospective cohort studies. Eur. J. Epidemiol. 2017, 32, 269–287. [Google Scholar] [CrossRef] [PubMed]
- Corte-Real, J.; Bohn, T. Interaction of divalent minerals with liposoluble nutrients and phytochemicals during digestion and influences on their bioavailability—A review. Food Chem. 2018, 252, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Drehmer, M.; Pereira, M.A.; Schmidt, M.I.; Alvim, S.; Lotufo, P.A.; Luft, V.C.; Duncan, B.B. Total and Full-Fat, but Not Low-Fat, Dairy Product Intakes are Inversely Associated with Metabolic Syndrome in Adults. J. Nutr. 2016, 146, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Elwood, P.C.; Pickering, J.E.; Givens, D.I.; Gallacher, J.E. The consumption of milk and dairy foods and the incidence of vascular disease and diabetes: An overview of the evidence. Lipids 2010, 45, 925–939. [Google Scholar] [CrossRef] [PubMed]
- German, J.B.; Gibson, R.A.; Krauss, R.M.; Nestel, P.; Lamarche, B.; van Staveren, W.A.; Steijns, J.M.; de Groot, L.C.P.G.M.; Lock, A.L.; Destaillats, F. A reappraisal of the impact of dairy foods and milk fat on cardiovascular disease risk. Eur. J. Nutr. 2009, 48, 191–203. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Otto, M.C.; Mozaffarian, D.; Kromhout, D.; Bertoni, A.G.; Sibley, C.T.; Jacobs, D.R.; Nettleton, J.A. Dietary intake of saturated fat by food source and incident cardiovascular disease: The Multi-Ethnic Study of Atherosclerosis. Am. J. Clin. Nutr. 2012, 96, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Yakoob, M.Y.; Shi, P.; Willett, W.C.; Rexrode, K.M.; Campos, H.; Orav, E.J.; Hu, F.B.; Mozaffarian, D. Circulating Biomarkers of Dairy Fat and Risk of Incident Diabetes Mellitus Among Men and Women in the United States in Two Large Prospective Cohorts. Circulation 2016, 133, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- Vlachopoulos, C.; Aznaouridis, K.; Stefanadis, C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2010, 55, 1318–1327. [Google Scholar] [CrossRef] [PubMed]
- Selmer, R.; Kristiansen, I.; Haglerod, A.; Graff-Iversen, S.; Larsen, H.; Meyer, H.; Bonaa, K.; Thelle, D. Cost and health consequences of reducing the population intake of salt. J. Epidemiol. Community Health 2000, 54, 697–702. [Google Scholar] [CrossRef] [PubMed]
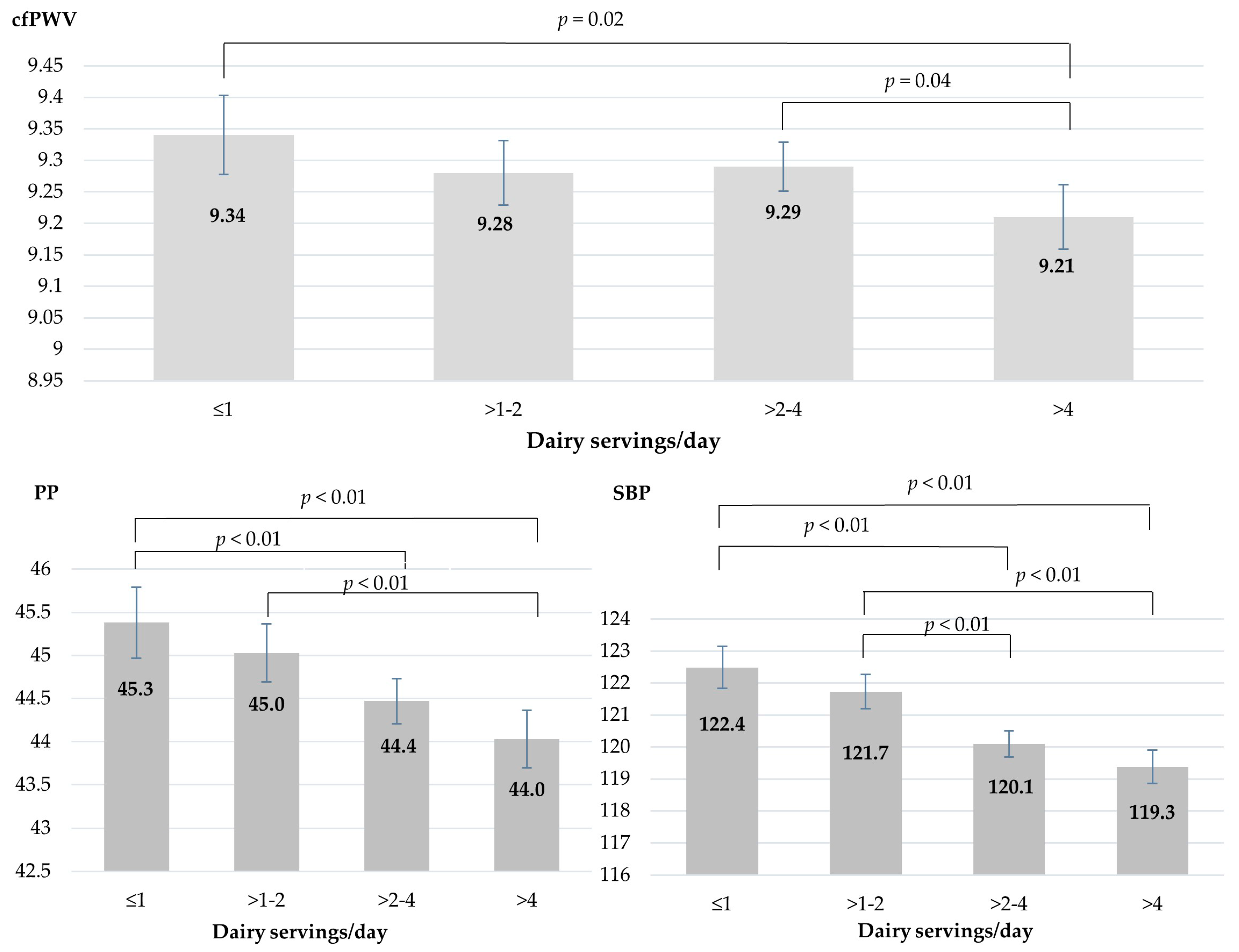
| Dairy Products, Servings/Day | Categories of Dairy Consumption (Servings/Day) | ||||
|---|---|---|---|---|---|
| ≤1 (n = 2036) | >1–2 (n = 2862) | >2–4 (n = 4700) | >4 (n = 3294) | p | |
| Dairy 2 | 0.52 | 1.53 | 2.90 | 5.49 | |
| Low-fat dairy 2 | 0.10 | 0.60 | 1.27 | 2.80 | |
| Skimmed milk, low-fat milk | 0.06 ± 0.18 | 0.24 ± 0.40 | 0.52 ± 0.79 | 1.00 ± 1.34 | <0.001 |
| Low-fat yogurt | 0.02 ± 0.09 | 0.08 ± 0.23 | 0.15 ± 0.35 | 0.21 ± 0.45 | <0.001 |
| Low-fat cheese | 0.13 ± 0.19 | 0.36 ± 0.39 | 0.77 ± 0.75 | 1.79 ± 1.77 | <0.001 |
| Full-fat dairy 2 | 0.23 | 0.93 | 1.40 | 2.80 | |
| Whole milk | 0.12 ± 0.23 | 0.30 ± 0.43 | 0.49 ± 0.79 | 0.82 ± 1.37 | <0.001 |
| Regular yogurt | 0.04 ± 0.11 | 0.11 ± 0.23 | 0.20 ± 0.38 | 0.30 ± 0.54 | <0.001 |
| Regular cheese | 0.14 ± 0.19 | 0.32 ± 0.37 | 0.56 ± 0.64 | 1.22 ± 1.42 | <0.001 |
| Fermented dairy 2 | 0.23 | 0.90 | 1.67 | 3.20 | |
| Yogurt (regular, low-fat) | 0.06 ± 0.13 | 0.19 ± 0.29 | 0.35 ± 0.45 | 0.52 ± 0.61 | <0.001 |
| Cheese (regular, low-fat) | 0.26 ± 0.26 | 0.68 ± 0.47 | 1.32 ± 0.84 | 3.00 ± 1.99 | <0.001 |
| Butter | 0.04 ± 0.13 | 0.12 ± 0.28 | 0.26 ± 0.52 | 0.67 ± 1.08 | <0.001 |
| Characteristics of Participants | Categories of Dairy Consumption (Servings/Day) | p | |||
|---|---|---|---|---|---|
| ≤1 (n = 2036) | >1–2 (n = 2862) | >2–4 (n = 4700) | >4 (n = 3294) | ||
| Age, years | 51.2 ± 8.3 | 51.3 ± 8.9 | 51.7 ± 9.0 | 52.3 ± 9.2 | <0.001 |
| Sex, n (%) | |||||
| Men | 1111 (54.6) | 1356 (47.4) | 1924 (40.9) | 1339 (40.9) | |
| Women | 925 (45.4) | 1506 (52.6) | 2776 (59.1) | 2001 (59.1) | <0.001 |
| Race, n (%) | |||||
| White | 845 (41.5) | 1368 (47.8) | 2646 (56.3) | 1963 (59.6) | |
| Other | 1191 (58.5) | 1494 (52.2) | 2054 (43.7) | 1331 (40.4) | <0.001 |
| Educational level, n (%) | |||||
| Completed secondary school | 872 (42.8) | 1081 (37.8) | 1516 (32.3) | 923 (28.0) | |
| University degree | 728 (35.8) | 1399 (48.9) | 2743 (58.4) | 2133 (64.8) | <0.001 |
| Weight, kg | 73.1 ± 14.2 | 73.7 ± 14.6 | 72.6 ± 14.7 | 73.2 ± 15.1 | 0.019 |
| BMI, kg/m2 | 26.8 ± 4.5 | 27.0 ± 4.6 | 26.7 ± 4.6 | 26.8 ± 4.6 | 0.111 |
| Waist circumference, cm | 91.1 ± 12.2 | 91.1 ± 12.3 | 90.1 ± 12.5 | 90.4 ± 12.7 | 0.001 |
| Smoking status, n (%) | |||||
| Never smoker | 1033 (50.7) | 1642 (57.4) | 2829 (60.2) | 1980 (60.1) | |
| Ex-smoker | 631 (31.0) | 830 (29.0) | 1361 (29.0) | 943 (28.6) | |
| Current smoker | 372 (18.3) | 390 (13.6) | 510 (10.8) | 371 (11.3) | <0.001 |
| Alcohol intake, g ethanol/day | 71.8 ± 142.7 | 55.7 ± 118.3 | 47.0 ± 91.2 | 47.2 ± 90.0 | <0.001 |
| Physical activity, min/week | 467.1 ± 884.2 | 557.5 ± 954.0 | 623.8 ± 1051.8 | 725.2 ± 1158.7 | <0.001 |
| cfPWV, m/s | 9.51 ± 1.90 | 9.33 ± 1.81 | 9.22 ± 1.79 | 9.17 ± 1.74 | <0.001 |
| Systolic blood pressure, mm Hg | 123.7 ± 18.7 | 122.0 ± 17.5 | 119.4 ± 16.5 | 119.2 ± 15.8 | <0.001 |
| Diastolic blood pressure, mm Hg | 78.0 ± 11.3 | 77.0 ± 10.9 | 75.2 ± 10.3 | 75.1 ± 10.2 | <0.001 |
| Mean blood pressure, mm Hg | 95.4 ± 12.9 | 94.0 ± 12.0 | 91.9 ± 11.4 | 91.8 ± 11.3 | <0.001 |
| Pulse pressure, mm Hg | 45.8 ± 11.5 | 45.0 ± 10.7 | 44.2 ± 10.3 | 44.1 ± 9.9 | <0.001 |
| Fasting glucose, mg/dL | 114.5 ± 35.6 | 111.3 ± 29.1 | 110.0 ± 27.8 | 109.6 ± 26.8 | <0.001 |
| Total cholesterol, mg/dL | 218.0 ± 43.4 | 215.9 ± 41.7 | 214.8 ± 41.3 | 214.7 ± 41.0 | 0.019 |
| Drugs, n (%) | |||||
| Antidiabetic drugs | 170 (8.3) | 217 (7.6) | 334 (7.1) | 231 (7.0) | 0.247 |
| Lipid-lowering drugs | 208 (10.2) | 318 (11.1) | 572 (12.2) | 403 (12.2) | 0.066 |
| Antihypertensive drugs | 561 (27.6) | 813 (28.4) | 1235 (26.3) | 842 (25.6) | 0.055 |
| Food groups, g/day | |||||
| Fruit | 460.7 ± 405.3 | 504.3 ± 407.9 | 548.6 ± 401.8 | 616.9 ± 452.2 | <0.001 |
| Vegetables | 191.1 ± 148.8 | 198.5 ± 136.7 | 217.5 ± 142.1 | 240.9 ± 159.4 | <0.001 |
| Unprocessed meat | 153.7 ± 126.7 | 161.0 ± 112.4 | 165.6 ± 114.3 | 179.3 ± 124.5 | <0.001 |
| Processed meat | 18.7 ± 23.2 | 20.1 ± 22.6 | 21.3 ± 23.0 | 27.1 ± 28.6 | <0.001 |
| Fish | 46.8 ± 61.2 | 50.5 ± 61.3 | 50.2 ± 58.5 | 52.2 ± 59.9 | 0.016 |
| Whole grains | 30.4 ± 68.7 | 37.3 ± 71.1 | 44.6 ± 70.3 | 52.6 ± 77.0 | <0.001 |
| Outcome | Categories of Dairy Consumption (Servings/Day) | p2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ≤1 | >1–2 | >2–4 | >4 | |||||||
| n = 2036 | n = 2862 | n = 4700 | n = 3294 | |||||||
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | |||
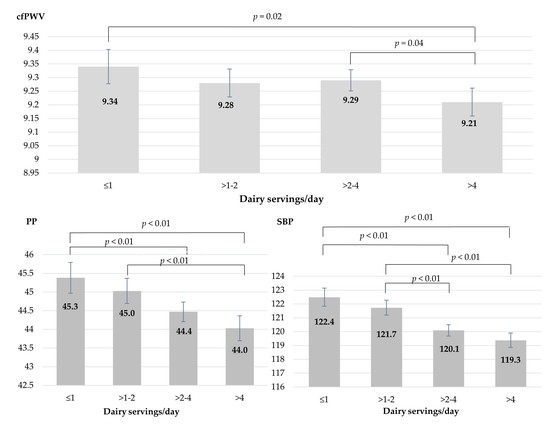
| cfPWV | Model 1 | 9.43 | 9.36–9.49 | 9.33 | 9.27–9.38 | 9.26 | 9.22–9.31 | 9.17 | 9.12–9.22 | <0.001 ‡ |
| Model 2 | 9.43 | 9.36–9.49 | 9.32 | 9.26–9.38 | 9.27 | 9.22–9.31 | 9.17 | 9.12–9.22 | <0.001 ‡ | |
| Model 3 | 9.34 | 9.28–9.40 | 9.28 | 9.23–9.33 | 9.29 | 9.25–9.34 | 9.21 | 9.16–9.26 | 0.006 ‡ | |
| Model 4 | 9.34 | 9.27–9.40 | 9.28 | 9.23–9.33 | 9.29 | 9.26–9.34 | 9.21 | 9.16–9.26 | 0.014 ‡ | |
| PP | Model 1 | 45.3 | 44.9–45.7 | 44.9 | 44.6–45.3 | 44.4 | 44.2–44.7 | 44.1 | 43.8–44.5 | <0.001 ‡ |
| Model 2 | 45.3 | 44.8–45.7 | 44.9 | 44.6–45.3 | 44.4 | 44.2–44.7 | 44.2 | 43.9–44.5 | <0.001 ‡ | |
| Model 3 | 45.1 | 44.7–45.5 | 44.9 | 44.5–45.2 | 44.4 | 44.2–44.7 | 44.3 | 44.0–44.6 | 0.003 ‡ | |
| Model 4 | 45.3 | 44.9–45.8 | 45.0 | 44.7–45.3 | 44.4 | 44.2–44.7 | 44.0 | 43.7–44.4 | <0.001 ‡ | |
| SBP | Model 1 | 122.4 | 121.7–123.0 | 121.6 | 121.1–122.2 | 119.9 | 119.5–120.4 | 119.6 | 119.1–120.1 | <0.001 ‡ |
| Model 2 | 122.2 | 121.6–122.9 | 121.5 | 121.0–122.1 | 120.0 | 119.6–120.4 | 119.7 | 119.2–120.2 | <0.001 ‡ | |
| Model 3 | 122.0 | 121.3–122.6 | 121.4 | 120.9–121.9 | 120.0 | 119.6–120.5 | 119.9 | 119.4–120.5 | <0.001 ‡ | |
| Model 4 | 122.4 | 121.8–123.1 | 121.7 | 121.2–122.2 | 120.1 | 119.7–120.5 | 119.3 | 118.8–119.9 | <0.001 ‡ | |
| Subgroups of Dairy, Servings/Day 1 | cfPWV (m/s) | PP (mmHg) | SBP (mmHg) |
|---|---|---|---|
| Low-fat dairy | −0.02 (−0.04, −0.01) | −0.3 (−0.35, −0.15) | −0.4 (−0.58, −0.26) |
| Full-fat dairy (without butter) | −0.00 (−0.02, 0.01) | −0.0 (−0.16, 0.06) | −0.2 (−0.40, −0.06) |
| Fermented dairy | −0.02 (−0.04, −0.01) | −0.3 (−0.43, −0.21) | −0.5 (−0.66, −0.33) |
| Milk | 0.01 (−0.01, 0.03) | −0.0 (−0.19, 0.12) | −0.4 (−0.61, −0.12) |
| Cheese | −0.02 (−0.04, −0.01) | −0.4 (−0.47, −0.24) | −0.5 (−0.69, −0.33) |
| Yogurt | −0.02 (−0.07, 0.03) | −0.1 (−0.47, 0.24) | −0.5 (−1.05, 0.08) |
| Butter | −0.05 (−0.09, −0.02) | 0.0 (−0.22, 0.25) | −0.1 (−0.51, 0.24) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, A.G.; Mill, J.G.; Cade, N.V.; Velasquez-Melendez, G.; Matos, S.M.A.; Molina, M.D.C.B. Associations of Dairy Intake with Arterial Stiffness in Brazilian Adults: The Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). Nutrients 2018, 10, 701. https://doi.org/10.3390/nu10060701
Ribeiro AG, Mill JG, Cade NV, Velasquez-Melendez G, Matos SMA, Molina MDCB. Associations of Dairy Intake with Arterial Stiffness in Brazilian Adults: The Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). Nutrients. 2018; 10(6):701. https://doi.org/10.3390/nu10060701
Chicago/Turabian StyleRibeiro, Amanda Gomes, José Geraldo Mill, Nágela Valadão Cade, Gustavo Velasquez-Melendez, Sheila Maria Alvim Matos, and Maria Del Carmen Bisi Molina. 2018. "Associations of Dairy Intake with Arterial Stiffness in Brazilian Adults: The Brazilian Longitudinal Study of Adult Health (ELSA-Brasil)" Nutrients 10, no. 6: 701. https://doi.org/10.3390/nu10060701