Milk Fermented by Specific Lactobacillus Strains Regulates the Serum Levels of IL-6, TNF-α and IL-10 Cytokines in a LPS-Stimulated Murine Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrates and Chemicals
2.2. Strains and Growth Conditions
2.3. Preparation of Fermented Milk
2.4. Proteolytic and Acidifying Activity
2.5. Assay in a Murine Model
2.6. Cytokine Determinations
2.7. Isolation of Peptide Fractions by Reversed-Phase HPLC
2.8. Analysis of Peptides by Tandem Mass Spectrometry
2.9. Statistical Analysis
3. Results and Discussion
3.1. Strain Selection
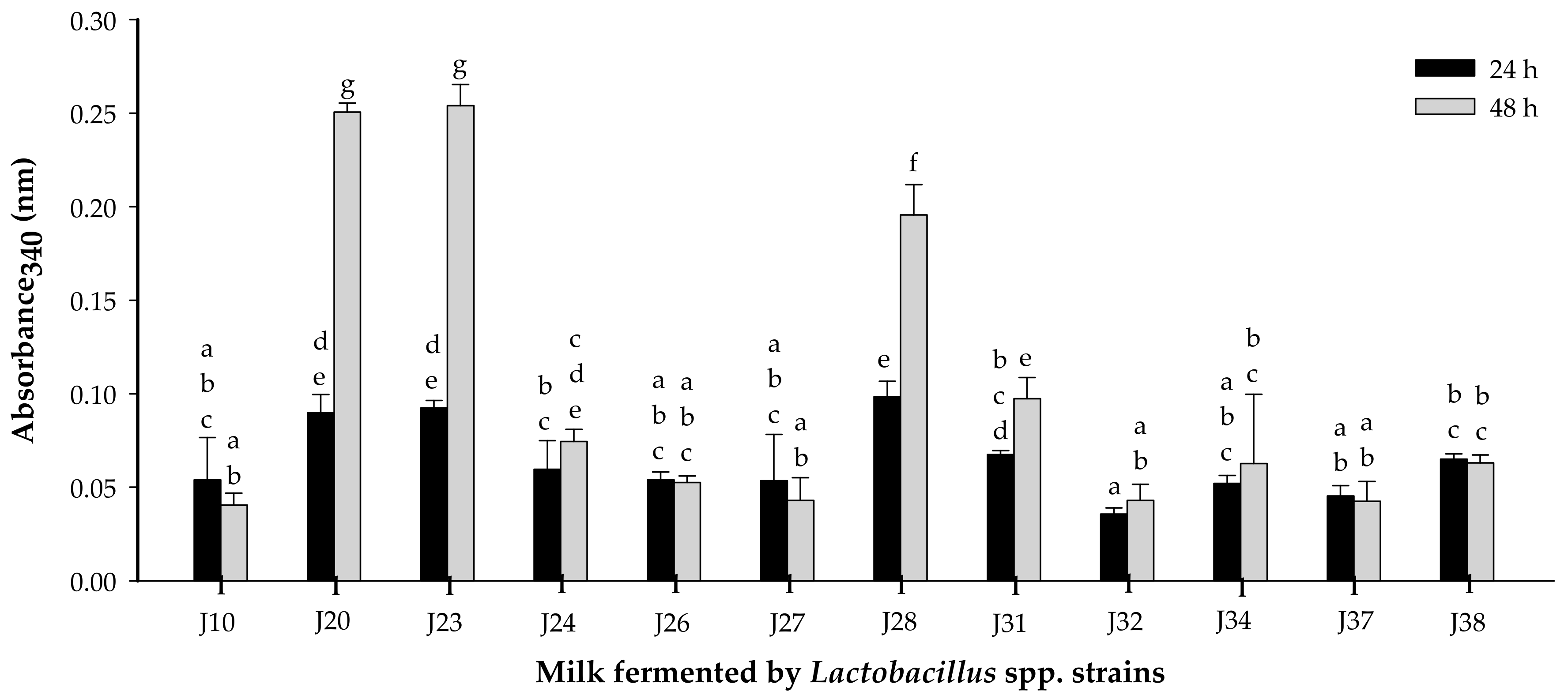
3.1.1. Proteolytic Activity
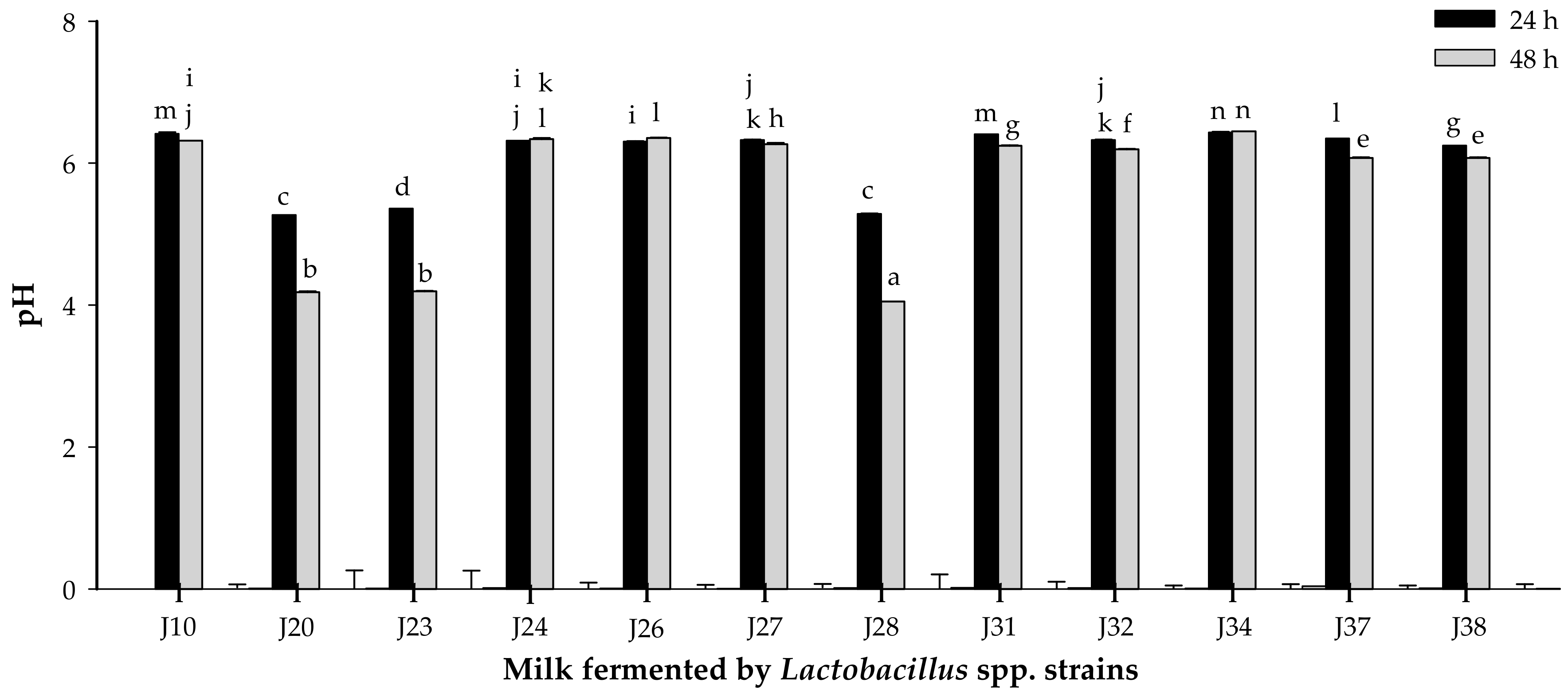
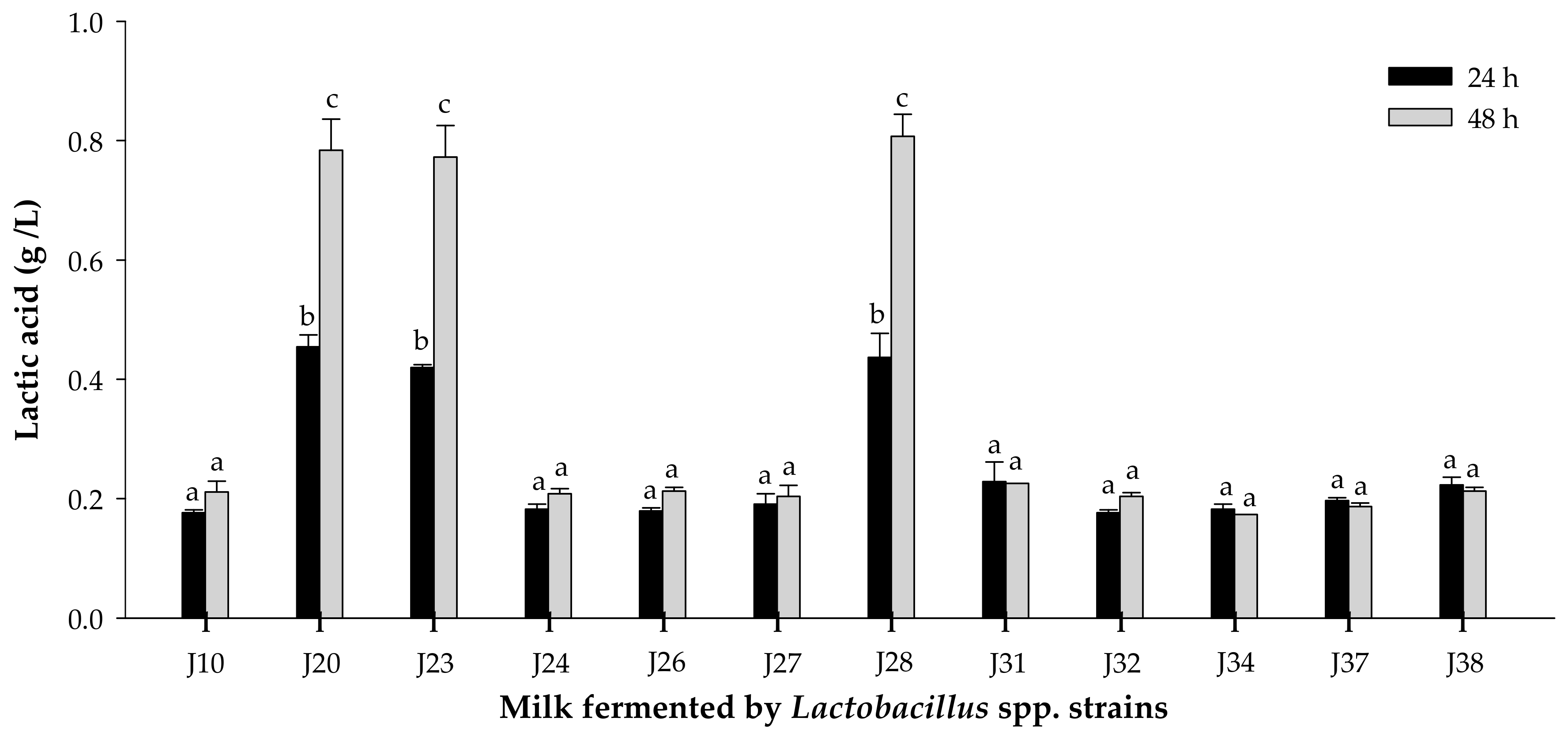
3.1.2. Acidifying Activity
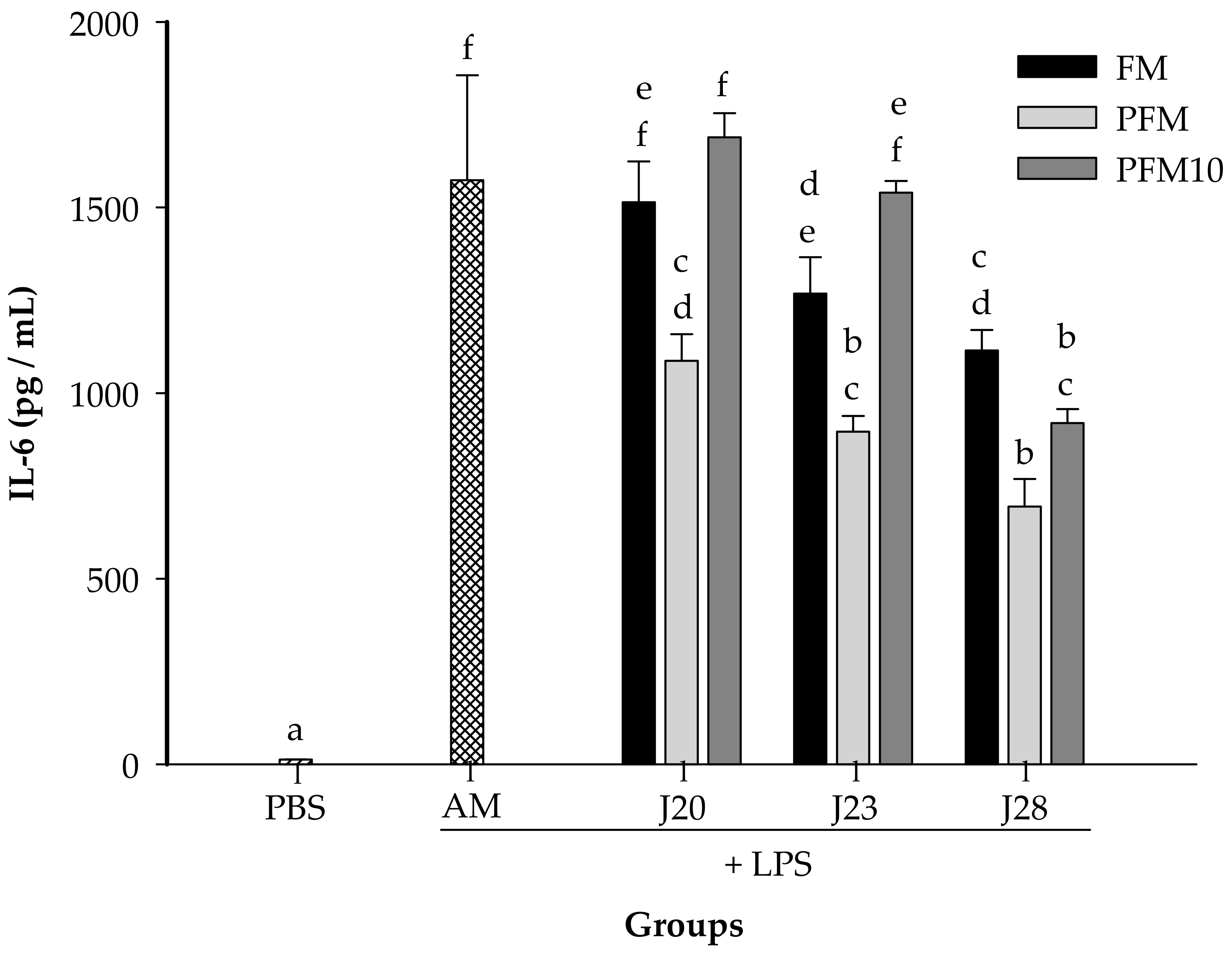
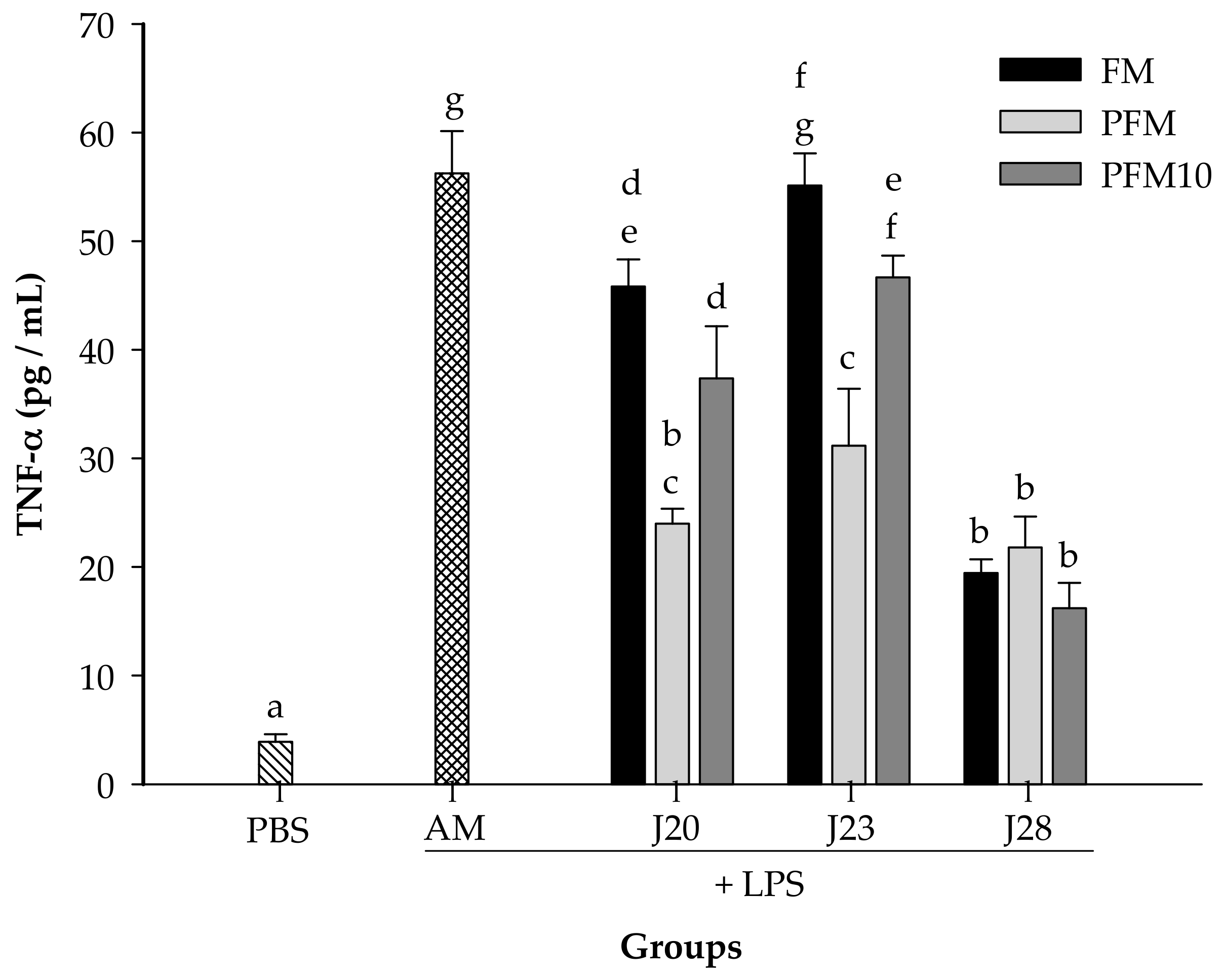
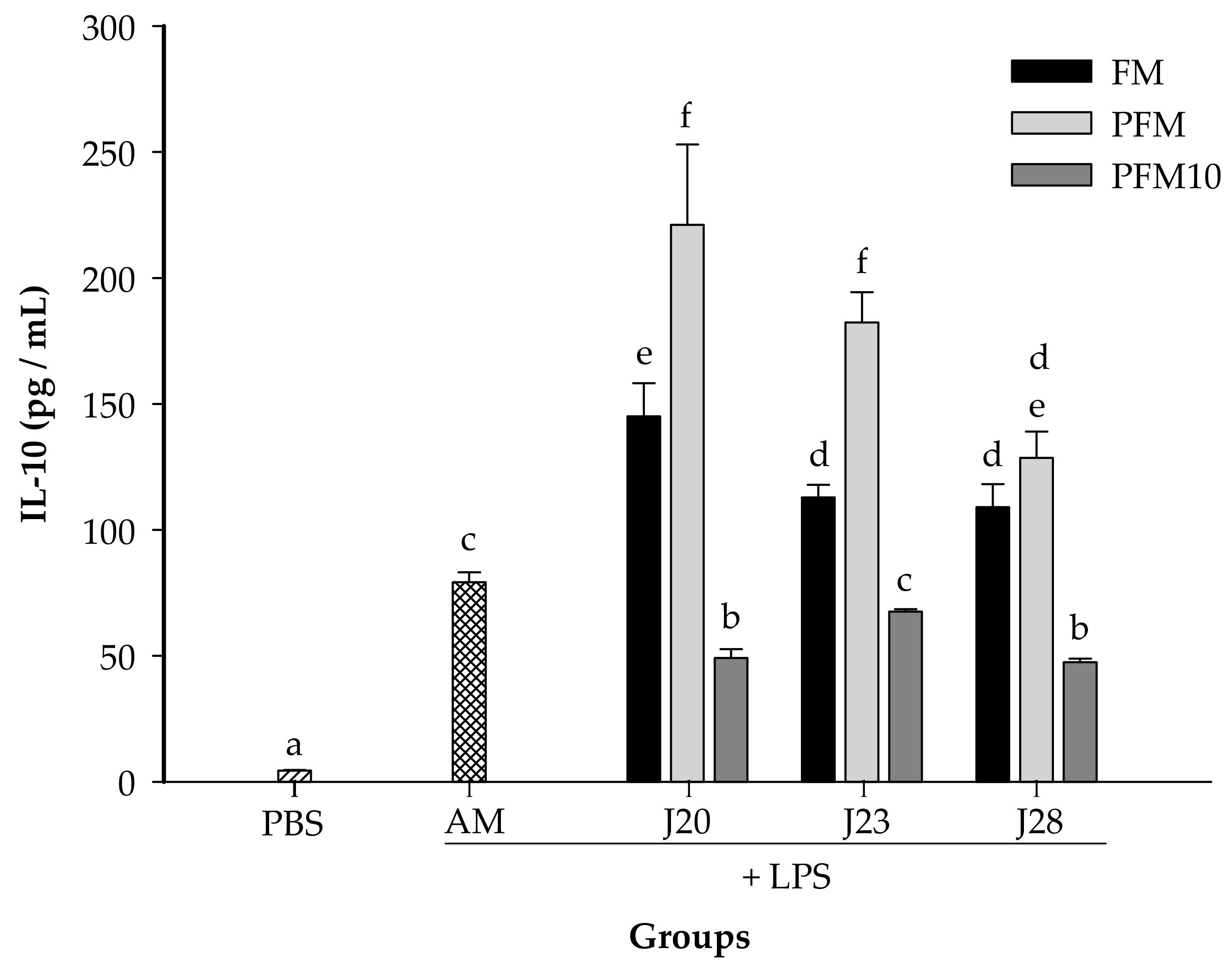
3.2. Cytokine Analysis in an LPS-Stimulated Murine Model
3.3. Identification of Peptides in Milk Fermented by Lactobacillus Fermentum J28 with Potential Regulatory Effect on Cytokine Production
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Shiby, V.K.; Mishra, H.N. Fermented Milks and Milk Products as Functional Foods—A Review. Crit. Rev. Food Sci. Nutr. 2013, 53, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.H.; Lin, S.L.; Ou, C.C.; Lu, Y.C.; Huang, H.Y.; Lin, M.Y. Antiinflammatory effect of lactobacilli bacteria on HepG2 cells is through cross-regulation of TLR4 and NOD2 signalling. J. Funct. Foods 2013, 5, 820–828. [Google Scholar] [CrossRef]
- Juarez, G.E.; Villena, J.; Salva, S.; Font de Valdez, G.; Rodriguez, A.V. Lactobacillus reuteri CRL1101 beneficially modulate lipopolysaccharide-mediated inflammatory response in a mouse model of endotoxic shock. J. Funct. Foods 2013, 5, 1761–1773. [Google Scholar] [CrossRef]
- Maldonado Galdeano, C.; Novotny Núñez, I.; de Moreno de LeBlanc, A.; Carmuega, E.; Weill, R.; Perdigón, G. Impact of a probiotic fermented milk in the gut ecosystem and in the systemic immunity using a non-severe protein-energy-malnutrition model in mice. BMC Gastroenterol. 2011, 11, 64. [Google Scholar] [CrossRef] [PubMed]
- Agyei, D.; Ongkudon, C.M.; Wei, C.Y.; Chan, A.S.; Danquah, M.K. Bioprocess challenges to the isolation and purification of bioactive peptides. Food Bioprod. Process. 2016, 98, 244–256. [Google Scholar] [CrossRef]
- LeBlanc, J.; Fliss, I.; Matar, C. Induction of a humoral immune response following an Escherichia coli O157:H7 infection with an immunomodulatory peptidic fraction derived from Lactobacillus helveticus-fermented milk. Clin. Diagn. Lab. Immunol. 2004, 11, 1171–1181. [Google Scholar] [CrossRef] [PubMed]
- Vinderola, G.; Matar, C.; Palacios, J.; Perdigón, G. Mucosal immunomodulation by the non-bacterial fraction of milk fermented by Lactobacillus helveticus R389. Int. J. Food Microbiol. 2007, 115, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Matar, C.; Valdez, J.C.; Medina, M.; Rachid, M.; Perdigon, G. Immunomodulating effects of milks fermented by Lactobacillus helveticus and its nonproteolytic variant. J. Dairy Res. 2001, 68, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Tellez, A.; Corredig, M.; Turner, P.; Morales, R.; Griffiths, M.W. A peptidic fraction from milk fermented with L. helveticus protects mice against Salmonella infection. Int. Dairy J. 2011, 21, 607–614. [Google Scholar] [CrossRef]
- Sharma, R.; Kapila, R.; Kapasiya, M.; Saliganti, V.; Dass, G.; Kapila, S. Dietary supplementation of milk fermented with probiotic Lactobacillus fermentum enhances systemic immune response and antioxidant capacity in aging mice. Nutr. Res. 2014, 34, 968–981. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.; Wu, J.; Li, X.; Men, X.; Xu, Z. Probiotics and probiotic metabolic product improved intestinal function and ameliorated lps-induced injury in rats. Curr. Microbiol. 2017, 74, 1306–1315. [Google Scholar] [CrossRef] [PubMed]
- Koscik, R.J.E.; Reid, G.; Kim, S.O.; Li, W.; Challis, J.R.G.; Bocking, A.D. Effect of Lactobacillus rhamnosus GR-1 Supernatant on cytokine and chemokine output from human amnion cells treated with lipoteichoic acid and lipopolysaccharide. Reprod. Sci. 2017, 25, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Shigemori, S.; Namai, F.; Yamamoto, Y.; Nigar, S.; Sato, T.; Ogita, T.; Shimosato, T. Genetically modified Lactococcus lactis producing a green fluorescent protein–bovine lactoferrin fusion protein suppresses proinflammatory cytokine expression in lipopolysaccharide-stimulated RAW 264.7 cells. J. Dairy Sci. 2017, 100, 7007–7015. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Proinflammatory cytokines. Chest 2000, 118, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, M.; Vuopio-Varkila, J.; Varkila, K. Production of human tumor necrosis factor alpha, interleukin-6, and interleukin-10 is induced by lactic acid bacteria. Infect. Immun. 1996, 64, 5403–5405. [Google Scholar] [PubMed]
- Gabay, C. Interleukin-6 and chronic inflammation. Arthritis Res. Ther. 2006, 8, S3. [Google Scholar] [CrossRef] [PubMed]
- Louis, H.; LeMoine, O.; Peny, M.O.; Quertinmont, E.; Fokan, D.; Goldman, M.; Devière, J. Production and role of interleukin-10 in concanavalin A induced hepatitis in mice. Hepatology 1997, 25, 1382–1389. [Google Scholar] [CrossRef] [PubMed]
- Sang, H.; Wallis, G.L.; Stewart, C.A.; Kotake, Y. Expression of cytokines and activation of transcription factors in lipopolysaccharide-administered rats and their inhibition by phenyl N-tert-butylnitrone (PBN). Arch. Biochem. Biophys. 1999, 363, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Heredia, C.P.Y.; Méndez-Romero, J.I.; Hernández-Mendoza, A.; Acedo-Félix, E.; González-Córdova, A.F.; Vallejo-Cordoba, B. Antimicrobial activity and partial characterization of bacteriocin-like inhibitory substances produced by Lactobacillus spp. isolated from artisanal Mexican cheese. J. Dairy Sci. 2015, 98, 8285–8293. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1987, 150, 76–85. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC, 17th ed.; Association of Analytical Communities: Gaithersburg, MD, USA, 2000. [Google Scholar]
- National Research Council (NRC). Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar] [CrossRef]
- Perkins, D.; Pappin, D.J.; Creasy, D.M.; Cottrell, J.S. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis 1999, 20, 3551–3567. [Google Scholar] [CrossRef]
- Shihata, A.; Shah, N.P. Proteolytic profiles of yogurt and probiotic bacteria. Int. Dairy J. 2000, 10, 401–408. [Google Scholar] [CrossRef]
- Widyastuti, Y.; Rohmatussolihat; Febrisiantosa, A. The role of lactic acid bacteria in milk fermentation. Food Nutr. Sci. 2014, 5, 435–442. [Google Scholar] [CrossRef]
- Zhou, J.; Ma, L.; Xu, H.; Gao, Y.; Jin, Y.; Zhao, L.; David, X.A.L.; Zhan, D.; Zhang, S. Immunomodulating effects of casein-derived peptides QEPVL and QEPV on lymphocytes in vitro and in vivo. Food Funct. 2014, 5, 2061–2069. [Google Scholar]
- Meisel, H.; Bockelmann, W. Bioactive peptides encrypted in milk proteins: Proteolytic activation and thropho-functional properties. Antonie Leeuwenhoek 1999, 76, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Taverniti, V.; Guglielmetti, S. The immunomodulatory properties of probiotics microorganisms beyond their viability (ghost probiotics: Proposal of paraprobiotic concept). Genes Nutr. 2011, 6, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Cross, M.L.; Ganner, A.; Teilab, D.; Fray, L.M. Patterns of cytokine induction by gram-positive and gram-negative probiotic bacteria. FEMS Immunol. Med. Microbiol. 2004, 42, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Chuang, L.; Wu, K.G.; Pai, C.; Hsieh, P.S.; Tsai, J.J.; Yen, J.H.; Lin, M.Y. Heat-killed cells of lactobacilli skew the immune response toward T helper polarization in mouse splenocytes and dentritic cell-treated T cells. J. Agric. Food Chem. 2007, 55, 11080–11086. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rivera, L.; Ménard, O.; Recio, I.; Dupont, D. Peptide mapping during dynamic gastric digestion of heated and unheated skimmed milk powder. Food Res. Int. 2015, 77, 132–139. [Google Scholar] [CrossRef]
- Gill, H.S.; Doull, F.; Rutherfurd, K.J.; Cross, M.L. Immunoregulatory peptides in bovine milk. Br. J. Nutr. 2000, 84 (Suppl. 1), S111–S117. [Google Scholar] [CrossRef] [PubMed]
- Requena, P.; González, R.; López-Posadas, R.; Abadía-Molina, A.; Suárez, M.D.; Zarzuelo, A.; de Medina, F.S.; Martínez-Augustin, O. The intestinal antiinflammatory agent glycomacropeptide has immunomodulatory actions on rat splenocytes. Biochem. Pharmacol. 2010, 79, 1797–1804. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.; Gupta, N.; Smith, R.D.; Pevzner, P.A. Does trypsin cut before proline? J. Proteome Res. 2008, 7, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Díaz, A.; González-Córdova, A.F.; Hernández-Mendoza, A.; Reyes-Díaz, R.; Vallejo-Cordoba, B. Immunomodulation by hydrolysates and peptides derived from milk proteins. Int. J. Dairy Technol. 2018, 71, 1–9. [Google Scholar] [CrossRef]
- Meisel, H.; FitzGerald, R.J. Biofunctional peptides from milk proteins: Mineral binding and cytomodulatory effects. Curr. Pharm. Des. 2003, 9, 1289–1295. [Google Scholar] [PubMed]
- Haque, E.; Chand, R. Antihypertensive and antimicrobial bioactive peptides from milk proteins. Eur. Food Res. Technol. 2008, 227, 7–15. [Google Scholar] [CrossRef]
- Rodríguez-Figueroa, J.C.; González-Córdova, A.F.; Torres-Yanez, M.J.; Garcia, H.S.; Vallejo-Cordoba, B. Novel angiotensin I-converting enzyme inhibitory peptides produced in fermented milk by specific wild Lactococcus lactis strains. J. Dairy Sci. 2010, 95, 5536–5543. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Tian, B.; Brodkorb, A.; Huo, G. Production, analysis and in vivo evaluation of novel angiotensin-I-converting enzyme inhibitory peptides from bovine casein. Food Chem. 2010, 123, 779–786. [Google Scholar] [CrossRef]
- Hernández-Ledesma, B.; Amigo, L.; Ramos, M.; Recio, I. Angiotensin converting enzyme inhibitory activity in commercial fermented products. Formation of peptides under simulated gastrointestinal digestion. J. Agric. Food Chem. 2004, 52, 1504–1510. [Google Scholar] [CrossRef] [PubMed]
- Rival, S.G.; Boeriu, C.G.; Wichers, H.J. Caseins and casein hydrolysates. 2. Antioxidative properties and relevance to lipoxygenase inhibition. J. Agric. Food Chem. 2001, 49, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, V.P.; Kapila, S.; Kemgang, T.S.; Kapila, R. Antioxidative peptide derived from enzymatic digestion of buffalo casein. Int. Dairy J. 2015, 42, 1–5. [Google Scholar] [CrossRef]
- Vij, R.; Reddi, S.; Kapila, S.; Kapila, R. Transepithelial transport of milk derived bioactive peptide VLPVPQK. Food Chem. 2016, 190, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.; Xing, M.; Cui, L.; Deng, Y.; Xu, Y.; Huang, M.; Zhang, S. Antioxidant, antihypertensive, and immunomodulatory activities of peptide fractions from fermented skim milk with Lactobacillus delbrueckii ssp. bulgaricus LB340. J. Dairy Res. 2011, 78, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.; Tamaya, K.; Seki, E.; Osajima, K.; Matsumo, K.; Kawasaki, T. Absorption of Val-Tyr with in vitro angiotensin i-converting enzyme inhibitory activity into the circulating blood system of mild hypertensive subjects. Biol. Pharm. Bull. 2002, 25, 1228–1230. [Google Scholar] [CrossRef] [PubMed]
- Kagawa, K.; Matsutaka, H.; Fukuhama, C.; Watanabe, Y.; Fujino, H. Globin digest, acidic protease hydrolysate, inhibits dietary hypertriglyceridemia and Val-Val-Tyr-Pro, one of its constituents, possesses most superior effect. Life Sci. 1996, 58, 1745–1755. [Google Scholar] [CrossRef]
- Kayser, H.; Meisel, H. Stimulation of human peripheral blood lymphocytes by bioactive peptides derived from bovine milk proteins. FEBS Lett. 1996, 383, 18–20. [Google Scholar] [CrossRef]
- Van Amersfoort, E.S.; Van Berkel, T.J.C.; Kuiper, J. Receptors, mediators, and mechanisms involved in bacterial sepsis and septic shock. Clin. Microbiol. Rev. 2003, 16, 379–414. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Gao, D.; Chen, B.; Mao, X. Endotoxin-binding peptides derived from casein glycomacropeptide inhibit lipopolysaccharide-stimulated inflammatory responses via blockade of NF-κβ activation in macrophages. Nutrients 2015, 7, 3119–3137. [Google Scholar] [CrossRef] [PubMed]
- Iskandar, M.M.; Dauletbaev, N.; Kubow, S.; Mawji, N.; Lands, L.C. Whey protein hydrolysates decrease IL-8 secretion in lipopolysaccharide (LPS)-stimulated respiratory epithelial cells by affecting LPS binding to Toll-like receptor 4. Br. J. Nutr. 2013, 110, 58–68. [Google Scholar] [CrossRef] [PubMed]


| Sample a | Experimental Mass | Theoretical Mass | Molecular Ion (m/z) Selected for MS/MS b(Charge) | Protein Fragment | Sequence |
|---|---|---|---|---|---|
| F1 | 1622.75 | 1623.26 | 541.1 (+3) | α-La (f100–113) | LDDDLTDDIMCVKK |
| 2028.52 | 2028.09 | 2029.5 (+1) | β-Lg (f48–65) | LDAQSAPLRVYVEELKPT | |
| F2 | 1877.74 | 1878.87 | 1878.7 (+1) | αS1-CN (50–66) | EKVNELSKDIGSESTED |
| 708.21 | 708.34 | 237.1 (+3) | α-La (f33–39) | DLKGYGG | |
| F3 | 1029.86 | 1030.65 | 344.3 (+3) | β-Lg (f91–99) | KTKIPAVFK |
| 729.22 | 729.43 | 244.1 (+3) | Lactotransferrin (f67–73) | AIAEKKA | |
| 3409.04 | 3409.73 | 1705.5 (+2) | Lactotransferrin (f73–104) | ADAVTLDGGMVFEAGRDPYKLRPVAAEIYGTK | |
| 2036.38 | 2036.87 | 2037.4 (+1) | Lactotransferrin (f564–581) | NDTVWENTNGESTADWAK | |
| 810.65 | 811.40 | 407.3 (+2) | Serotransferrin (f668–674) | AKKTYDS | |
| 831.44 | 832.42 | 832.4 (+1) | Serotransferrin (f683–689) | AMTNLRQ | |
| F4 | 967.94 | 968.47 | 484.9 (+2) | κ-CN (f55–62) | RYPSYGLN |
| 949.23 | 949.59 | 317.4 (+3) | β-CN (185–193) | VLPVPQKAV | |
| 2236.12 | 2237.17 | 2237.1 (+1) | Lactotransferrin (f628–647) | QVLLHQQALFGKNGKNCPDK | |
| F5 | 916.00 | 916.45 | 459.0 (+2) | β-Lg (f117–123) | KYLLFCM |
| 1133.62 | 1133.50 | 284.4 (+4) | Lactotransferrin (f523–533) | LCAGDDQGLDK |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reyes-Díaz, A.; Mata-Haro, V.; Hernández, J.; González-Córdova, A.F.; Hernández-Mendoza, A.; Reyes-Díaz, R.; Torres-Llanez, M.J.; Beltrán-Barrientos, L.M.; Vallejo-Cordoba, B. Milk Fermented by Specific Lactobacillus Strains Regulates the Serum Levels of IL-6, TNF-α and IL-10 Cytokines in a LPS-Stimulated Murine Model. Nutrients 2018, 10, 691. https://doi.org/10.3390/nu10060691
Reyes-Díaz A, Mata-Haro V, Hernández J, González-Córdova AF, Hernández-Mendoza A, Reyes-Díaz R, Torres-Llanez MJ, Beltrán-Barrientos LM, Vallejo-Cordoba B. Milk Fermented by Specific Lactobacillus Strains Regulates the Serum Levels of IL-6, TNF-α and IL-10 Cytokines in a LPS-Stimulated Murine Model. Nutrients. 2018; 10(6):691. https://doi.org/10.3390/nu10060691
Chicago/Turabian StyleReyes-Díaz, Aline, Verónica Mata-Haro, Jesús Hernández, Aarón F. González-Córdova, Adrián Hernández-Mendoza, Ricardo Reyes-Díaz, María J. Torres-Llanez, Lilia M. Beltrán-Barrientos, and Belinda Vallejo-Cordoba. 2018. "Milk Fermented by Specific Lactobacillus Strains Regulates the Serum Levels of IL-6, TNF-α and IL-10 Cytokines in a LPS-Stimulated Murine Model" Nutrients 10, no. 6: 691. https://doi.org/10.3390/nu10060691






