Vitamin D Status and Immune Health Outcomes in a Cross-Sectional Study and a Randomized Trial of Healthy Young Children
Abstract
:1. Introduction
2. Methods
Study Population
3. Study 1
3.1. Assessments
3.1.1. Blood Sampling, Vitamin D Status, and Immune Outcomes
3.1.2. Dietary Assessment, Demographics and Anthropometry
3.2. Statistical Analyses
4. Study 2
4.1. Assessments
4.1.1. Blood Sampling, Vitamin D Status, and Immune Outcomes
4.1.2 Dietary Assessment.
4.1.3. Demographics, Illnesses, UVB, and Anthropometry
4.2. Statistical Analyses
5. Results
6. Discussion
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Garriguet, D. Canadians’ eating habits. Health Rep. 2007, 18, 17–32. [Google Scholar] [PubMed]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J. Clin. Endocrinol. Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Prietl, B.; Treiber, G.; Pieber, T.R.; Amrein, K. Vitamin D and immune function. Nutrients 2013, 5, 2502–2521. [Google Scholar] [CrossRef] [PubMed]
- Janz, T.; Pearson, C. Vitamin D Blood Levels of Canadians; Statistics Canada: Ottawa, ON, Canada, 2013. Available online: http://www.statcan.gc.ca/pub/82-624-x/2013001/article/11727-eng.htm (accessed on 1 December 2015).
- Schwalfenberg, G.K. A review of the critical role of vitamin D in the functioning of the immune system and the clinical implications of vitamin D deficiency. Mol. Nutr. Food Res. 2011, 55, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, A.K.; Amento, E.P.; Serog, B.; Glimcher, L.H. 1,25-dihydroxyvitamin D3 inhibits antigen-induced T cell activation. J. Immunol. 1984, 133, 1748–1754. [Google Scholar] [PubMed]
- Adams, J.S.; Sharma, O.P.; Gacad, M.A.; Singer, F.R. Metabolism of 25-hydroxyvitamin D3 by cultured pulmonary alveolar macrophages in sarcoidosis. J. Clin. Investig. 1983, 72, 1856–1860. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.; Hughes, A.L.; Zhang, G. Rapid evolution and diversification of mammalian α-defensins as revealed by comparative analysis of rodent and primate genes. Physiol. Genom. 2004, 20, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lemire, J.M.; Adams, J.S.; Kermani-Arab, V.; Bakke, A.C.; Sakai, R.; Jordan, S.C. 1,25-dihydroxyvitamin D3 suppresses human T helper/inducer lymphocyte activity in vitro. J. Immunol. 1985, 134, 3032–3035. [Google Scholar] [PubMed]
- Cantorna, M.T.; Snyder, L.; Lin, Y.D.; Yang, L. Vitamin D and 1,25(OH)2D regulation of T cells. Nutrients 2015, 7, 3011–3021. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine, Food and Nutrition Board, Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. Dietary Reference Intakes for Calcium and Vitamin D; Del Valle, H.B., Yaktine, A.L., Taylor, C.L., Ross, A.C., Eds.; National Academies Press: Washington, DC, USA, 2011.
- Yakoob, M.Y.; Salam, R.A.; Khan, F.R.; Bhutta, Z.A. Vitamin D supplementation for preventing infections in children under five years of age. Cochrane Database Syst. Rev. 2016, 11, Cd008824. [Google Scholar] [CrossRef] [PubMed]
- Camargo, C.A.; Ganmaa, D.; Frazier, A.L.; Kirchberg, F.F.; Stuart, J.J.; Kleinman, K.; Sumberzul, N.; Rich-Edwards, J.W. Randomized trial of vitamin D supplementation and risk of acute respiratory infection in mongolia. Pediatrics 2012, 130, e561–e567. [Google Scholar] [CrossRef] [PubMed]
- Bock, G.; Prietl, B.; Mader, J.K.; Holler, E.; Wolf, M.; Pilz, S.; Graninger, W.B.; Obermayer-Pietsch, B.M.; Pieber, T.R. The effect of vitamin D supplementation on peripheral regulatory T cells and β cell function in healthy humans: A randomized controlled trial. Diabetes Metab. Res. Rev. 2011, 27, 942–945. [Google Scholar] [CrossRef] [PubMed]
- Milliken, S.V.; Wassall, H.; Lewis, B.J.; Logie, J.; Barker, R.N.; Macdonald, H.; Vickers, M.A.; Ormerod, A.D. Effects of ultraviolet light on human serum 25-hydroxyvitamin D and systemic immune function. J. Allergy Clin. Immunol. 2012, 129, 1554–1561. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Wan, Z.; Han, S.F.; Li, B.Y.; Zhang, Z.L.; Qin, L.Q. Effect of vitamin D supplementation on the level of circulating high-sensitivity c-reactive protein: A meta-analysis of randomized controlled trials. Nutrients 2014, 6, 2206–2216. [Google Scholar] [CrossRef] [PubMed]
- El Hayek, J.; Pham, T.T.; Finch, S.; Hazell, T.J.; Jean-Philippe, S.; Vanstone, C.A.; Agellon, S.; Rodd, C.; Rauch, F.; Weiler, H.A. Vitamin D status in Montreal preschoolers is satisfactory despite low vitamin D intake. J. Nutr. 2013, 143, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Brett, N.R.; Lavery, P.; Agellon, S.; Vanstone, C.A.; Maguire, J.L.; Rauch, F.; Weiler, H.A. Dietary vitamin D dose-response in healthy children 2 to 8 y of age: A 12-weeks randomized controlled trial using fortified foods. Am. J. Clin. Nutr. 2016, 103, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Canadian Institutes of Health Research; Natural Science and Engineering Research Council; Social Sciences and Humanities Research Council. Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans. Available online: https://www.google.com.hk/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&ved=0ahUKEwiSkOeU0KDbAhWGjJQKHbGvARsQFgglMAA&url=http%3A%2F%2Fwww.pre.ethics.gc.ca%2Fpdf%2Feng%2Ftcps2%2FTCPS_2_FINAL_Web.pdf&usg=AOvVaw0pa3Qv9lA2vu6QbNttaHCU (accessed on 20 May 2015).
- Bingham, S.; Gill, C.; Welch, A.; Day, K.; Cassidy, A.; Khaw, K.; Sneyd, M.; Key, T.; Roe, L.; Day, N. Comparison of dietary assessment methods in nutritional epidemiology: Weighed records v. 24 h recalls, food-frequency questionnaires and estimated-diet records. Br. J. Nutr. 1994, 72, 619–643. [Google Scholar] [CrossRef] [PubMed]
- Webb, A.R.; Engelsen, O. Ultraviolet exposure scenarios: Risks of erythema from recommendations on cutaneous vitamin D synthesis. In Sunlight, Vitamin D and Skin Cancer; Springer: New York, NY, USA, 2008; pp. 72–85. [Google Scholar]
- World Health Organization. Child Growth Standards. Available online: http://www.who.int/childgrowth/standards/bmi_for_age/en/ (accessed on 14 December 2014).
- Field, C.J.; Van Aerdel, J.E.; Robinson, L.E.; Clandinin, M.T. Effect of providing a formula supplemented with long-chain polyunsaturated fatty acids on immunity in full-term neonates. Br. J. Nutr. 2008, 99, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, A.; Song, L.Y.; Wilkening, C.; Sevin, A.; Blais, B.; Louzao, R.; Stein, D.; Defechereux, P.; Durand, D.; Riedel, E.; et al. Optimization and limitations of use of cryopreserved peripheral blood mononuclear cells for functional and phenotypic t-cell characterization. Clin. Vaccine Immunol. 2009, 16, 1176–1186. [Google Scholar] [CrossRef] [PubMed]
- Field, C.J.; Thomson, C.A.; Van Aerde, J.E.; Parrott, A.; Lien, E.; Clandinin, M.T. Lower proportion of cd45r0+ cells and deficient interleukin-10 production by formula-fed infants, compared with human-fed, is corrected with supplementation of long-chain polyunsaturated fatty acids. J. Pediatr. Gastroenterol. Nutr. 2000, 31, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Richard, C.; Wadowski, M.; Goruk, S.; Cameron, L.; Sharma, A.M.; Field, C.J. Individuals with obesity and type 2 diabetes have additional immune dysfunction compared with obese individuals who are metabolically healthy. BMJ Open Diabetes Res. Care 2017, 5, e000379. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, B.; Young, N.L.; Dick, P.T.; Ipp, M.M.; Dutkowski, R.; Davies, H.D.; Langley, J.M.; Greenberg, S.; Stephens, D.; Wang, E.E. Canadian acute respiratory illness and flu scale (CARIFS): Development of a valid measure for childhood respiratory infections. J. Clin. Epidemiol. 2000, 53, 793–799. [Google Scholar] [CrossRef]
- Shea, S.; Aymong, E.; Zybert, P.; Shamoon, H.; Tracy, R.P.; Deckelbaum, R.J.; Basch, C.E. Obesity, fasting plasma insulin, and C-reactive protein levels in healthy children. Obesity 2003, 11, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Castell, J.V.; Gomez-Lechon, M.J.; David, M.; Andus, T.; Geiger, T.; Trullenque, R.; Fabra, R.; Heinrich, P.C. Interleukin-6 is the major regulator of acute phase protein synthesis in adult human hepatocytes. FEBS Lett. 1989, 242, 237–239. [Google Scholar] [CrossRef]
- Dorn, L.D.; Gayles, J.G.; Engeland, C.G.; Houts, R.; Cizza, G.; Denson, L.A. Cytokine patterns in healthy adolescent girls: Heterogeneity captured by variable-and person-centered statistical strategies. Psychosom. Med. 2016, 78, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Scheett, T.P.; Mills, P.J.; Ziegler, M.G.; Stoppani, J.; Cooper, D.M. Effect of exercise on cytokines and growth mediators in prepubertal children. Pediatr. Res. 1999, 46, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.R.; Ha, C.D.; Jin, Y.Y.; Kang, H.S. The effect of physical activity on serum Il-6 and vaspin levels in late elementary school children. J. Exerc. Nutr. Biochem. 2015, 19, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Petersen, A.M.W.; Pedersen, B.K. The anti-inflammatory effect of exercise. J. Appl. Physiol. 2005, 98, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-M.; An, J. Cytokines, inflammation and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Cannell, J.J.; Vieth, R.; Umhau, J.C.; Holick, M.F.; Grant, W.B.; Madronich, S.; Garland, C.F.; Giovannucci, E. Epidemic influenza and vitamin D. Epidemiol. Infect. 2006, 134, 1129–1140. [Google Scholar] [CrossRef] [PubMed]
- Hart, P.H.; Gorman, S.; Finlay-Jones, J.J. Modulation of the immune system by UV radiation: More than just the effects of vitamin D? Nat. Rev. Immunol. 2011, 11, 584–596. [Google Scholar] [CrossRef] [PubMed]
- Chadha, M.K.; Fakih, M.; Muindi, J.; Tian, L.; Mashtare, T.; Johnson, C.S.; Trump, D. Effect of 25-hydroxyvitamin D status on serological response to influenza vaccine in prostate cancer patients. Prostate 2011, 71, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Whiting, S.J.; Langlois, K.A.; Vatanparast, H.; Greene-Finestone, L.S. The vitamin D status of Canadians relative to the 2011 dietary reference intakes: An examination in children and adults with and without supplement use. Am. J. Clin. Nutr. 2011, 94, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Thornton, K.A.; Marin, C.; Mora-Plazas, M.; Villamor, E. Vitamin D deficiency associated with increased incidence of gastrointestinal and ear infections in school-age children. Pediatr. Infect. Dis. J. 2013, 32, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Science, M.; Maguire, J.L.; Russell, M.L.; Smieja, M.; Walter, S.D.; Loeb, M. Low serum 25-hydroxyvitamin D level and risk of upper respiratory tract infection in children and adolescents. Clin. Infect. Dis. 2013, 57, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Konowalchuk, J.D.; Rieger, A.M.; Kiemele, M.D.; Ayres, D.C.; Barreda, D.R. Modulation of weanling pig cellular immunity in response to diet supplementation with 25-hydroxyvitamin D3. Vet. Immunol. Immunopathol. 2013, 155, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.H. Il-2, regulatory T cells, and tolerance. J. Immunol. 2004, 172, 3983–3988. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Leung, D.Y.; Richers, B.N.; Liu, Y.; Remigio, L.K.; Riches, D.W.; Goleva, E. Vitamin d inhibits monocyte/macrophage proinflammatory cytokine production by targeting mapk phosphatase-1. J. Immunol. 2012, 188, 2127–2135. [Google Scholar] [CrossRef] [PubMed]
- Schleithoff, S.S.; Zittermann, A.; Tenderich, G.; Berthold, H.K.; Stehle, P.; Koerfer, R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: A double-blind, randomized, placebo-controlled trial. Am. J. Clin. Nutr. 2006, 83, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Aglipay, M.; Birken, C.S.; Parkin, P.C.; Loeb, M.B.; Thorpe, K.; Chen, Y.; Laupacis, A.; Mamdani, M.; Macarthur, C.; Hoch, J.S. Effect of high-dose vs. standard-dose wintertime vitamin D supplementation on viral upper respiratory tract infections in young healthy children. JAMA 2017, 318, 245–254. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Study 1 (Cross sectional over 1 year) | Study 2 (Baseline of 12 weeks winter trial) | |
|---|---|---|---|
| 2–5 years old | 2–8 years old | 6–8 years old subgroup | |
| n | 457 | 77 | 22 |
| Age (years) | 3.7 ± 1.0 | 5.1 ± 1.9 | 7.5 ± 0.7 |
| Range: 1.8–5.9 | Range: 1.9–8.7 | Range: 6.0–8.7 | |
| Male n (%) | 242 (53%) | 40 (52%) | 12 (55%) |
| Ethnicity, White n (%) | 242 (53%) | 56 (72.7%) | 12 (65%) |
| Family income: n (%) | |||
| >$65,000 1 | 264 (58%) 2 | 43 (56%) | 12 (55%) |
| Not disclosed | 37 (8%) | 5 (6%) | 2 (9%) |
| BMI z-score | 0.50 ±1.00 | 0.51 ± 0.89 | 0.65 ± 1.09 |
| Vitamin D intake 3 (IU/day): | |||
| Total | 397 (299–486) | ||
| Without Supplements | 236 (152–320) | 202 (148–316) | 259 (158–404) |
| Total 25(OH)D (nmol/L) | 78.3 ± 27.3 | 59.3 ± 12.7 | 57.2 ± 11.5 |
| Vitamin D category: | |||
| <30 nmol/L | 3 (1%) | 1 (1%) | 0 |
| <50 nmol/L | 51 (11%) | 17 (22%) | 6 (27%) |
| 50.0–74.9 nmol/L | 189 (41%) | 50 (65%) | 14 (64%) |
| 75.0–124.9 nmol/L | 182 (40%) | 9 (12%) | 2 (9%) |
| ≥125 nmol/L | 32 (7%) | 0 | 0 |
| Control (n = 24) | EAR (n = 25) | RDA (n = 25) | Overall (n = 74) | p-Value 4 | |
|---|---|---|---|---|---|
| Number of children reported getting ill 1 | 14 (58%) | 12 (48%) | 13 (52%) | 39 (53%) | 0.68 |
| Duration of illness (days) 2 | 4 (3–6) | 5.5 (2.75–12) | 3 (2.5–6) | 4 (3–6) | 0.17 |
| Days of school/daycare missed 2 | 2 (1–3) | 1 (1–3) | 2 (1–3) | 1.5 (1–3) | 0.25 |
| Days of work missed by parents 2 | 2 (1–2) | 1 (1–1) | 1 (1–2) | 1.5 (1–2) | 0.52 |
| CARIFS score 3 | 31.1 ± 13.2 | 29.8 ± 12.2 | 39.5 ± 7.5 | 33.6 ± 12.1 | 0.07 |
| 0 week 2 | 12 weeks 2 | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|
| Outcome | CTRL n = 7 | EAR n = 7 | RDA n = 9 | CTRL n = 7 | EAR n = 7 | RDA n = 9 | Group | Visit |
| 25(OH)D (nmol/L) | 55.0 ± 11.9 | 56.1 ± 9.2 | 59.9 ± 13.4 | 55.8 ± 11.9 | 64.1 ± 10.0* | 63.7 ± 12.4 * | 0.02 | 0.47 |
| White blood cell fraction 1 | ||||||||
| WBC (5.5–15.5 × 109/L) (109/L) 3 | 5.35 ± 1.07 | 5.42 ± 0.83 | 5.99 ± 1.13 | 5.89 ± 0.44 | 6.45 ± 1.20 | 5.45 ± 0.81 | 0.76 | 0.22 |
| Neutrophil (1.50–8.50 × 109/L) (109/L) 3 | 2.45 ± 0.55 | 2.40 ± 0.54 | 2.42 ± 0.64 | 2.83 ± 0.70 | 3.16 ± 0.88 | 2.51 ± 0.91 | 0.31 | 0.06 |
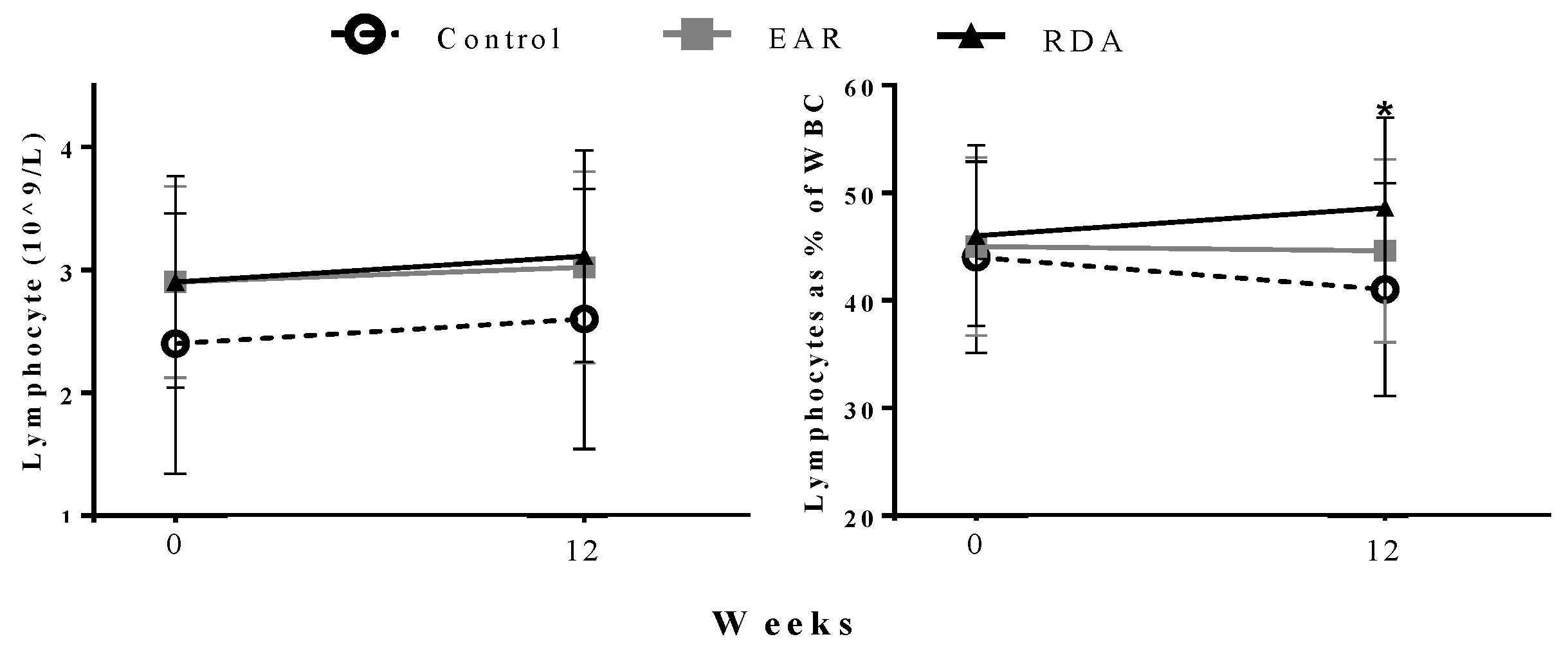
| Lymphocyte (2.00–8.00 × 109/L) (109/L) 3 | 2.30 ± 0.51 | 2.37 ± 0.40 | 2.78 ± 0.88 | 2.08 ± 0.36 | 2.55 ± 0.52 | 2.86 ± 0.67 | 0.11 | 0.97 |
| Lymphocyte % of WBC (36–52%) 3 | 43.6 ± 10.2 | 43.9 ± 6.4 | 45.7 ± 8.3 | 37.0 ± 6.1 | 40.0 ± 6.3 | 51.7 ± 8.3 * | 0.01 | 0.72 |
| Monocyte (0.10–0.80 × 109/L) 3 (109/L) | 0.48 ± 20.0 | 0.42 ± 10.1 | 0.43 ± 15.0 | 0.44 ± 13.9 | 0.48 ± 11.6 | 0.44 ± 13.0 | 0.85 | 0.75 |
| Total CD3+ 4 | 1.6 ± 0.4 | 1.6 ± 0.7 | 1.9 ± 0.6 | 1.4 ± 0.4 | 1.7 ± 0.5 | 2.0 ± 0.6 | 0.16 | 0.91 |
| Total CD4+ 4 | 0.9 ± 0.2 | 1.1 ± 0.3 | 1.1 ± 0.5 | 0.7 ± 0.1 | 1.1 ± 0.3 | 1.2 ± 0.5 | 0.13 | 0.84 |
| Total CD8+ 4 | 0.6 ± 0.1 | 0.6 ± 0.2 | 0.6 ± 0.2 | 0.5 ± 0.2 | 0.6 ± 0.2 | 0.7 ± 0.2 | 0.53 | 0.90 |
| Total CD25+ 4 | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.1 ± 0.0 | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.97 | 0.35 |
| B cells (CD19+) 4 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.1 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.31 | 0.71 |
| T regulatory (CD4+25+) 4 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.04 ± 0.1 | 0.1 ± 0.0 | 0.1 ± 0.1 | 0.67 | 0.83 |
| T helper (CD3+4+) 4 | 0.8 ± 0.2 | 1.0 ± 0.3 | 1.1 ± 0.4 | 0.8 ± 0.1 | 1.0 ± 0.3 | 1.2 ± 0.5 | 0.23 | 0.65 |
| T suppressor (CD3+8+) 4 | 0.5 ± 0.1 | 0.5 ± 0.2 | 0.6 ± 0.2 | 0.5 ± 0.1 | 0.6 ± 0.2 | 0.6 ± 0.2 | 0.58 | 0.60 |
| 0 week | 12 weeks | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|
| Cell Type | CTRL (n = 7) | EAR (n = 7) | RDA (n = 9) | CTRL (n = 7) | EAR (n = 7) | RDA (n = 9) | Group | Visit |
| Monocytes (CD14+) | 14.4 (12.7–16.4) | 13.6 (11.6–17.7) | 14.8 (12.5–21.2) | 14.4 (13.1–14.8) | 14.9 (14.2–16.7) | 16.5 (13.0–19.9) | 0.10 | 0.84 |
| B cells (CD19+) | 9.1 (7.7–13.4) | 8.0 (5.1–11.4) | 6.2 (4.8–10.2) | 5.4 (3.4–10.4) | 6.7 5.7–9.4) | 9.3 (5.7–13.5) | 0.27 | 0.48 |
| T Regulatory (CD4+CD25+) | 2.4 (0.3–3.0) | 1.9 (1.0–3.0) | 2.1 (1.0–2.4) | 2.5 (1.1–3.0) | 2.3 (2.3–2.3) | 2.5 (0.4–3.3) | 0.96 | 0.78 |
| T Helper (CD3+CD4+) | 31.6 (26.7–34.0) | 41.7 (36.4–46.4) | 35.7 (29.3–37.5) | 39.6 (31.9–46.7) | 41.4 (38.4–42.0) | 35.5 (33.3–48.2) | 0.39 | 0.34 |
| T Suppresser (CD3+CD8+) | 18.8 (17.2–23.2) | 19.8 (15.6–24.3) | 21.7 (16.5–24.5) | 21.6 (20.6–24.3) | 21.4 (19.6–22.3) | 21.0 (15.2–24.8) | 0.80 | 0.41 |
| CD4:CD8 | 1.5 (1.1–1.6) | 1.7 (1.6–1.9) | 1.7 (1.3–2.3) | 1.7 (1.3–1.9) | 1.7 (1.6–1.8) | 1.7 (1.2–2.2) | 0.56 | 0.80 |
| Total CD3+ | 66.7 (58.2–71.7) | 63.0 (43.8–76.1) | 68.7 (59.7–74.0) | 63.6 (58.8–68.7) | 67.3 (63.6–71.7) | 65.0 (60.3–72.8) | 0.94 | 0.82 |
| Total CD4+ | 36.2 (29.2–47.2) | 42.5 (37.1–47.7) | 40.1 (33.3–50.3) | 34.9 (29.8–39.6) | 40.1 (37.0–43.9) | 37.8 (28.7–49.0) | 0.31 | 0.54 |
| Total CD8+ | 24.4 (20.7–28.6) | 24.6 (22.2–28.2) | 23.0 (21.0–27.1) | 24.0 (20.2–27.3) | 24.4 (22.2–27.6) | 22.4 (20.8–25.9) | 0.55 | 0.80 |
| Total CD25+ | 4.4 (0.8–6.9) | 3.4 (1.5–5.4) | 3.0 (1.8–4.2) | 2.9 (0.9–4.1) | 2.9 (1.4–3.9) | 3.2(0.5–5.3) | 0.80 | 0.42 |
| Factor | Concentration | p-Value | Concentration corrected for lymphocyte concentration (1 × 109/L) | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| CTRL (n = 7) | EAR (n = 7) | RDA (n = 9) | Group | CTRL (n = 7) | EAR (n = 7) | RDA (n = 9) | Group | |
| TNFα (pg/mL) | 593 (419–959) | 414 (239–455) | 541 (301–1141) | 0.34 | 345 (250–446) | 126 (104–207) | 190 (99–393) | 0.28 |
| IL-2 (pg/mL) | 40 (39–87) | 53 (30–54) | 44 (12–102) | 0.73 | 26 (17–46) | 16 (13–28) | 13 (4–43) | 0.82 |
| IL-4 (pg/mL) | 2.8 (1.7–3.7) | 2.3 (1.8–2.4) | 2.0 (1.4–2.4) | 0.38 | 1.2 (1.3–1.9) | 0.8 (0.7–1.1) | 0.6 (0.5–0.8) | 0.19 |
| IL-6 (pg/mL) | 265 (94–422) | 45* (10–56) | 93* (78–176) | 0.008 | 167 (41–249) | 16 * (5–20) | 30 * (25–76) | 0.002 |
| IL-10 (pg/mL) | 17 (14–26) | 11 (9–17) | 13 (5–21) | 0.80 | 9 (8–14) | 4 (3–8) | 4 (2–9) | 0.35 |
| Cathelicidin (ng/mL) | 26 (17–34) | 27 (25–26) | 31 (25–38) | 0.50 | N/A | N/A | N/A | N/A |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brett, N.R.; Lavery, P.; Agellon, S.; Vanstone, C.A.; Goruk, S.; Field, C.J.; Weiler, H.A. Vitamin D Status and Immune Health Outcomes in a Cross-Sectional Study and a Randomized Trial of Healthy Young Children. Nutrients 2018, 10, 680. https://doi.org/10.3390/nu10060680
Brett NR, Lavery P, Agellon S, Vanstone CA, Goruk S, Field CJ, Weiler HA. Vitamin D Status and Immune Health Outcomes in a Cross-Sectional Study and a Randomized Trial of Healthy Young Children. Nutrients. 2018; 10(6):680. https://doi.org/10.3390/nu10060680
Chicago/Turabian StyleBrett, Neil R., Paula Lavery, Sherry Agellon, Catherine A. Vanstone, Susan Goruk, Catherine J. Field, and Hope A. Weiler. 2018. "Vitamin D Status and Immune Health Outcomes in a Cross-Sectional Study and a Randomized Trial of Healthy Young Children" Nutrients 10, no. 6: 680. https://doi.org/10.3390/nu10060680





