Relationship of a Special Acidified Milk Protein Drink with Cognitive Performance: A Randomized, Double-Blind, Placebo-Controlled, Crossover Study in Healthy Young Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
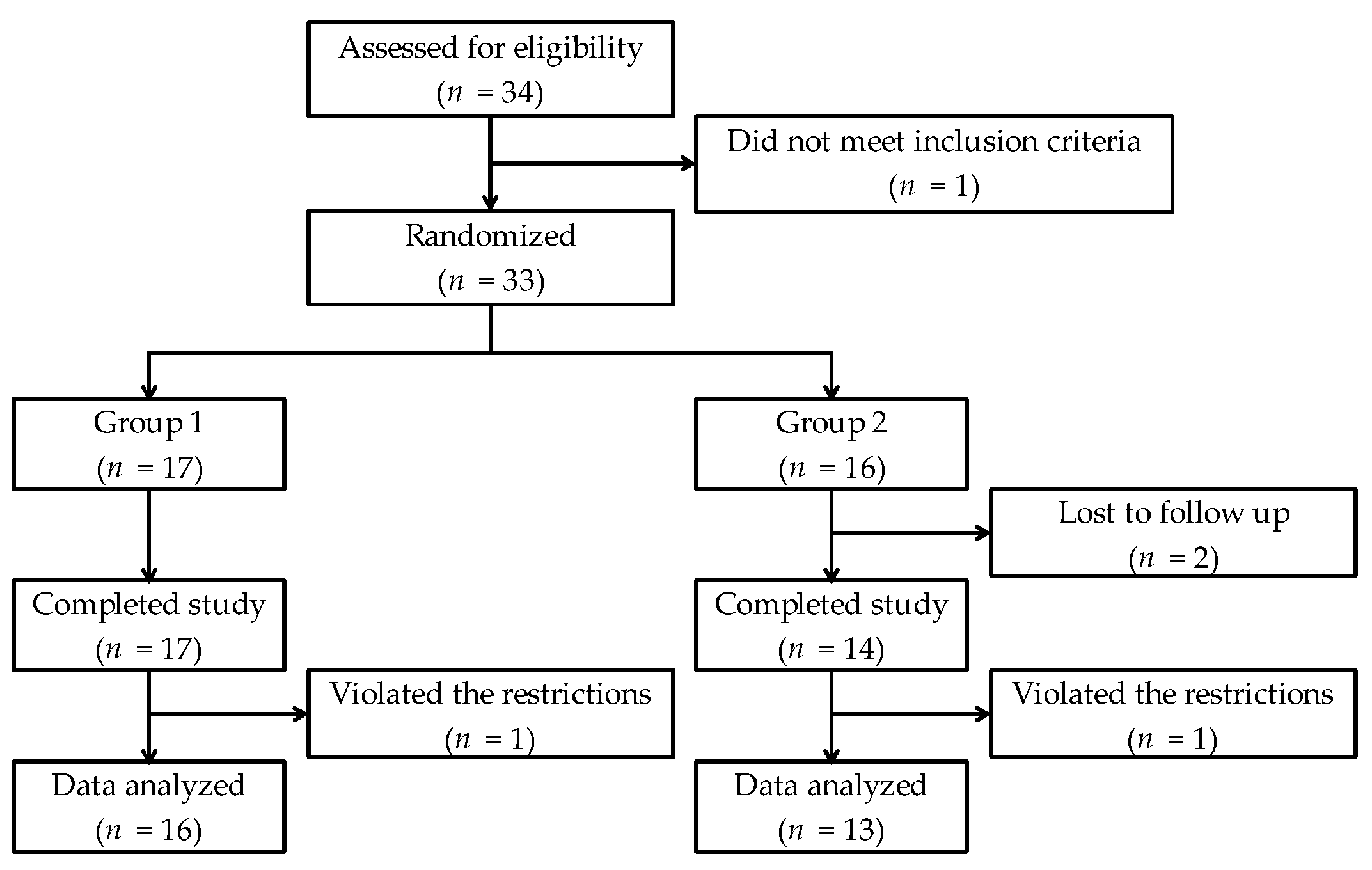
2.2. Subjects
2.3. Test Drinks
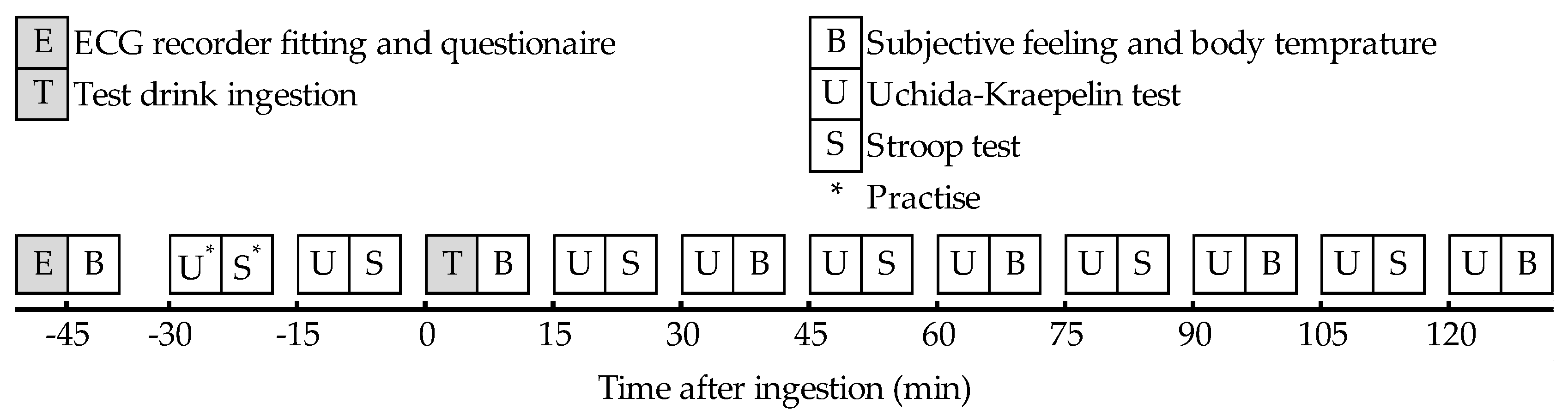
2.4. Study Procedure
2.5. Psychological Measurement
2.5.1. Uchida–Kraepelin Test (UKT)
2.5.2. Stroop Test
2.5.3. Subjective Feeling
2.6. Physiological Measurements
2.6.1. Heart Rate Variability (HRV)
2.6.2. Body Temperature
2.7. Statistical Analysis
3. Results
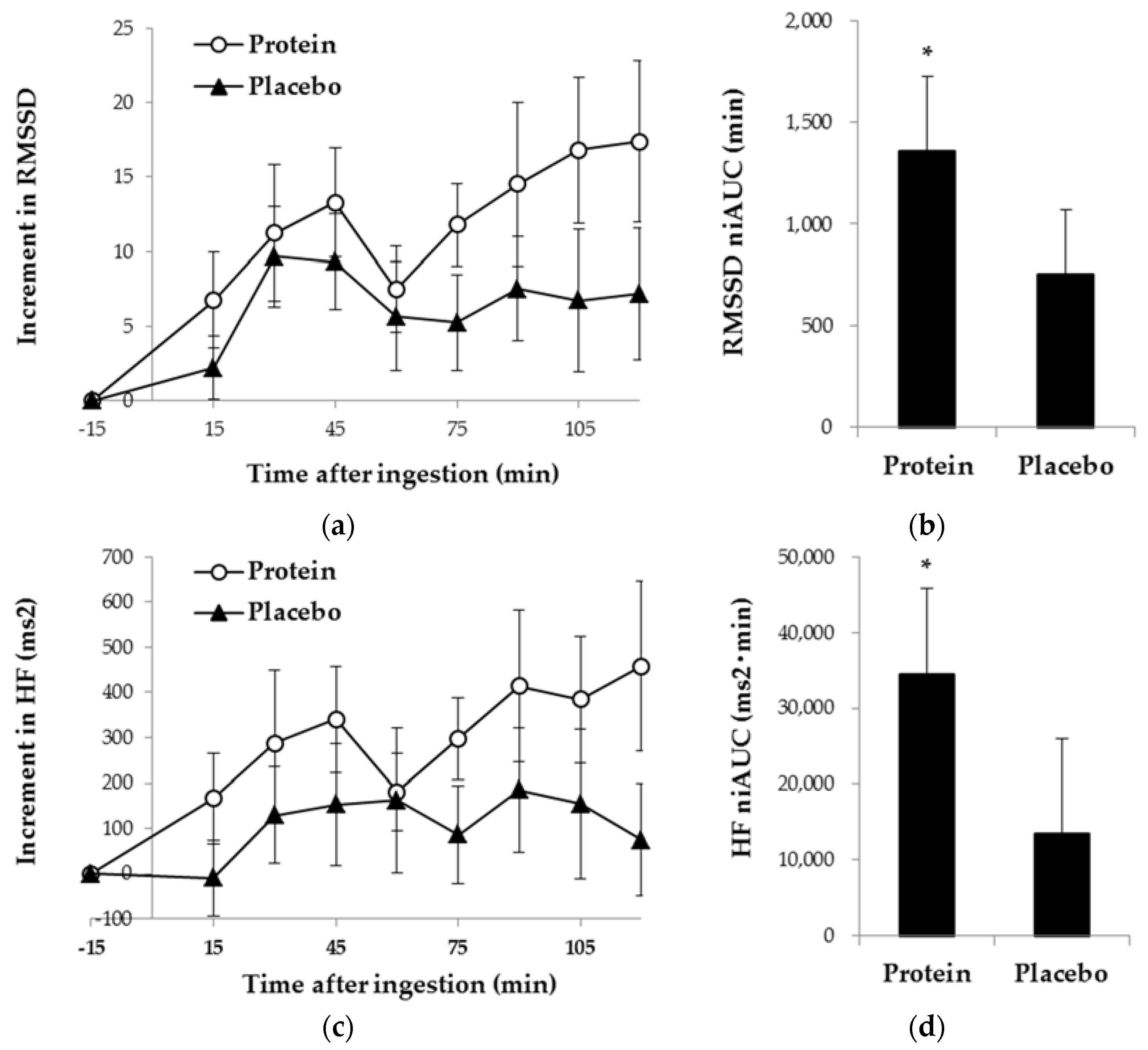
3.1. Psychological Measurements
3.2. Physiological Measurements
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nicklas, T.A.; O’Neil, C.E.; Berenson, G.S. Nutrient contribution of breakfast, secular trends, and the role of ready-to-eat cereals: A review of data from the bogalusa heart study. Am. J. Clin. Nutr. 1998, 67, 757s–763s. [Google Scholar] [CrossRef] [PubMed]
- Dykstra, H.; Davey, A.; Fisher, J.O.; Polonsky, H.; Sherman, S.; Abel, M.L.; Dale, L.C.; Foster, G.D.; Bauer, K.W. Breakfast-skipping and selecting low-nutritional-quality foods for breakfast are common among low-income urban children, regardless of food security status. J. Nutr. 2016, 146, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Edefonti, V.; Rosato, V.; Parpinel, M.; Nebbia, G.; Fiorica, L.; Fossali, E.; Ferraroni, M.; Decarli, A.; Agostoni, C. The effect of breakfast composition and energy contribution on cognitive and academic performance: A systematic review. Am. J. Clin. Nutr. 2014, 100, 626–656. [Google Scholar] [CrossRef] [PubMed]
- Hoyland, A.; Lawton, C.L.; Dye, L. Acute effects of macronutrient manipulations on cognitive test performance in healthy young adults: A systematic research review. Neurosci. Biobehav. Rev. 2008, 32, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Messier, C. Glucose improvement of memory: A review. Eur. J. Pharmacol. 2004, 490, 33–57. [Google Scholar] [CrossRef] [PubMed]
- Riby, L.M. The impact of age and task domain on cognitive performance: A meta-analytic review of the glucose facilitation effect. Brain Impair. 2004, 5, 145–165. [Google Scholar] [CrossRef]
- Muldoon, M.F.; Ryan, C.M.; Yao, J.K.; Conklin, S.M.; Manuck, S.B. Long-chain omega-3 fatty acids and optimization of cognitive performance. Mil. Med. 2014, 179, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Stonehouse, W. Does consumption of lc omega-3 pufa enhance cognitive performance in healthy school-aged children and throughout adulthood? Evidence from clinical trials. Nutrients 2014, 6, 2730–2758. [Google Scholar] [CrossRef] [PubMed]
- Ota, M.; Matsuo, J.; Ishida, I.; Hattori, K.; Teraishi, T.; Tonouchi, H.; Ashida, K.; Takahashi, T.; Kunugi, H. Effect of a ketogenic meal on cognitive function in elderly adults: Potential for cognitive enhancement. Psychopharmacology 2016, 233, 3797–3802. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, L.H.; Kondrup, J.; Zellner, M.; Tetens, I.; Roth, E. Effect of a high protein meat diet on muscle and cognitive functions: A randomised controlled dietary intervention trial in healthy men. Clin. Nutr. 2011, 30, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Van der Zwaluw, N.L.; van de Rest, O.; Tieland, M.; Adam, J.J.; Hiddink, G.J.; van Loon, L.J.; de Groot, L.C. The impact of protein supplementation on cognitive performance in frail elderly. Eur. J. Nutr. 2014, 53, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.; Colombani, P.C.; Langhans, W.; Wenk, C. Carbohydrate to protein ratio in food and cognitive performance in the morning. Phys. Behav. 2002, 75, 411–423. [Google Scholar] [CrossRef]
- Zeng, Y.-C.; Li, S.-M.; Xiong, G.-L.; Su, H.-M.; Wan, J.-C. Influences of protein to energy ratios in breakfast on mood, alertness and attention in the healthy undergraduate students. Health 2011, 3, 383. [Google Scholar] [CrossRef]
- Beasley, J.M.; Deierlein, A.; Morland, K.; Granieri, E.; Spark, A. Is meeting the recommended dietary allowance (RDA) for protein related to body composition among older adults? Results from the cardiovascular health of seniors and built environment study. J. Nutr. Health Aging 2016, 20, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Sumida, S.; Iwamoto, J.; Kamide, N.; Otani, T. Evaluation of bone, nutrition, and physical function in shorinji kempo athletes. Open Access J. Sports Med. 2012, 3, 107. [Google Scholar] [PubMed]
- Park, K.M.; Fulgoni, V.L. The association between dairy product consumption and cognitive function in the national health and nutrition examination survey. Br. J. Nutr. 2013, 109, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Crichton, G.E.; Elias, M.F.; Dore, G.A.; Robbins, M.A. Relation between dairy food intake and cognitive function: The maine-syracuse longitudinal study. Int. Dairy J. 2012, 22, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.R.; Hawkins, M.A.; Updegraff, J.; Gunstad, J.; Spitznagel, M.B. Baseline glucoregulatory function moderates the effect of dairy milk and fruit juice on postprandial cognition in healthy young adults. Eur. J. Nutr. 2017. [Google Scholar] [CrossRef] [PubMed]
- Rutherfurd, S.M.; Fanning, A.C.; Miller, B.J.; Moughan, P.J. Protein digestibility-corrected amino acid scores and digestible indispensable amino acid scores differentially describe protein quality in growing male rats. J. Nutr. 2015, 145, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, G. The protein digestibility-corrected amino acid score method overestimates quality of proteins containing antinutritional factors and of poorly digestible proteins supplemented with limiting amino acids in rats. J. Nutr. 1997, 127, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Mahe, S.; Roos, N.; Benamouzig, R.; Davin, L.; Luengo, C.; Gagnon, L.; Gausserges, N.; Rautureau, J.; Tome, D. Gastrojejunal kinetics and the digestion of (15N)beta-lactoglobulin and casein in humans: The influence of the nature and quantity of the protein. Am. J. Clin. Nutr. 1996, 63, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, K.; Kanda, A.; Tagawa, R.; Sanbongi, C.; Ikegami, S.; Itoh, H. Post-exercise muscle protein synthesis in rats after ingestion of acidified bovine milk compared with skim milk. Nutrients 2017, 9, E1071. [Google Scholar]
- Higuchi, T.; Hamada, K.; Imazuya, S.; Irie, S. The effect of breakfast omission and breakfast type on body temperature, mood and intellectual performance. J. Jpn. Soc. Clin. Nutr. 2007, 29, 35–43. [Google Scholar]
- Wright, K.P., Jr.; Hull, J.T.; Czeisler, C.A. Relationship between alertness, performance, and body temperature in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 283, R1370–R1377. [Google Scholar] [CrossRef] [PubMed]
- Cariou, M.; Galy, E.; Melan, C. Differential 24-hour variation of alertness and subjective tension in process controllers: Investigation of the relationship with body temperature and heart rate. Chronobiol. Int. 2008, 25, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Holzman, J.B.; Bridgett, D.J. Heart rate variability indices as bio-markers of top-down self-regulatory mechanisms: A meta-analytic review. Neurosci. Biobehav. Rev. 2017, 74, 233–255. [Google Scholar] [CrossRef] [PubMed]
- Draghici, A.E.; Taylor, J.A. The physiological basis and measurement of heart rate variability in humans. J. Physiol. Anthropol. 2016, 35, 22. [Google Scholar] [CrossRef] [PubMed]
- Sundstrom Poromaa, I.; Gingnell, M. Menstrual cycle influence on cognitive function and emotion processing-from a reproductive perspective. Front. Neurosci. 2014, 8, 380. [Google Scholar]
- Jones, B.; Kenward, M.G. Design and Analysis of Cross-Over Trials, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Wellek, S.; Blettner, M. On the proper use of the crossover design in clinical trials: Part 18 of a series on evaluation of scientific publications. Deutsch. Ärzteblatt Int. 2012, 109, 276. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Galioto, R.; Spitznagel, M.B. The effects of breakfast and breakfast composition on cognition in adults. Adv. Nutr. 2016, 7, 576s–589s. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.; Hassinger, L.; Davis, J.; Devor, S.T.; DiSilvestro, R.A. A randomized, double blind, placebo controlled study of spirulina supplementation on indices of mental and physical fatigue in men. Int. J. Food Sci. Nutr. 2016, 67, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Fujimaki, K.; Takemoto, H.; Morinobu, S. Cortical activation changes and sub-threshold affective symptoms are associated with social functioning in a non-clinical population: A multi-channel near-infrared spectroscopy study. Psychiatry Res. 2016, 248, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, M.; Ishizaki, T.; Maruyama, T.; Takatsuka, Y.; Kuboki, T. Effect of dried-bonito broth on mental fatigue and mental task performance in subjects with a high fatigue score. Physiol. Behav. 2007, 92, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Yasumasu, T.; Reyes Del Paso, G.A.; Takahara, K.; Nakashima, Y. Reduced baroreflex cardiac sensitivity predicts increased cognitive performance. Psychophysiology 2006, 43, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Sorqvist, P. On interpretation and task selection in studies on the effects of noise on cognitive performance. Front. Psychol. 2014, 5, 1249. [Google Scholar] [PubMed]
- Feder, K.P.; Majnemer, A. Handwriting development, competency, and intervention. Dev. Med. Child Neurol. 2007, 49, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.A.; Emory, E. Executive function and the frontal lobes: A meta-analytic review. Neuropsychol. Rev. 2006, 16, 17–42. [Google Scholar] [CrossRef] [PubMed]
- Halton, T.L.; Hu, F.B. The effects of high protein diets on thermogenesis, satiety and weight loss: A critical review. J. Am. Coll. Nutr. 2004, 23, 373–385. [Google Scholar] [CrossRef] [PubMed]
- McCraty, R.; Atkinson, M.; Tomasino, D.; Bradley, R.T. The Coherent Heart Heart-Brain Interactions, Psychophysiological Coherence, and the Emergence of System-Wide Order. Available online: http://203.187.160.134:9011/www.integral-review.org/c3pr90ntc0td/issues/vol_5_no_2_mccraty_et_al_the_coherent_heart.pdf (accessed on 13 April 2018).
- Shaffer, F.; McCraty, R.; Zerr, C.L. A healthy heart is not a metronome: An integrative review of the heart’s anatomy and heart rate variability. Front. Psychol. 2014, 5, 1040. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.L.; Johnsen, B.H.; Sollers, J.J., 3rd; Stenvik, K.; Thayer, J.F. Heart rate variability and its relation to prefrontal cognitive function: The effects of training and detraining. Eur. J. Appl. Physiol. 2004, 93, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.L.; Johnsen, B.H.; Thayer, J.F. Vagal influence on working memory and attention. Int. J. Psychophysiol. 2003, 48, 263–274. [Google Scholar] [CrossRef]
- Hansen, A.L.; Johnsen, B.H.; Thayer, J.F. Relationship between heart rate variability and cognitive function during threat of shock. Anxiety Stress Coping 2009, 22, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Saus, E.-R.; Johnsen, B.H.; Eid, J.; Riisem, P.K.; Andersen, R.; Thayer, J.F. The effect of brief situational awareness training in a police shooting simulator: An experimental study. Mil. Psychol. 2006, 18, S3. [Google Scholar] [CrossRef]
- Thayer, J.F.; Hansen, A.L.; Sollers, J.; Johnsen, B.H. Heart rate variability as an index of prefrontal neural function in military settings. Biomonit. Physiol. Cognit. Perform. During Mil. Oper. 2005, 5797, 71–77. [Google Scholar]
- Johnsen, B.H.; Thayer, J.F.; Laberg, J.C.; Wormnes, B.; Raadal, M.; Skaret, E.; Kvale, G.; Berg, E. Attentional and physiological characteristics of patients with dental anxiety. J. Anxiety Dis. 2003, 17, 75–87. [Google Scholar] [CrossRef]
- Richard Jennings, J.; Allen, B.; Gianaros, P.J.; Thayer, J.F.; Manuck, S.B. Focusing neurovisceral integration: Cognition, heart rate variability, and cerebral blood flow. Psychophysiology 2015, 52, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.; Thayer, J.F.; Khalsa, S.S.; Lane, R.D. The hierarchical basis of neurovisceral integration. Neurosci. Biobehav. Rev. 2017, 75, 274–296. [Google Scholar] [CrossRef] [PubMed]
- Fernstrom, J.D. Large neutral amino acids: Dietary effects on brain neurochemistry and function. Amino Acids 2013, 45, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Disilvio, B.; Fernstrom, M.H.; Fernstrom, J.D. Meal ingestion, amino acids and brain neurotransmitters: Effects of dietary protein source on serotonin and catecholamine synthesis rates. Physiol. Behav. 2009, 98, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, T.A.; Nguyen, J.C.; Polglaze, K.E.; Bertrand, P.P. Influence of tryptophan and serotonin on mood and cognition with a possible role of the gut-brain axis. Nutrients 2016, 8, 56. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Iwamoto, M.; Ogata, T.; Washida, K.; Sekine, K.; Takase, M.; Park, B.J.; Morikawa, T.; Miyazaki, Y. Effects of milk casein-derived peptides on absolute oxyhaemoglobin concentrations in the prefrontal area and on work efficiency after mental stress loading in male students. J. Int. Med. Res. 2008, 36, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Iwamoto, M.; Washida, K.; Sekine, K.; Takase, M.; Park, B.J.; Morikawa, T.; Miyazaki, Y. Influences of casein hydrolysate ingestion on cerebral activity, autonomic nerve activity, and anxiety. J. Physiol. Anthropol. 2010, 29, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Parsons, M.W.; Gold, P.E. Glucose enhancement of memory in elderly humans: An inverted-U dose-response curve. Neurobiol. Aging 1992, 13, 401–404. [Google Scholar] [CrossRef]
- Rizzo, M.R.; Marfella, R.; Barbieri, M.; Boccardi, V.; Vestini, F.; Lettieri, B.; Canonico, S.; Paolisso, G. Relationships between daily acute glucose fluctuations and cognitive performance among aged type 2 diabetic patients. Diabetes Care 2010, 33, 2169–2174. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.; Colombani, P.C.; Langhans, W.; Wenk, C. Cognitive performance and its relationship with postprandial metabolic changes after ingestion of different macronutrients in the morning. Br. J. Nutr. 2001, 85, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, M.A.; Gunstad, J.; Calvo, D.; Spitznagel, M.B. Higher fasting glucose is associated with poorer cognition among healthy young adults. Health Psychol. 2016, 35, 199. [Google Scholar] [CrossRef] [PubMed]
- Cook, R.L.; O’Dwyer, N.J.; Donges, C.E.; Parker, H.M.; Cheng, H.L.; Steinbeck, K.S.; Cox, E.P.; Franklin, J.L.; Garg, M.L.; Rooney, K.B.; et al. Relationship between obesity and cognitive function in young women: The food, mood and mind study. J. Obes. 2017, 2017, 5923862. [Google Scholar] [CrossRef] [PubMed]

| Acidified Milk Protein Drink | Placebo Drink | |
|---|---|---|
| Basic ingredients (%) 1 | ||
| Milk protein 2 | 4.4 | |
| Glucose 3 | 4.6 | 8.5 |
| Trehalose 4 | 1.0 | 1.0 |
| Fermented cellulose 5 | 0.05 | |
| Pectin 6 | 0.1 | |
| Soybean polysaccharide 7 | 0.45 | 0.45 |
| Citric acid 8 | 0.32 | 0.13 |
| Malic acid 9 | 0.27 | 0.11 |
| Sodium citrate 10 | 0.27 | |
| Vitamin B6 11 | 0.00019 | 0.00019 |
| Water | 88.7 | 89.0 |
| Nutrient (g) | ||
| Carbohydrate | 28 | 42 |
| Protein | 16 | 0 |
| Fat | 0.3 | 0.9 |
| Energy (kcal) | 176 | 176 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saito, Y.; Murata, N.; Noma, T.; Itoh, H.; Kayano, M.; Nakamura, K.; Urashima, T. Relationship of a Special Acidified Milk Protein Drink with Cognitive Performance: A Randomized, Double-Blind, Placebo-Controlled, Crossover Study in Healthy Young Adults. Nutrients 2018, 10, 574. https://doi.org/10.3390/nu10050574
Saito Y, Murata N, Noma T, Itoh H, Kayano M, Nakamura K, Urashima T. Relationship of a Special Acidified Milk Protein Drink with Cognitive Performance: A Randomized, Double-Blind, Placebo-Controlled, Crossover Study in Healthy Young Adults. Nutrients. 2018; 10(5):574. https://doi.org/10.3390/nu10050574
Chicago/Turabian StyleSaito, Yoshie, Natsuko Murata, Teruyuki Noma, Hiroyuki Itoh, Mitsunori Kayano, Kimihide Nakamura, and Tadasu Urashima. 2018. "Relationship of a Special Acidified Milk Protein Drink with Cognitive Performance: A Randomized, Double-Blind, Placebo-Controlled, Crossover Study in Healthy Young Adults" Nutrients 10, no. 5: 574. https://doi.org/10.3390/nu10050574





