Postnatal Growth Disadvantage of the Small for Gestational Age Preterm Twins
Abstract
:1. Introduction
2. Methods
2.1. Study Design
2.2. Statistics
3. Results
3.1. Postnatal Weight Characteristics
3.2. Postnatal Head Circumference Characteristics
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- American College of Obstetricians and Gynecologists. ACOG Practice bulletin No. 134: Fetal Growth Restriction. Obstet. Gynecol. 2013, 121, 1122–1133. [Google Scholar]
- Miller, J.; Chauhan, S.P.; Abuhamad, A.Z. Discordant twins: Diagnosis, evaluation and management. Am. J. Obstet Gynecol. 2012, 206, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Brennan, A.M.; Murphy, B.P.; Kiely, M.E. Optimising preterm nutrition: Present and future. Proc. Nutr. Soc. 2016, 75, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Raiten, D.J.; Steiber, A.L.; Hand, R.K. Executive summary: Evaluation of the evidence to support practice guidelines for nutritional care of preterm infants—The Pre-B Project 1–4. Am. J. Clin. Nutr. 2016, 103, 599S–605S. [Google Scholar] [CrossRef] [PubMed]
- Iacobelli, S.; Viaud, M.; Lapillonne, A.; Robillard, P.Y.; Gouyon, J.B.; Bonsante, F.; NUTRIQUAL Group. Nutrition practice, compliance to guidelines and postnatal growth in moderately premature babies: The NUTRIQUAL French survey. BMC Pediatr. 2015, 15, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gill, A.; Yu, V.Y.; Bajuk, B.; Astbury, J. Postnatal growth in infants born before 30 weeks’ gestation. Arch. Dis. Child. 1986, 61, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, S.G.; Quimiro, C.L.; Anderson, J.V.; Hall, R.T. Postnatal Weight Changes in Low Birth Weight Infants. Pediatrics 1987, 79, 702–705. [Google Scholar] [PubMed]
- Ehrenkranz, R.A. Growth in the Neonatal Intensive Care Unit Influences Neurodevelopmental and Growth Outcomes of Extremely Low Birth Weight Infants. Pediatrics 2006, 117, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.H.; Thomas, P.; Peabody, J. Extrauterine Growth Restriction Remains a Serious Problem in Prematurely Born Neonates. Pediatrics 2003, 111, 986–990. [Google Scholar] [CrossRef] [PubMed]
- Franz, A.R.; Pohlandt, F.; Bode, H.; Mihatsch, W.A.; Sander, S.; Kron, M.; Steinmacher, J. Intrauterine, Early Neonatal, and Postdischarge Growth and Neurodevelopmental Outcome at 5.4 Years in Extremely Preterm Infants After Intensive Neonatal Nutritional Support. Pediatrics 2009, 123, e101–e109. [Google Scholar] [CrossRef] [PubMed]
- Casey, P.H.; Whiteside-Mansell, L.; Barrett, K.; Bradley, R.H.; Gargus, R. Impact of Prenatal and/or Postnatal Growth Problems in Low Birth Weight Preterm Infants on School-Age Outcomes: An 8-Year Longitudinal Evaluation. Pediatrics 2006, 118, 1078–1086. [Google Scholar] [CrossRef] [PubMed]
- Horta, B.L.; Sibbritt, D.W.; Lima, R.C.; Victora, C.G. Weight catch-up and achieved schooling at 18 years of age in Brazilian males. Eur. J. Clin. Nutr. 2009, 63, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Pylipow, M.; Spector, L.G.; Puumala, S.E.; Boys, C.; Cohen, J.; Georgieff, M.K. Early Postnatal Weight Gain, Intellectual Performance, and Body Mass Index at 7 Years of Age in Term Infants with Intrauterine Growth Restriction. J. Pediatr. 2009, 154, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Martorell, R.; Horta, B.L.; Adair, L.S.; Stein, A.D.; Richter, L.; Fall, C.H.D.; Bhargava, S.K.; Biswas, S.K.; Perez, L.; Barros, F.C.; et al. Weight Gain in the First Two Years of Life Is an Important Predictor of Schooling Outcomes in Pooled Analyses from Five Birth Cohorts from Low- and Middle-Income Countries. J. Nutr. 2010, 140, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Varella, M.H.; Moss, W.J. Early growth patterns are associated with intelligence quotient scores in children born small-for-gestational age. Early Hum. Dev. 2015, 91, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Fenton, T.R.; Kim, J.H. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013, 13, 59. [Google Scholar] [CrossRef] [PubMed]
- West, J.; Manchester, B.; Wright, J.; Lawlor, D.A.; Waiblinger, D. Reliability of routine clinical measurements of neonatal circumferences and research measurements of neonatal skinfold thicknesses: Findings from the Born in Bradford study. Paediatr. Perinat. Epidemiol. 2011, 25, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.S.; Wong, K.Y.; Merko, S.; Bishara, R.; Dunn, M.; Asztalos, E.; Darling, P.B. Postnatal growth failure in preterm infants: Ascertainment and relation to long-term outcome. J. Perinat. Med. 2006, 34, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Kliegman, R.M.; Walsh, M.C. Neonatal necrotizing enterocolitis: Pathogenesis, classification, and spectrum of illness. Curr. Probl. Pediatr. 1987, 17, 219–288. [Google Scholar] [CrossRef]
- Ofek Shlomai, N.; Reichman, B.; Lerner-Geva, L.; Boyko, V.; Bar-Oz, B. Population-based study shows improved postnatal growth in preterm very-low-birthweight infants between 1995 and 2010. Acta Paediatr. 2014, 103, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Griffin, I.J.; Tancredi, D.J.; Bertino, E.; Lee, H.C.; Profit, J. Postnatal growth failure in very low birthweight infants born between 2005 and 2012. Arch. Dis. Child. Fetal Neonatal Ed. 2016, 101, F50–F55. [Google Scholar] [CrossRef] [PubMed]
- Leppanen, M.; Lapinleimu, H.; Lind, A.; Matomaki, J.; Lehtonen, L.; Haataja, L.; Rautava, P.; PIPARI Study Group. Antenatal and Postnatal Growth and 5-Year Cognitive Outcome in Very Preterm Infants. Pediatrics 2014, 133, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Lemons, J.A.; Bauer, C.R.; Oh, W.; Korones, S.B.; Papile, L.A.; Stoll, B.J.; Verter, J.; Temprosa, M.; Wright, L.L.; Ehrenkranz, R.A.; et al. Very low birth weight outcomes of the National Institute of Child health and human development neonatal research network, January 1995 through December 1996. NICHD Neonatal Research Network. Pediatrics 2001, 107, E1. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, M.; Itabashi, K.; Sato, Y.; Hibino, S.; Mizuno, K. Extrauterine growth restriction in preterm infants of gestational age ≤32 weeks. Pediatr. Int. 2008, 50, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Radmacher, P.G.; Looney, S.W.; Rafail, S.T.; Adamkin, D.H. Prediction of extrauterine growth retardation (EUGR) in VVLBW infants. J. Perinatol. 2003, 23, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Marks, K.; Reichman, B.; Lusky, A.; Zmora, E. Fetal growth and postnatal growth failure in very-low-birthweight infants. Acta Paediatr. 2006, 95, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Cooke, R.J.; Ainsworth, S.B.; Fenton, A.C. Postnatal growth retardation: A universal problem in preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 2004, 89, 428–430. [Google Scholar] [CrossRef] [PubMed]
- Cooke, R. Postnatal growth in preterm infants: Have we got it right? J. Perinatol. 2005, 25, S12–S14. [Google Scholar] [CrossRef] [PubMed]
- Guellec, I.; Lapillonne, A.; Marret, S.; Picaud, J.C.; Mitanchez, D.; Charkaluk, M.L.; Fresson, J.; Arnaud, C.; Flamand, C.; Cambonie, G.; et al. Effect of Intra- and Extrauterine Growth on Long-Term Neurologic Outcomes of Very Preterm Infants. J. Pediatr. 2016, 175, 93.e1–99.e1. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Chen, Y.; Ye, J.; Ouyang, F.; Jiang, F.; Zhang, J. The optimal postnatal growth trajectory for term small for gestational age babies: A prospective cohort study. J. Pediatr. 2015, 166, 54.e3–58.e3. [Google Scholar] [CrossRef] [PubMed]
- Çamurdan, M.O.; Çamurdan, A.D.; Polat, S.; Beyazova, U. Growth patterns of large, small, and appropriate for gestational age infants: Impacts of long-term breastfeeding: A retrospective cohort study. J. Pediatr. Endocrinol. Metab. 2011, 24, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.R.; Brown, Y.F.; Ehrenkranz, R.A.; O’Shea, T.M.; Allred, E.N.; Belfort, M.B.; McCormick, M.C.; Leviton, A.; Extremely Low Gestational Age Newborns Study Investigators. Nutritional Practices and Growth Velocity in the First Month of Life in Extremely Premature Infants. Pediatrics 2009, 124, 649–657. [Google Scholar]
- Neubauer, V.; Griesmaier, E.; Pehböck-Walser, N.; Pupp-Peglow, U.; Kiechl-Kohlendorfer, U. Poor postnatal head growth in very preterm infants is associated with impaired neurodevelopment outcome. Acta Paediatr. Int. J. Paediatr. 2013, 102, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Cheong, J.L.Y.; Hunt, R.W.; Anderson, P.J.; Howard, K.; Thompson, D.K.; Wang, H.X.; Bear, M.J.; Inder, T.E.; Doyle, L.W. Head Growth in Preterm Infants: Correlation with Magnetic Resonance Imaging and Neurodevelopmental Outcome. Pediatrics 2008, 121, e1534–e1540. [Google Scholar] [CrossRef] [PubMed]
- Ranke, M.B.; Krägeloh-Mann, I.; Vollmer, B. Growth, head growth, and neurocognitive outcome in children born very preterm: Methodological aspects and selected results. Dev. Med. Child. Neurol. 2015, 57, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Raghuram, K.; Yang, J.; Church, P.T.; Cieslak, Z.; Synnes, A.; Mukerji, A.; Shah, P.S.; Canadian Neonatal Network; Canadian Neonatal Follow-Up Network Investigators. Head Growth Trajectory and Neurodevelopmental Outcomes in Preterm Neonates. Pediatrics 2017, 140, e20170216. [Google Scholar] [CrossRef] [PubMed]
- Regev, R.H.; Arnon, S.; Litmanovitz, I.; Bauer-Rusek, S.; Boyko, V.; Lerner-Geva, L.; Reichman, B.; Israel Neonatal Network. Association between neonatal morbidities and head growth from birth until discharge in very-low-birthweight infants born preterm: A population-based study. Dev. Med. Child. Neurol. 2016, 58, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; McKay, K.; Rosenkrantz, T.S.; Hussain, N. Comparisons of mortality and pre-discharge respiratory outcomes in small-for-gestational-age and appropriate-for-gestational-age premature infants. BMC Pediatr. 2004, 4, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giapros, V.; Drougia, A.; Krallis, N.; Theocharis, P.; Andronikou, S. Morbidity and mortality patterns in small-for-gestational age infants born preterm. J. Matern. Fetal Neonatal Med. 2012, 25, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Turitz, A.L.; Gyamfi-Bannerman, C. Comparison of Respiratory Outcomes between Preterm Small-For-Gestational-Age and Appropriate-For-Gestational-Age Infants. Am. J. Perinatol. 2017, 34, 283–288. [Google Scholar] [PubMed]
- Villar, J.; Giuliani, F.; Bhutta, Z.A.; Bertino, E.; Ohuma, E.O.; Ismail, L.C.; Altman, D.G.; Victora, C.; Noble, J.A.; Gravett, M.G.; et al. Postnatal growth standards for preterm infants: The Preterm Postnatal Follow-up Study of the INTERGROWTH-21stProject. Lancet Glob. Health 2015, 3, e681–e691. [Google Scholar] [CrossRef]
- Tuzun, F.; Yucesoy, E.; Baysal, B.; Kumral, A.; Duman, N.; Ozkan, H. Comparison of INTERGROWTH-21 and Fenton growth standards to assess size at birth and extrauterine growth in very preterm infants. J. Matern. Fetal Neonatal Med. 2017, 1–6. [Google Scholar] [CrossRef] [PubMed]


| Maternal Characteristics | n = 83 |
|---|---|
| Median maternal age at delivery, years (min–max) | 32.5 (21–52) |
| In vitro fertilization, n (%) | 41 (49.4) |
| First pregnancy, n (%) | 49 (59.0) |
| Gestational diabetes, n (%) | 10 (12.0) |
| Prenatal steroids, n (%) | 68 (81.9) |
| Maternal hypertension during pregnancy, n (%) | 14 (16.9) |
| Fetal indication for delivery, n (%) | 40 (48.2) |
| Spontaneous preterm labor, n (%) | 32 (38.6) |
| Surgical delivery, n (%) | 71 (85.5) |
| Median gestational age, weeks (min–max), | 33.6 (30.9–34.9) |
| Discordance < 10%, n (%) | 3 (3.6) |
| Discordance 11–15%, n (%) | 9 (10.8) |
| Discordance 16–20%, n (%) | 14 (16.9) |
| Discordance ≥ 20%, n (%) | 57 (68.7) |
| SGA | AGA | p Value | |
|---|---|---|---|
| Male, n (%) | 48 (57.8) | 49 (59.0) | 0.077 |
| Minimal weight loss in % mean (SD) | 7.6 (13.1) | 8.8 (3.7) | 0.404 |
| Apgar 1 < 6, n (%) | 10 (12.0) | 4 (4.8) | <0.001 |
| Hypoglycemia (<45 mg/dL within the first 72 h), n (%) | 12 (14.5) | 3 (3.6) | 0.009 |
| Admission temperature, mean (SD) (Celsius) | 35.4 (0.7) | 35.8 (1.3) | <0.001 |
| Day of life to birth weight, mean (SD) | 9.1 (4.5) | 12.0 (4.6) | <0.001 |
| Duration of antibiotics (d), mean (SD) | 4.0 (5.7) | 3.1 (5.2) | 0.253 |
| Hospitalization duration (d), mean (SD) | 37.6 (19.7) | 23.2 (11.7) | <0.001 |
| Duration of fluids infusion (d), mean (SD) | 9.8 (9.1) | 5.1 (4.8) | <0.001 |
| Day of first enteral feeding | 3.1 (3.3) | 2.0 (1.1) | 0.007 |
| Total parenteral nutrition, n (%) | 65 (83.5) | 48 (60.8) | <0.001 |
| Total parenteral nutrition (d), mean (SD) | 9.0 (8.5) | 4.3 (4.8) | <0.001 |
| Any breastmilk at day of life 14, n (%) | 67 (71.3) | 64 (68.1) | 0.581 |
| Any breastmilk at 36 weeks, n (%) | 60 (63.2) | 60 (63.2) | 1 |
| Proven sepsis, n (%) | 16 (19.3) | 3 (3.6) | 0.002 |
| Oxygen treatment, n (%) | 22 (26.5) | 37 (44.6) | 0.006 |
| Duration of oxygen treatment (d), | 2.6 (11.9) | 2.2 (3.4) | 0.733 |
| Oxygen at 36 weeks, n (%) | 2 (2.4) | 0 | - |
| Necrotizing enterocolitis, n (%) | 4 (4.8) | 1 (1.2) | 0.375 |
| Severe morbidity *, n (%) | 19 (22.9) | 4 (4.8) | 0.001 |
| Weight Characteristics | SGA | AGA | p Value |
|---|---|---|---|
| Birth weight (g), median (Min–Max) Mean (SD) | 1284 (656–1830) 1291 (289) | 1906 (1192–2625) 1909 (304) | <0.001 |
| Birth weight (g), n (%) | |||
| >1500 | 22 (26.5) | 74 (89.2) | |
| 1000–1499 | 47 (56.6) | 9 (10.8) | <0.001 |
| <1000 | 14 (16.9) | 0 | |
| Birth weight z-score, mean (SD) | −1.90 ± 0.47 | −0.33 ± 0.50 | <0.001 |
| Weight at 36 wks (g), mean (SD) | 1548 ± 257 | 2138 ± 234 | <0.001 |
| Weight at 36 wks z-score, mean (SD) | −2.80 ± 0.72 | −1.29 ± 0.58 | <0.001 |
| Weight at 36 wks < 10%, n (%) | 83 (100) | 44 (53.0) | - |
| Weight at 36 wks < 3%, n (%) | 72 (90.0) | 10 (12.5) | <0.001 |
| ∆ z score weight from birth to 36 weeks, mean (SD) | −0.90 ± 0.46 | −0.96 ± 0.44 | 0.247 |
| ∆ z score decrease of 0 to 0.99 | 55 (66.3) | 50 (60.2) | |
| ∆ z score decrease of 1 to 1.99 | 25 (30.1) | 31 (37.3) | 0.012 |
| ∆ z score decrease of ≥ 2 | 3 (3.6) | 2 (2.4) | |
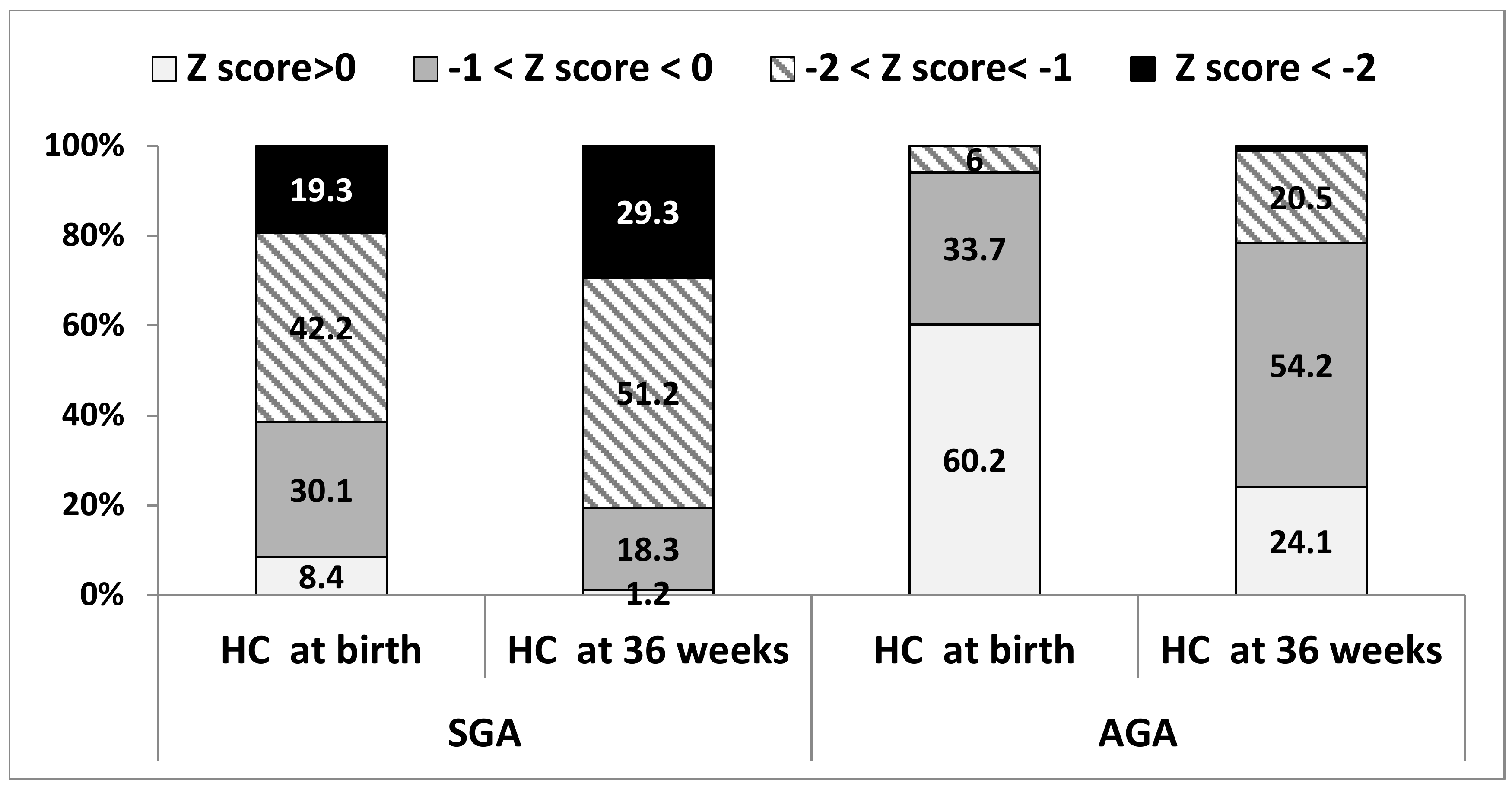
| HC at birth z-score, mean (SD) | −1.03 ± 2.42 | 0.26 ± 0.74 | <0.001 |
| HC at 36 wks z-score, mean (SD) | −1.67 ± 0.85 | −0.51 ± 0.65 | <0.001 |
| HC at birth < 10%, n (%) | 47 (57.3) | 1 (1.2) | <0.001 |
| HC at 36 wks < 10%, n (%) | 52 (63.4) | 9 (11.0) | <0.001 |
| ∆ z score HC from birth to 36 weeks, mean (SD) | −0.39 ± 0.72 | −0.76 ± 0.66 | 0.001 |
| ∆ z score decrease of 0 to 0.99 | 47 (59.5) | 55 (67.1) | |
| ∆ z score decrease of 1 to 1.99 | 6 (7.6) | 17 (20.7) | |
| ∆ z score decrease of ≥2 | 5 (6.3) | 3 (3.7) | 0.395 |
| Increased | 21 (26.6) | 7 (8.5) |
| SGA | AGA | |||||||
|---|---|---|---|---|---|---|---|---|
| Risk of Moderate or Severe PNGF (Unadjusted) | Risk of Moderate or Severe PNGF (Adjusted) | Risk of Moderate or Severe PNGF (Unadjusted) | Risk of Moderate or Severe PNGF (Adjusted) | |||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Gestational age | 0.30 | 0.18–0.52 | 0.26 | 0.0–0.69 | 0.70 | 0.47–1.06 | 0.49 | 0.20–1.21 |
| Birth weight | 0.99 | 0.99–0.99 | 1.01 | 0.99–1.00 | 1.00 | 0.99–1.00 | 1.01 | 1.00–1.01 |
| Gender | 2.53 | 1.00–6.42 | 4.28 | 0.97–18.80 | 1.36 | 0.56–3.32 | 0.27 | 0.07–1.02 |
| Sepsis | 3.25 | 1.06–9.97 | 3.01 | 0.63–14.47 | - | - | - | - |
| Oxygen treatment | 5.88 | 2.05–16.85 | 1.78 | 0.38–8.42 | 2.41 | 0.98–5.93 | 1.63 | 0.38–1.02 |
| Fluid infusion duration | 1.09 | 1.03–1.16 | 1.05 | 0.97–1.15 | 1.22 | 1.07–1.38 | 1.23 | 0.97–1.57 |
| Days to regain birth weight | 1.21 | 1.05–1.38 | 1.22 | 1.00–1.48 | 1.40 | 1.19–1.64 | 1.34 | 1.12–1.61 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morag, I.; Stern Levkovitz, O.; Siman-Tov, M.; Frisch, M.; Pinhas-Hamiel, O.; Strauss, T. Postnatal Growth Disadvantage of the Small for Gestational Age Preterm Twins. Nutrients 2018, 10, 476. https://doi.org/10.3390/nu10040476
Morag I, Stern Levkovitz O, Siman-Tov M, Frisch M, Pinhas-Hamiel O, Strauss T. Postnatal Growth Disadvantage of the Small for Gestational Age Preterm Twins. Nutrients. 2018; 10(4):476. https://doi.org/10.3390/nu10040476
Chicago/Turabian StyleMorag, Iris, Orly Stern Levkovitz, Maya Siman-Tov, Mor Frisch, Orit Pinhas-Hamiel, and Tzipi Strauss. 2018. "Postnatal Growth Disadvantage of the Small for Gestational Age Preterm Twins" Nutrients 10, no. 4: 476. https://doi.org/10.3390/nu10040476





