Dietary Pattern and Plasma BCAA-Variations in Healthy Men and Women—Results from the KarMeN Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. BCAA Measurement
2.3. Dietary Assessment
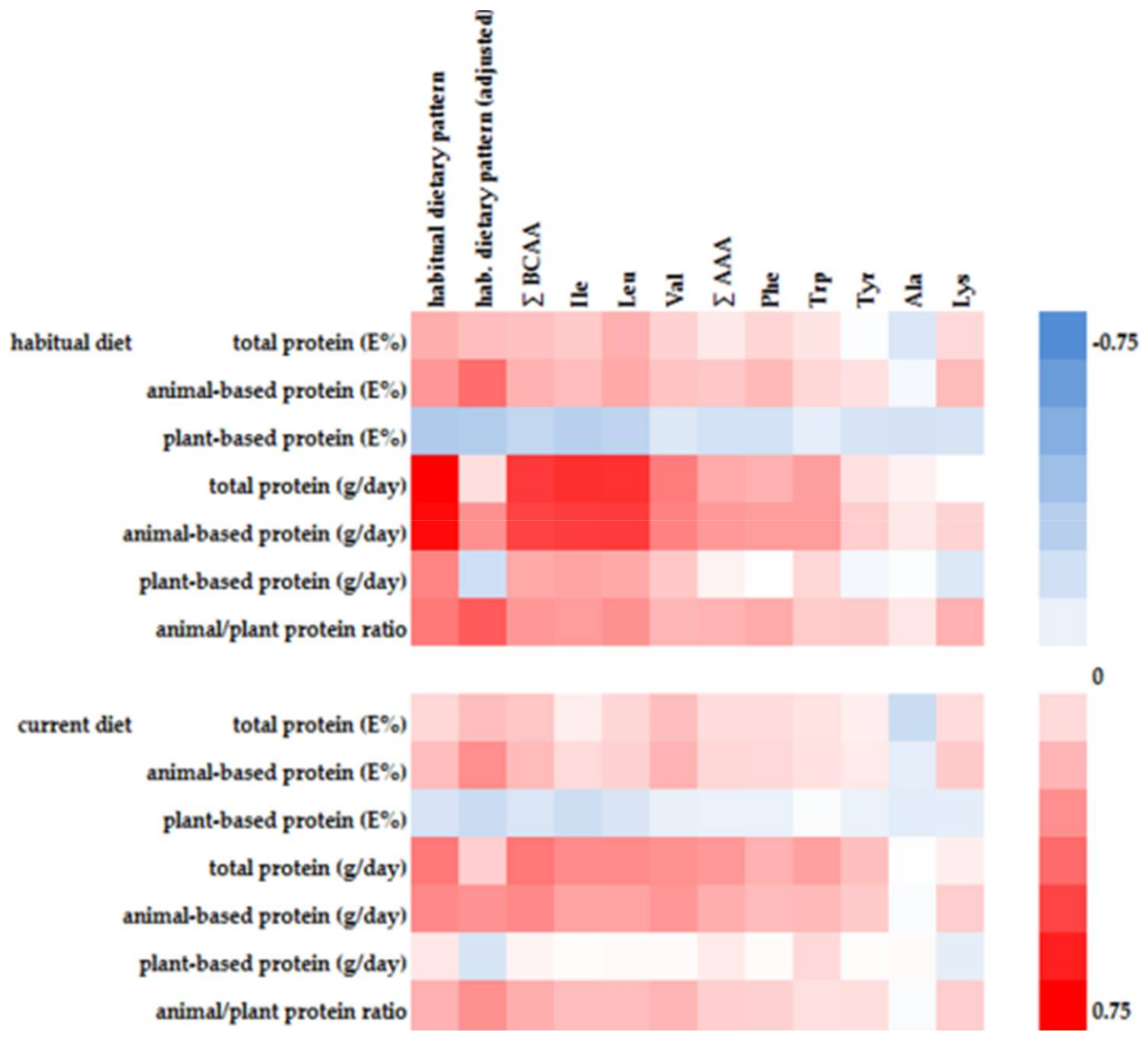
2.4. Statistical Analysis
3. Results
3.1. Study Population
3.2. Dietary Pattern Analysis
3.3. Associations of Plasma Amino Acid Concentrations with Dietary Pattern Score
4. Discussion
4.1. Current vs. Habitual Diet
4.2. Plasma AA Concentrations and Dietary AA Composition
4.3. Dietary Protein Source of a BCAA-Rich Diet
4.4. Additional Nutrients of a BCAA-Rich Diet
4.5. Indirect Dietary Contributions
4.6. Limitations and Strengths
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Zheng, Y.; Li, Y.; Qi, Q.; Hruby, A.; Manson, J.E.; Willett, W.C.; Wolpin, B.M.; Hu, F.B.; Qi, L. Cumulative consumption of branched-chain amino acids and incidence of type 2 diabetes. Int. J. Epidemiol. 2016, 45, 1482–1492. [Google Scholar] [CrossRef] [PubMed]
- Jennings, A.; MacGregor, A.; Pallister, T.; Spector, T.; Cassidy, A. Associations between branched chain amino acid intake and biomarkers of adiposity and cardiometabolic health independent of genetic factors: A twin study. Int. J. Cardiol. 2016, 223, 992–998. [Google Scholar] [CrossRef] [PubMed]
- Mangge, H.; Zelzer, S.; Prüller, F.; Schnedl, W.J.; Weghuber, D.; Enko, D.; Bergsten, P.; Haybaeck, J.; Meinitzer, A. Branched-chain amino acids are associated with cardiometabolic risk profiles found already in lean, overweight and obese young. J. Nutr. Biochem. 2016, 32, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Rietman, A.; Schwarz, J.; Tome, D.; Kok, F.J.; Mensink, M. High dietary protein intake, reducing or eliciting insulin resistance? Eur. J. Clin. Nutr. 2014, 68, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Cortiella, J.; Matthews, D.E.; Hoerr, R.A.; Bier, D.M.; Young, V.R. Leucine kinetics at graded intakes in young men: Quantitative fate of dietary leucine. Am. J. Clin. Nutr. 1988, 48, 998–1009. [Google Scholar] [CrossRef] [PubMed]
- Biolo, G.; Tessari, P.; Inchiostro, S.; Bruttomesso, D.; Fongher, C.; Sabadin, L.; Fratton, M.G.; Valerio, A.; Tiengo, A. Leucine and phenylalanine kinetics during mixed meal ingestion: A multiple tracer approach. Am. J. Physiol. 1992, 262, E455–E463. [Google Scholar] [CrossRef] [PubMed]
- Meguid, M.M.; Matthews, D.E.; Bier, D.M.; Meredith, C.N.; Young, V.R. Valine kinetics at graded valine intakes in young men. Am. J. Clin. Nutr. 1986, 43, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.H.; Crosslin, D.R.; Haynes, C.S.; Nelson, S.; Turer, C.B.; Stevens, R.D.; Muehlbauer, M.J.; Wenner, B.R.; Bain, J.R.; Laferrère, B.; et al. Branched-chain amino acid levels are associated with improvement in insulin resistance with weight loss. Diabetologia 2012, 55, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.A.; Rinaldi, S.; Scalbert, A.; Ferrari, P.; Achaintre, D.; Gunter, M.J.; Appleby, P.N.; Key, T.J.; Travis, R.C. Plasma concentrations and intakes of amino acids in male meat-eaters, fish-eaters, vegetarians and vegans: A cross-sectional analysis in the EPIC-Oxford cohort. Eur. J. Clin. Nutr. 2016, 70, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Han, Q.; Liu, Y.; Sun, C.; Gang, X.; Wang, G. The Relationship between Branched-Chain Amino Acid Related Metabolomic Signature and Insulin Resistance: A Systematic Review. J. Diabetes Res. 2016, 2016, 2794591. [Google Scholar] [CrossRef] [PubMed]
- Batch, B.C.; Hyland, K.; Svetkey, L.P. Branch chain amino acids: Biomarkers of health and disease. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Fiehn, O.; Garvey, W.T.; Newman, J.W.; Lok, K.H.; Hoppel, C.L.; Adams, S.H. Plasma Metabolomic Profiles Reflective of Glucose Homeostasis in Non-Diabetic and Type 2 Diabetic Obese African-American Women. PLoS ONE 2010, 5, e15234. [Google Scholar] [CrossRef] [PubMed]
- Lackey, D.E.; Lynch, C.J.; Olson, K.C.; Mostaedi, R.; Ali, M.; Smith, W.H.; Karpe, F.; Humphreys, S.; Bedinger, D.H.; Dunn, T.N.; et al. Regulation of adipose branched-chain amino acid catabolism enzyme expression and cross-adipose amino acid flux in human obesity. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E1175–E1187. [Google Scholar] [CrossRef] [PubMed]
- Giesbertz, P.; Daniel, H. Branched-chain amino acids as biomarkers in diabetes. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, J.F.; Wang, T.J. Branched-Chain Amino Acids and Cardiovascular Disease: Does Diet Matter? Clin. Chem. 2016, 62, 545–547. [Google Scholar] [CrossRef] [PubMed]
- Tai, E.S.; Tan, M.L.S.; Stevens, R.D.; Low, Y.L.; Muehlbauer, M.J.; Goh, D.L.M.; Ilkayeva, O.R.; Wenner, B.R.; Bain, J.R.; Lee, J.J.; et al. Insulin resistance is associated with a metabolic profile of altered protein metabolism in Chinese and Asian-Indian men. Diabetologia 2010, 53, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Cummings, N.E.; Arriola Apelo, S.I.; Neuman, J.C.; Kasza, I.; Schmidt, B.A.; Cava, E.; Spelta, F.; Tosti, V.; Syed, F.A.; et al. Decreased Consumption of Branched-Chain Amino Acids Improves Metabolic Health. Cell Rep. 2016, 16, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Bub, A.; Kriebel, A.; Dörr, C.; Bandt, S.; Rist, M.; Roth, A.; Hummel, E.; Kulling, S.; Hoffmann, I.; Watzl, B. The Karlsruhe Metabolomics and Nutrition (KarMeN) Study: Protocol and Methods of a Cross-Sectional Study to Characterize the Metabolome of Healthy Men and Women. JMIR Res. Protoc. 2016, 5, e146. [Google Scholar] [CrossRef] [PubMed]
- Römisch-Margl, W.; Prehn, C.; Bogumil, R.; Röhring, C.; Suhre, K.; Adamski, J. Procedure for tissue sample preparation and metabolite extraction for high-throughput targeted metabolomics. Metabolomics 2012, 8, 133–142. [Google Scholar] [CrossRef]
- Biocrates Life Sciences AG. AbsoluteIDQ Kit—User Manual UM-P180-ABSCIEX-5; Biocrates Life Sciences AG: Innsbruck, Austria, 2012. [Google Scholar]
- Slimani, N.; Deharveng, G.; Charrondiere, R.U.; van Kappel, A.L.; Ocke, M.C.; Welch, A.; Lagiou, A.; van Liere, M.; Agudo, A.; Pala, V.; et al. Structure of the standardized computerized 24-h diet recall interview used as reference method in the 22 centers participating in the EPIC project. European Prospective Investigation into Cancer and Nutrition. Comput. Methods Programs Biomed. 1999, 58, 251–266. [Google Scholar] [CrossRef]
- Slimani, N.; Ferrari, P.; Ocke, M.; Welch, A.; Boeing, H.; Liere, M.; Pala, V.; Amiano, P.; Lagiou, A.; Mattisson, I.; et al. Standardization of the 24-hour diet recall calibration method used in the European prospective investigation into cancer and nutrition (EPIC): General concepts and preliminary results. Eur. J. Clin. Nutr. 2000, 54, 900–917. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, B.M.; Heuer, T.; Hoffmann, I. The German Nutrient Database: Effect of different versions on the calculated energy and nutrient intake of the German population. J. Food Compos. Anal. 2015, 42, 26–29. [Google Scholar] [CrossRef]
- Kipnis, V.; Midthune, D.; Buckman, D.W.; Dodd, K.W.; Guenther, P.M.; Krebs-Smith, S.M.; Subar, A.F.; Tooze, J.A.; Carroll, R.J.; Freedman, L.S. Modeling Data with Excess Zeros and Measurement Error: Application to Evaluating Relationships between Episodically Consumed Foods and Health Outcomes. Biometrics 2009, 65, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Tooze, J.A.; Midthune, D.; Dodd, K.W.; Freedman, L.S.; Krebs-Smith, S.M.; Subar, A.F.; Guenther, P.M.; Carroll, R.J.; Kipnis, V. A New Statistical Method for Estimating the Usual Intake of Episodically Consumed Foods with Application to Their Distribution. J. Am. Diet. Assoc. 2006, 106, 1575–1587. [Google Scholar] [CrossRef] [PubMed]
- Box, G.E.P.; Cox, D.R. An Analysis of Transformations. J. R. Stat. Soc. Ser. B (Methodol.) 1964, 26, 211–252. [Google Scholar]
- Hoffmann, K.; Schulze, M.B.; Schienkiewitz, A.; Nothlings, U.; Boeing, H. Application of a new statistical method to derive dietary patterns in nutritional epidemiology. Am. J. Epidemiol. 2004, 159, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Rietman, A.; Stanley, T.L.; Clish, C.; Mootha, V.; Mensink, M.; Grinspoon, S.K.; Makimura, H. Associations between plasma branched-chain amino acids, β-aminoisobutyric acid and body composition. J. Nutr. Sci. 2016, 5, e6. [Google Scholar] [CrossRef] [PubMed]
- Floegel, A.; von Ruesten, A.; Drogan, D.; Schulze, M.B.; Prehn, C.; Adamski, J.; Pischon, T.; Boeing, H. Variation of serum metabolites related to habitual diet: A targeted metabolomic approach in EPIC-Potsdam. Eur. J. Clin. Nutr. 2013, 67, 1100–1108. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, N.; Mahbub, M.H.; Takahashi, H.; Hase, R.; Ishimaru, Y.; Sunagawa, H.; Amano, H.; Kobayashi-Miura, M.; Kanda, H.; Fujita, Y.; et al. Plasma free amino acid profiles evaluate risk of metabolic syndrome, diabetes, dyslipidemia, and hypertension in a large Asian population. Environ. Health Prev. Med. 2017, 22, 35. [Google Scholar] [CrossRef] [PubMed]
- Guasch-Ferre, M.; Hruby, A.; Toledo, E.; Clish, C.B.; Martinez-Gonzalez, M.A.; Salas-Salvado, J.; Hu, F.B. Metabolomics in Prediabetes and Diabetes: A Systematic Review and Meta-analysis. Diabetes Care 2016, 39, 833–846. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, M.; Lewis, G.D.; Ericson, U.; Orho-Melander, M.; Hedblad, B.; Engstrom, G.; Ostling, G.; Clish, C.; Wang, T.J.; Gerszten, R.E.; et al. A diabetes-predictive amino acid score and future cardiovascular disease. Eur. Heart J. 2013, 34, 1982–1989. [Google Scholar] [CrossRef] [PubMed]
- Siomkajło, M.; Rybka, J.; Mierzchała-Pasierb, M.; Gamian, A.; Stankiewicz-Olczyk, J.; Bolanowski, M.; Daroszewski, J. Specific plasma amino acid disturbances associated with metabolic syndrome. Endocrine 2017, 58, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.T. Metabolism of BCAAs. In Branched Chain Amino Acids in Clinical Nutrition: Volume 1; Rajendram, R., Preedy, V.R., Eds.; Springer: New York, NY, USA, 2015; pp. 13–24. [Google Scholar]
- Vaz, F.M.; Wanders, R.J. Carnitine biosynthesis in mammals. Biochem. J. 2002, 361, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Azemati, B.; Rajaram, S.; Jaceldo-Siegl, K.; Sabate, J.; Shavlik, D.; Fraser, G.E.; Haddad, E.H. Animal-Protein Intake Is Associated with Insulin Resistance in Adventist Health Study 2 (AHS-2) Calibration Substudy Participants: A Cross-Sectional Analysis. Curr. Dev. Nutr. 2017, 1, e000299. [Google Scholar] [CrossRef]
- Mirmiran, P.; Bahadoran, Z.; Esfandyari, S.; Azizi, F. Dietary Protein and Amino Acid Profiles in Relation to Risk of Dysglycemia: Findings from a Prospective Population-Based Study. Nutrients 2017, 9, 971. [Google Scholar] [CrossRef] [PubMed]
- Malik, V.S.; Li, Y.; Tobias, D.K.; Pan, A.; Hu, F.B. Dietary Protein Intake and Risk of Type 2 Diabetes in US Men and Women. Am. J. Epidemiol. 2016, 183, 715–728. [Google Scholar] [CrossRef] [PubMed]
- Layman, D.K.; Walker, D.A. Potential importance of leucine in treatment of obesity and the metabolic syndrome. J. Nutr. 2006, 136, 319S–323S. [Google Scholar] [CrossRef] [PubMed]
- Bouchard-Mercier, A.; Rudkowska, I.; Lemieux, S.; Couture, P.; Vohl, M.C. The metabolic signature associated with the Western dietary pattern: A cross-sectional study. J. Nutr. 2013, 12, 158. [Google Scholar] [CrossRef] [PubMed]
- Bhupathiraju, S.N.; Tucker, K.L. Coronary heart disease prevention: Nutrients, foods, and dietary patterns. Clin. Chim. Acta 2011, 412, 1493–1514. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B.; Rimm, E.B.; Stampfer, M.J.; Ascherio, A.; Spiegelman, D.; Willett, W.C. Prospective study of major dietary patterns and risk of coronary heart disease in men. Am. J. Clin. Nutr. 2000, 72, 912–921. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.T.; Schulze, M.; Manson, J.E.; Willett, W.C.; Hu, F.B. Dietary patterns, meat intake, and the risk of type 2 diabetes in women. Arch. Intern. Med. 2004, 164, 2235–2240. [Google Scholar] [CrossRef] [PubMed]
- Aadland, E.K.; Graff, I.E.; Lavigne, C.; Eng, Ø.; Paquette, M.; Holthe, A.; Mellgren, G.; Madsen, L.; Jacques, H.; Liaset, B. Lean Seafood Intake Reduces Postprandial C-peptide and Lactate Concentrations in Healthy Adults in a Randomized Controlled Trial with a Crossover Design. J. Nutr. 2016, 146, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Aadland, E.K.; Lavigne, C.; Graff, I.E.; Eng, O.; Paquette, M.; Holthe, A.; Mellgren, G.; Jacques, H.; Liaset, B. Lean-seafood intake reduces cardiovascular lipid risk factors in healthy subjects: Results from a randomized controlled trial with a crossover design. Am. J. Clin. Nutr. 2015, 102, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, M.; Portune, K.J.; Steuer, N.; Lan, A.; Cerrudo, V.; Audebert, M.; Dumont, F.; Mancano, G.; Khodorova, N.; Andriamihaja, M.; et al. Quantity and source of dietary protein influence metabolite production by gut microbiota and rectal mucosa gene expression: A randomized, parallel, double-blind trial in overweight humans. Am. J. Clin. Nutr. 2017, 106, 1005–1019. [Google Scholar] [CrossRef] [PubMed]
- Sawicki, C.M.; Livingston, K.A.; Obin, M.; Roberts, S.B.; Chung, M.; McKeown, N.M. Dietary Fiber and the Human Gut Microbiota: Application of Evidence Mapping Methodology. Nutrients 2017, 9, 125. [Google Scholar] [CrossRef] [PubMed]
- Graf, D.; Di Cagno, R.; Fak, F.; Flint, H.J.; Nyman, M.; Saarela, M.; Watzl, B. Contribution of diet to the composition of the human gut microbiota. Microb. Ecol. Health Dis. 2015, 26, 26164. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.; Kitai, T.; Hazen, S.L. Gut Microbiota in Cardiovascular Health and Disease. Circ. Res. 2017, 120, 1183–1196. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, H.K.; Gudmundsdottir, V.; Nielsen, H.B.; Hyotylainen, T.; Nielsen, T.; Jensen, B.A.H.; Forslund, K.; Hildebrand, F.; Prifti, E.; Falony, G.; et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 2016, 535, 376. [Google Scholar] [CrossRef] [PubMed]
| Men (n = 171) | Women (n = 127) | |||
|---|---|---|---|---|
| Age (years) | 44.5 | ±17.9 | 51.6 | ±15 |
| Body weight (kg) | 79.2 | ±10.2 | 64.3 | ±8.2 |
| BMI (kg/m2) | 24.4 | ±2.7 | 23.1 | ±2.9 |
| Body fat (%) | 23.0 | ±6.4 | 33.4 | ±6.6 |
| Blood pressure (mmHg) | ||||
| Systolic | 128.3 | ±14.2 | 120.3 | ±17.7 |
| Diastolic | 84.8 | ±10.6 | 83.4 | ±12.0 |
| Cholesterol (mg/dL) | ||||
| HDL | 62 | ±14 | 77 | ±16 |
| LDL | 123 | ±41 | 129 | ±35 |
| Food Group (g/day) | Spearmans’ rho | p |
|---|---|---|
| Sauces | 0.59 | <0.0001 |
| Sausages and meat products smoked | 0.58 | <0.0001 |
| Meat and meat products unsmoked | 0.56 | <0.0001 |
| Ice cream | 0.29 | <0.0001 |
| Eggs | 0.25 | <0.0001 |
| Cereals and cereal products | −0.22 | 0.0001 |
| Nuts and seeds | −0.25 | <0.0001 |
| Mushrooms | −0.26 | <0.0001 |
| Pulses | −0.29 | <0.0001 |
| Variable | Q1 (n = 74) | Q2 (n = 75) | Q3 (n = 75) | Q4 (n = 74) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Min | Max | Min | Max | Min | Max | p | |
| Adjusted first habitual dietary pattern score | −2.5 | −0.43 | −0.42 | 0.06 | 0.07 | 0.50 | 0.51 | 1.93 | <0.0001 |
| Mean | ±SD | Mean | SD | Mean | SD | Mean | SD | ||
| Val | 196.7 | ±51.7 | 198.4 | ±43.9 | 204.0 | ±43.4 | 210.2 | ±44.7 | <0.0001 |
| Leu | 140.6 | ±28.5 | 141.3 | ±28.0 | 153.3 | ±32.5 | 161.3 | ±27.8 | 0.03 |
| Ile | 73.2 | ±15.3 | 73.8 | ±16.0 | 78.8 | ±18.1 | 83.8 | ±15.3 | <0.0001 |
| ∑ BCAA | 410.5 | ±86.2 | 413.5 | ±76.5 | 436.2 | ±81.3 | 455.3 | ±69.8 | 0.0002 |
| Phe | 55.9 | ±8.2 | 56.7 | ±6.7 | 59.0 | ±8.1 | 60.1 | ±8.9 | 0.0001 |
| Trp | 63.4 | ±12.8 | 62.8 | ±9.9 | 68.1 | ±13.4 | 67.7 | ±12.4 | 0.0010 |
| Tyr | 66.2 | ±13.5 | 67.9 | ±11.0 | 72.4 | ±16.1 | 70.3 | ±11.1 | 0.0003 |
| ∑ AAA | 185.5 | ±28.3 | 187.4 | ±20.6 | 199.5 | ±30.3 | 198.2 | ±25.7 | <0.0001 |
| Energy intake (kcal/day) | 2432 | ±460 | 2350 | ±464 | 2334 | ±457 | 2474 | ±497 | 0.90 |
| Macronutrient intake (E%) | |||||||||
| CHO | 45.4 | ±4.4 | 45.4 | ±4.5 | 43.9 | ±4.1 | 42.7 | ±4.7 | <0.0001 |
| Monosaccharides | 8.1 | ±2.4 | 8.2 | ±2.5 | 7.6 | ±2.2 | 7.3 | ±1.9 | 0.02 |
| Disaccharides | 11.7 | ±2.2 | 12.4 | ±2.4 | 11.9 | ±2.3 | 11.6 | ±2.3 | 0.75 |
| Polysaccharides | 23.1 | ±3.1 | 22.5 | ±3.7 | 22.1 | ±3.5 | 21.7 | ±4.1 | 0.003 |
| Fat | 36.7 | ±4.1 | 36.7 | ±3.9 | 37.4 | ±3.7 | 38.1 | ±3.8 | 0.001 |
| SFA | 15.7 | ±2.5 | 15.9 | ±2.2 | 16.6 | ±2.3 | 16.5 | ±2.0 | 0.0001 |
| MUFA | 12.2 | ±1.5 | 12.2 | ±1.7 | 12.5 | ±1.4 | 13.1 | ±1.8 | <0.0001 |
| PUFA | 5.9 | ±1.1 | 5.7 | ±1.0 | 5.6 | ±0.6 | 5.7 | ±0.8 | 0.047 |
| Protein | 14.3 | ±1.3 | 14.4 | ±1.2 | 14.6 | ±1.2 | 15.1 | ±1.5 | 0.0015 |
| Animal-based protein | 6.5 | ±1.6 | 7.2 | ±1.3 | 7.6 | ±1.4 | 8.4 | ±1.5 | <0.0001 |
| Plant-based protein | 5.2 | ±1.0 | 4.9 | ±0.8 | 4.7 | ±0.7 | 4.5 | ±0.7 | <0.0001 |
| Animal/plant protein ratio | 1.3 | ±0.5 | 1.5 | ±0.5 | 1.7 | ±0.5 | 1.9 | ±0.4 | <0.0001 |
| Alcohol | 2.41 | ±1.92 | 2.09 | ±2.07 | 3.43 | ±2.96 | 2.95 | ±2.71 | 0.05 |
| Dietary fiber (g/MJ) | 2.8 | ±0.6 | 2.7 | ±0.5 | 2.6 | ±0.5 | 2.4 | ±0.5 | <0.0001 |
| Spearmans’ rho | p | |
|---|---|---|
| Ala | 0.15 | 0.01 |
| Arg | 0.01 | 0.89 |
| Asn | −0.10 | 0.09 |
| Asp | 0.01 | 0.82 |
| Gln | −0.02 | 0.70 |
| His | 0.01 | 0.82 |
| Lys | 0.23 | <0.0001 |
| Met | 0.08 | 0.18 |
| Orn | 0.04 | 0.47 |
| Phe | 0.22 | 0.0001 |
| Pro | 0.08 | 0.18 |
| Ser | −0.03 | 0.67 |
| Thr | 0.04 | 0.51 |
| Trp | 0.17 | 0.003 |
| Tyr | 0.20 | 0.0005 |
| Ile | 0.31 | <0.0001 |
| Leu | 0.33 | <0.0001 |
| Val | 0.15 | 0.01 |
| ∑ BCAA | 0.27 | <0.0001 |
| ∑ AAA | 0.23 | <0.0001 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merz, B.; Frommherz, L.; Rist, M.J.; Kulling, S.E.; Bub, A.; Watzl, B. Dietary Pattern and Plasma BCAA-Variations in Healthy Men and Women—Results from the KarMeN Study. Nutrients 2018, 10, 623. https://doi.org/10.3390/nu10050623
Merz B, Frommherz L, Rist MJ, Kulling SE, Bub A, Watzl B. Dietary Pattern and Plasma BCAA-Variations in Healthy Men and Women—Results from the KarMeN Study. Nutrients. 2018; 10(5):623. https://doi.org/10.3390/nu10050623
Chicago/Turabian StyleMerz, Benedikt, Lara Frommherz, Manuela J. Rist, Sabine E. Kulling, Achim Bub, and Bernhard Watzl. 2018. "Dietary Pattern and Plasma BCAA-Variations in Healthy Men and Women—Results from the KarMeN Study" Nutrients 10, no. 5: 623. https://doi.org/10.3390/nu10050623





