The Anti-Periodontitis Effects of Ethanol Extract Prepared Using Lactobacillus paracasei subsp. paracasei NTU 101
Abstract
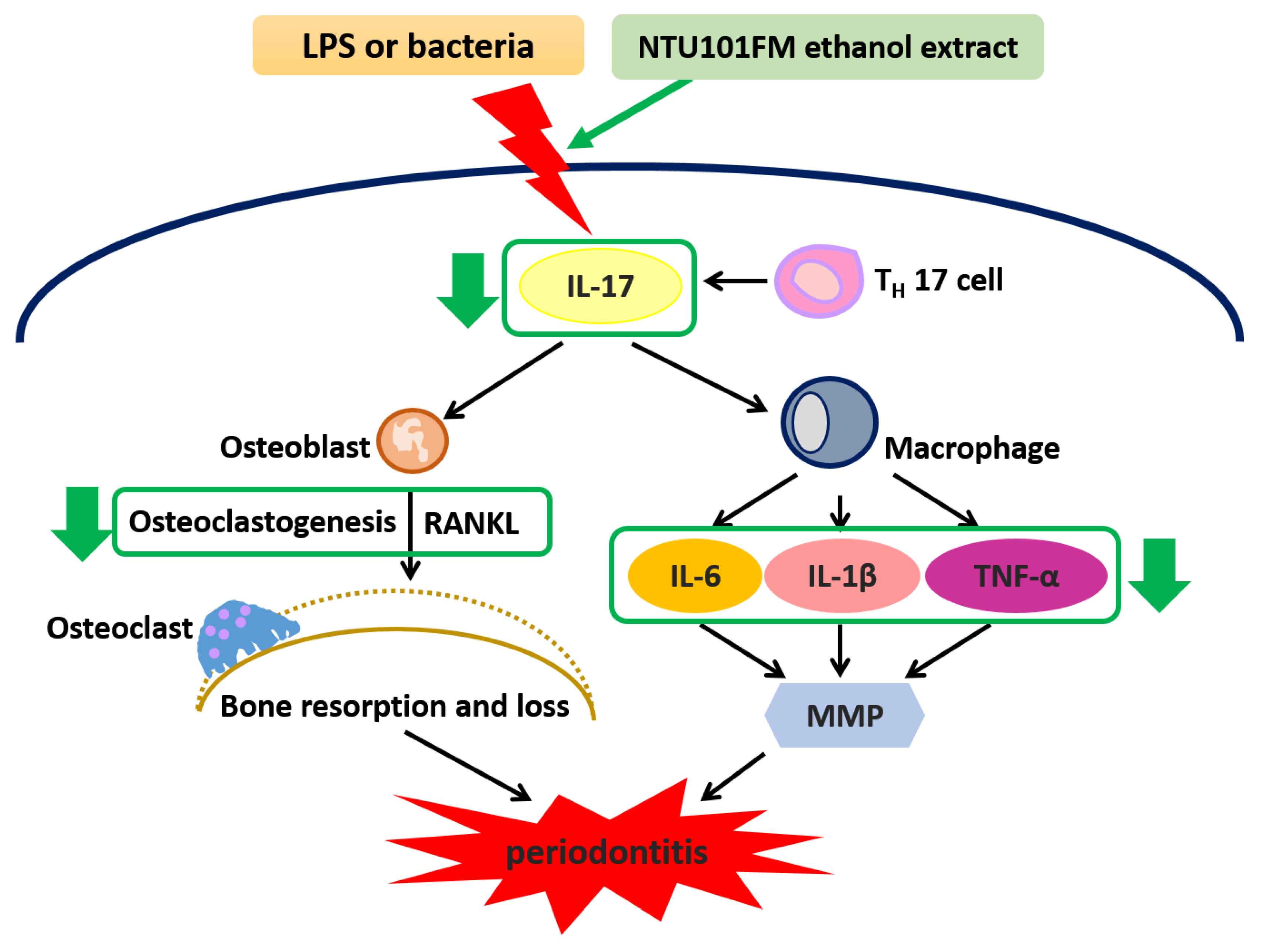
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fermented Skim Milk with NTU 101 and Extraction
2.3. Evaluation of the Anti-Oxidant Properties of NTU101FM
2.4. Anti-Microbial Activity of NTU101FM Ethanol Extract
2.5. Cell Culture and Cell Viability
2.6. Measurement of Nitric Oxide (NO) Production Levels
2.7. Measurement of Pro-Inflammatory Cytokine (IL-1β, IL-6, IL-17, and TNF-α) Levels
2.8. Osteoclast Differentiation, Tartrate-Resistant Acid Phosphatase (TRAP) Staining, and TRAP Activity
2.9. Assessment of Bone Resorptive Area by Pit Formation Assay
2.10. Statistical Analysis
3. Results and Discussion
3.1. The Anti-Oxidative Activities of NTU101FM
3.2. The Anti-Microbial Activities of NTU101FM
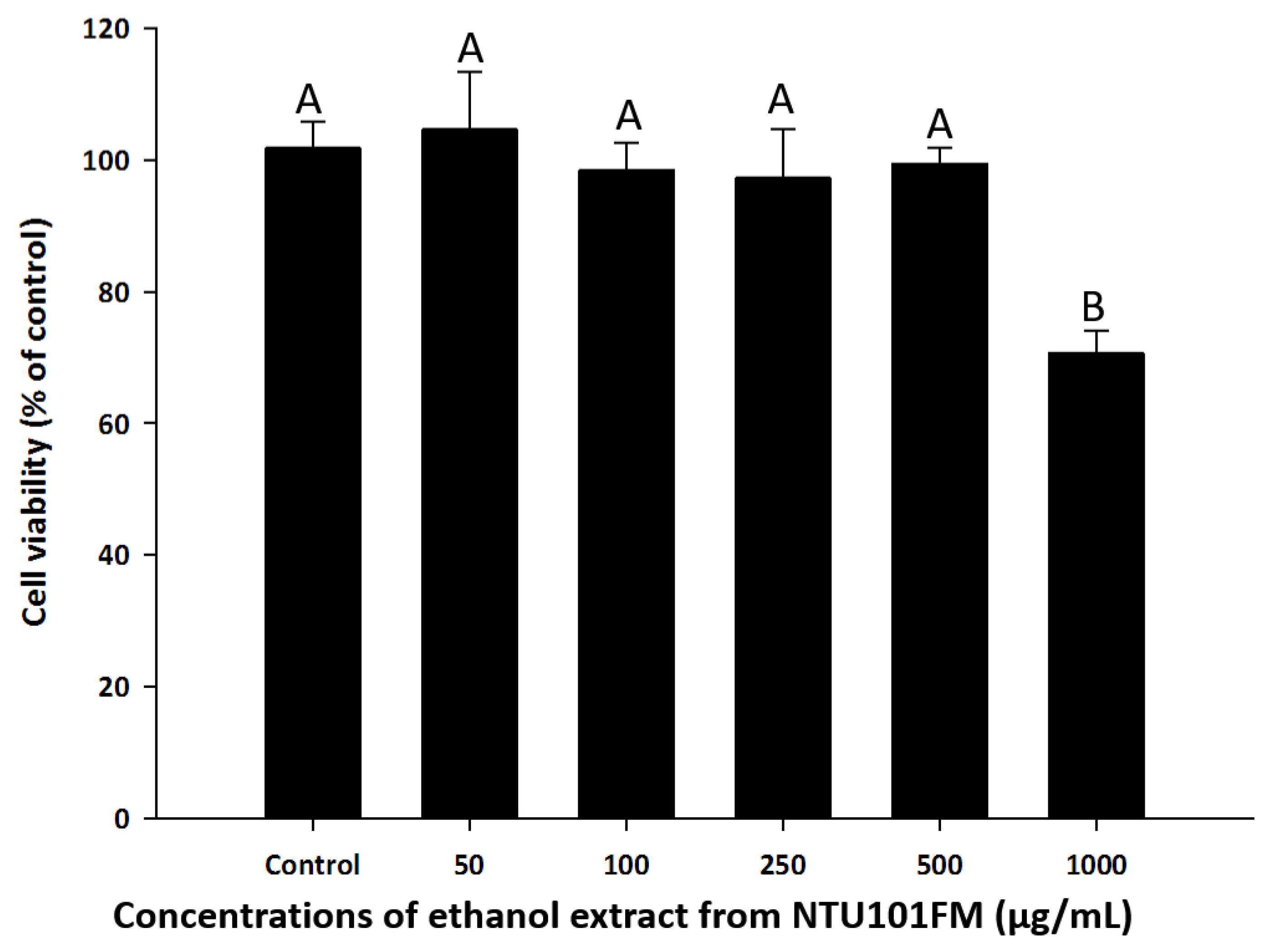
3.3. RAW 264.7 Cell Viability after Treatment with NTU101FM Ethanol Extract
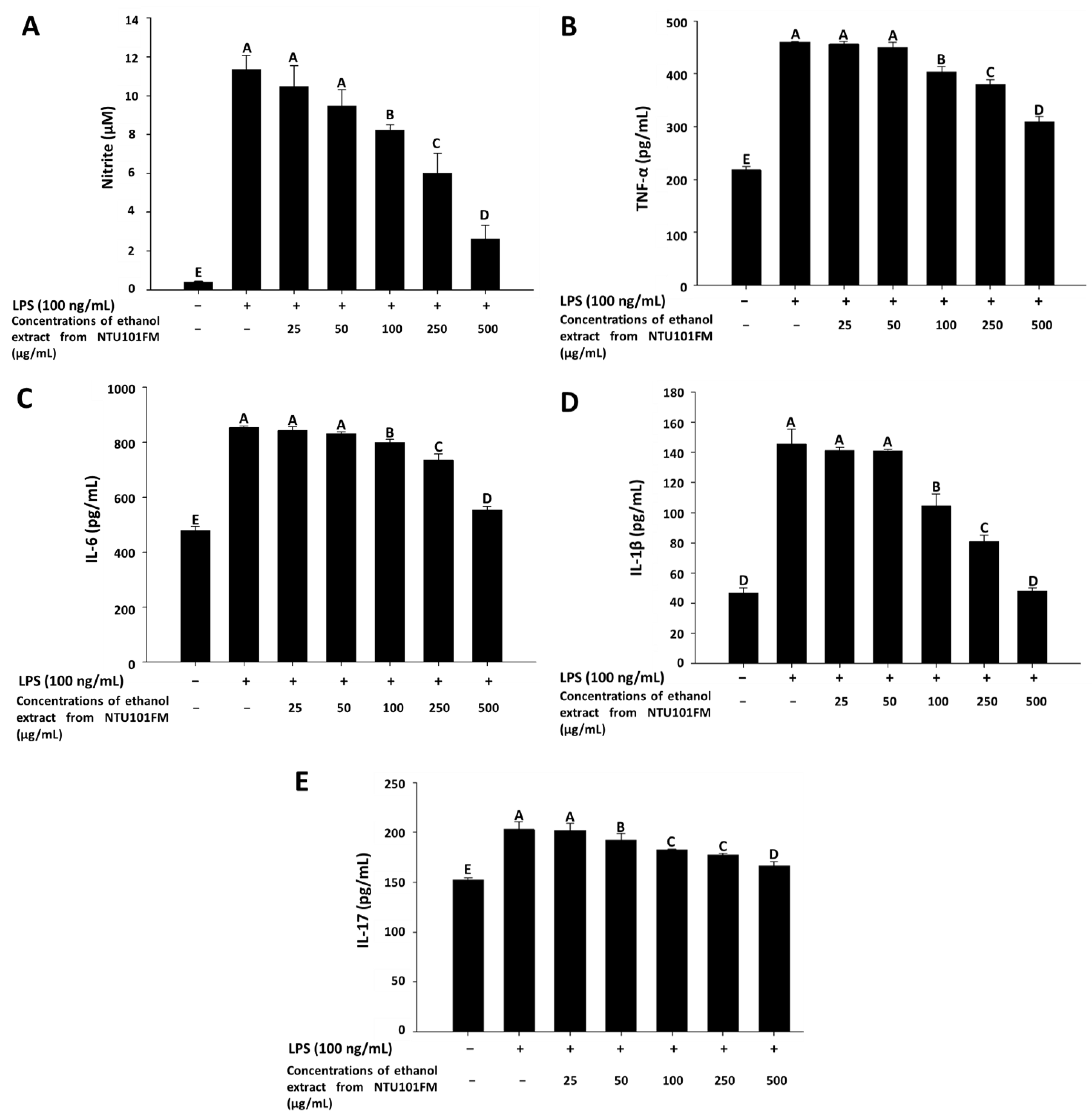
3.4. The Anti-Inflammatory Activities of NTU101FM Ethanol Extract on RAW 264.7 Cells
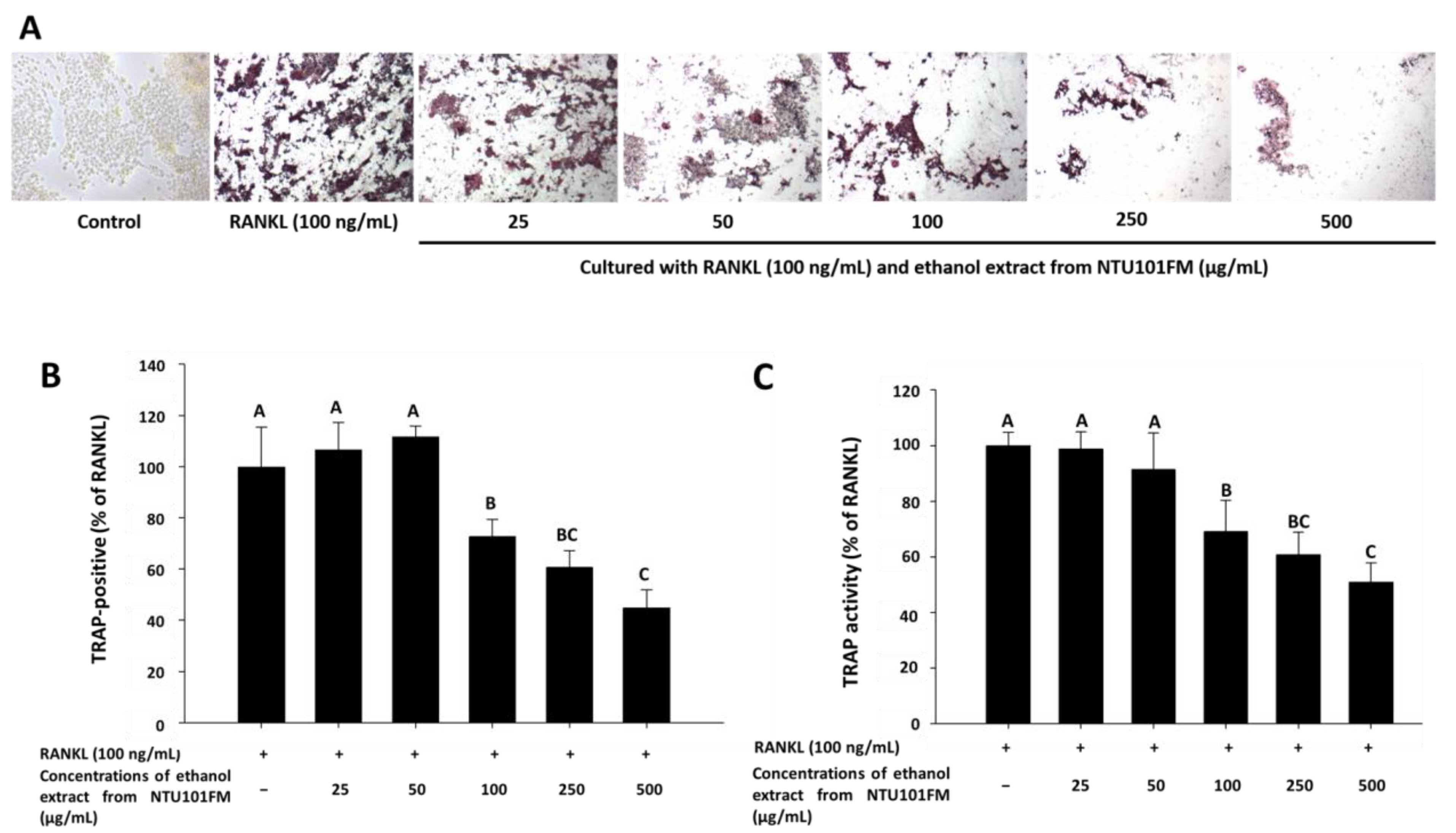
3.5. The Inhibitory Effects of NTU101FM Ethanol Extract on RANKL-Induced Osteoclastogenesis
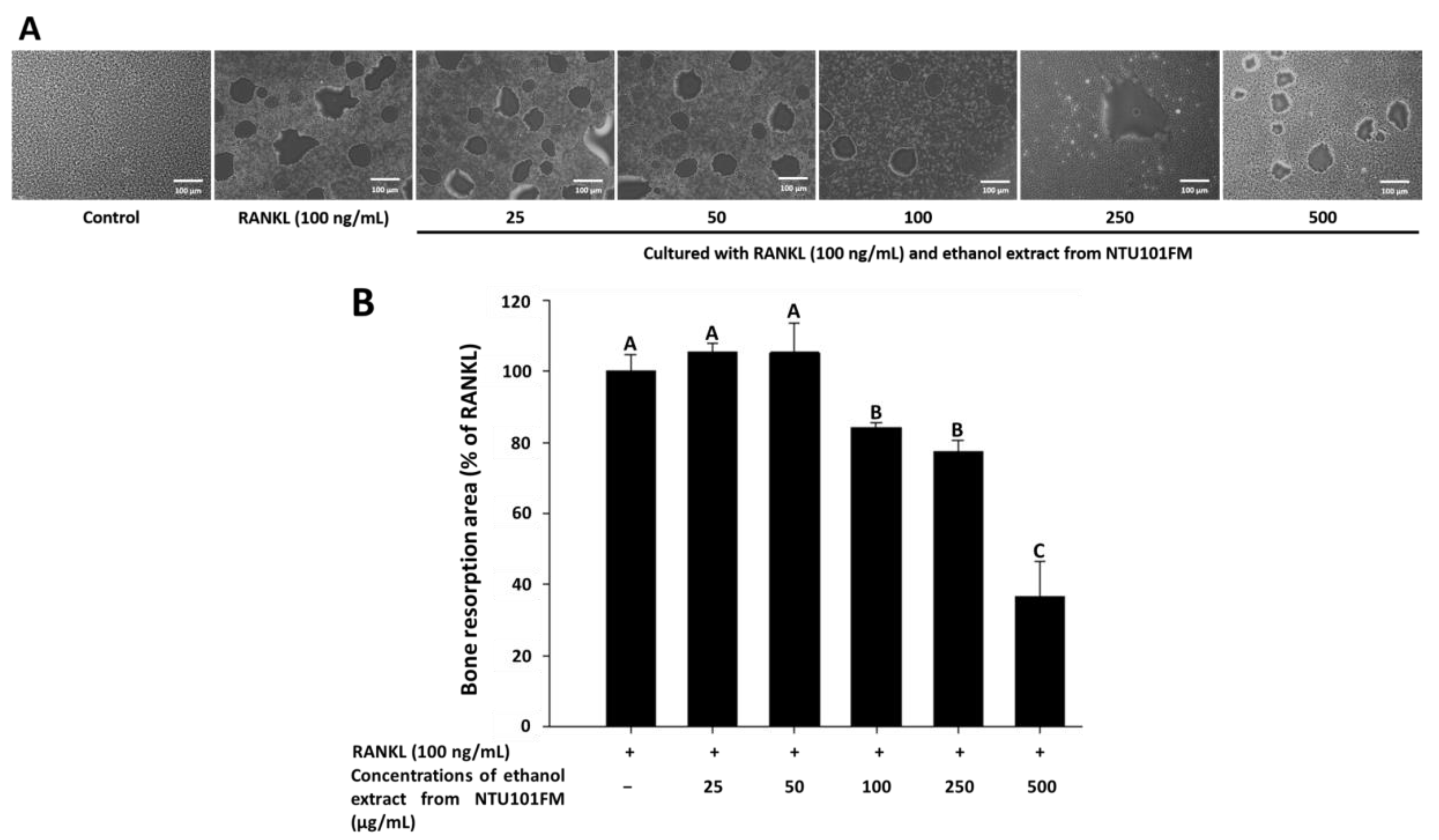
3.6. The Inhibitory Effects of NTU101FM Ethanol Extract on Bone Resorption
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ABL | alveolar bone loss |
| ALP | alkaline phosphatase |
| DMEM | Dulbecco’s modified Eagle’s medium |
| DMSO | dimethyl sulfoxide |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| ELISA | enzyme-linked immunosorbent assay |
| FBS | fetal bovine serum |
| IL | interleukin |
| LAB | lactic acid bacteria |
| LPS | lipopolysaccharide |
| MBCs | minimum bactericidal concentrations |
| MICs | minimum inhibitory concentrations |
| MTT | 3-(4,5-dimethylthiazol)-2-yl-2,5-diphenyltetrazolium bromide |
| NO | nitric oxide |
| NTU 101 | Lactobacillus paracasei subsp. paracasei NTU 101 |
| NTU101FM | NTU 101-fermented skim milk |
| PBS | phosphate-buffered saline |
| RANKL | receptor activator of nuclear factor-κB ligand |
| ROS | reactive oxygen species |
| TCA | trichloroacetic acid |
| TNF | tumor necrosis factor |
| TRAP | tartrate-resistant acid phosphatase |
References
- Lindhe, J.; Ranney, R.; Lamster, I.; Charles, A.; Chung, C.P.; Flemmig, T.; Kinane, D.; Listgarten, M.; Löe, H.; Schoor, R.; et al. Consensus report: Chronic periodontitis. Ann. Periodontol. 1999, 4, 38. [Google Scholar] [CrossRef]
- Wiebe, C.B.; Putnins, E.E. The periodontal disease classification system of the American academy of periodontology—An update. J. Can. Dent. Assoc. 2000, 66, 594–597. [Google Scholar] [PubMed]
- Golpasand Hagh, L.; Zakavi, F.; Hajizadeh, F.; Saleki, M. The association between hyperlipidemia and periodontal infection. Iran. Red Crescent Med. J. 2014, 16, e6577. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Mattheos, N.; Yao, Y.; Jia, Y.; Ma, L.; Gong, P. In vivo osteoprotegerin gene therapy preventing bone loss induced by periodontitis. J. Periodontal. Res. 2015, 50, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, R.; Kobayashi, T.; Sakai, F.; Hosoya, T.; Yamamoto, M.; Kurita-Ochiai, T. Oral administration of Lactobacillus gasseri SBT2055 is effective in preventing Porphyromonas gingivalis-accelerated periodontal disease. Sci. Rep. 2017, 7, 545. [Google Scholar] [CrossRef] [PubMed]
- Henderson, B.; Ward, J.M.; Ready, D. Aggregatibacter (Actinobacillus) actinomycetemcomitans: A triple A* periodontopathogen? Periodontology 2000 2010, 54, 78–105. [Google Scholar] [CrossRef] [PubMed]
- Moradi, J.; Abbasipour, F.; Zaringhalam, J.; Maleki, B.; Ziaee, N.; Khodadoustan, A.; Janahmadi, M. Anethole, a medicinal plant compound, decreases the production of pro-inflammatory TNF-alpha and IL-1beta in a rat model of LPS-induced periodontitis. Iran. J. Pharm. Res. 2014, 13, 1319–1325. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.A.; Lee, H.S.; Jung, Y.S.; Kim, S.W.; Lee, Y.W.; Chang, S.H.; Chung, H.J.; Kim, O.S.; Kim, Y.J. The effects of a novel botanical agent on lipopolysaccharide-induced alveolar bone loss in rats. J. Periodontol. 2013, 84, 1221–1229. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.L.; Matthews, J.B. The role of reactive oxygen and antioxidant species in periodontal tissue destruction. Periodontology 2000 2007, 43, 160–232. [Google Scholar] [CrossRef] [PubMed]
- Villa-Correa, Y.A.; Isaza-Guzman, D.M.; Tobon-Arroyave, S.I. Prognostic value of 8-hydroxy-2′-deoxyguanosine and human neutrophil elastase/alpha1-proteinase inhibitor complex as salivary biomarkers of oxidative stress in chronic periodontitis. J. Periodontol. 2015, 86, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, D.E.; Cha, J.H.; Bak, E.J.; Yoo, Y.J. Receptor activator of nuclear factor-κB ligand and sclerostin expression in osteocytes of alveolar bone in rats with ligature-induced periodontitis. J. Periodontol. 2014, 85, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Bizzini, B.; Pizzo, G.; Scapagnini, G.; Nuzzo, D.; Vasto, S. Probiotics and oral health. Curr. Pharm. Des. 2012, 18, 5522–5531. [Google Scholar] [CrossRef] [PubMed]
- Teanpaisan, R.; Dahlen, G. Use of polymerase chain reaction techniques and sodium dodecyl sulfate-polyacrylamide gel electrophoresis for differentiation of oral Lactobacillus species. Oral Microbiol. Immunol. 2006, 21, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Sookkhee, S.; Chulasiri, M.; Prachyabrued, W. Lactic acid bacteria from healthy oral cavity of Thai volunteers: Inhibition of oral pathogens. J. Appl. Microbiol. 2001, 90, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Krasse, P.; Carlsson, B.; Dahl, C.; Paulsson, A.; Nilsson, A.; Sinkiewicz, G. Decreased gum bleeding and reduced gingivitis by the probiotic Lactobacillus reuteri. Swed. Dent. J. 2006, 30, 55–60. [Google Scholar] [PubMed]
- Saha, S.; Tomaro-Duchesneau, C.; Tabrizian, M.; Prakash, S. Probiotics as oral health biotherapeutics. Expert Opin. Biol. Ther. 2012, 12, 1207–1220. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.J.; Feng, X.P.; Zhang, X.L.; Le, K.Y. Effect of Porphyromonas gingivalis and Lactobacillus acidophilus on secretion of IL1β, IL6, and IL8 by gingival epithelial cells. Inflammation 2012, 35, 1330–1337. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.M.; Chiu, C.H.; Pan, T.M. Fermentation of a milk-soymilk and Lycium chinense miller mixture using a new isolate of Lactobacillus paracasei subsp. paracasei NTU 101 and Bifidobacterium longum. J. Ind. Microbiol. Biotechnol. 2004, 31, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.F.; Pan, T.M. In vitro effects of lactic acid bacteria on cancer cell viability and antioxidant activity. J. Food Drug Anal. 2010, 18, 77–86. [Google Scholar]
- Chiang, S.S.; Pan, T.M. Beneficial effects of Lactobacillus paracasei subsp. paracasei NTU 101 and its fermented products. Appl. Microbiol. Biotechnol. 2012, 93, 903–916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, Y.T.; Cheng, P.C.; Pan, T.M. The immunomodulatory effects of lactic acid bacteria for improving immune functions and benefits. Appl. Microbiol. Biotechnol. 2012, 96, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.H.; Pan, T.M. Inhibitory effect of Lactobacillus paracasei subsp. paracasei NTU 101 on rat dental caries. J. Funct. Foods 2014, 10, 223–231. [Google Scholar] [CrossRef]
- Lin, T.H.; Lin, C.H.; Pan, T.M. The implication of probiotics in the prevention of dental caries. Appl. Microbiol. Biotechnol. 2018, 102, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Chua, M.T.; Tung, Y.T.; Chang, S.T. Antioxidant activities of ethanolic extracts from the twigs of Cinnamomum osmophloeum. Bioresour. Technol. 2008, 99, 1918–1925. [Google Scholar] [CrossRef] [PubMed]
- Yen, G.C.; Duh, P.D. Scavenging effect of methanolic extracts of peanut hulls on free-radical and active-oxygen species. J. Agric. Food Chem. 1994, 42, 629–632. [Google Scholar] [CrossRef]
- Erdogan-Orhan, I.; Sever-Yilmaz, B.; Altun, M.L.; Saltan, G. Radical quenching activity, ferric-reducing antioxidant power, and ferrous ion-chelating capacity of 16 Ballota species and their total phenol and flavonoid contents. J. Med. Food 2010, 13, 1537–1543. [Google Scholar] [CrossRef] [PubMed]
- Schillinger, U.; Lücke, F.K. Antibacterial activity of Lactobacillus sake isolated from meat. Appl. Environ. Microbiol. 1989, 55, 1901–1906. [Google Scholar] [PubMed]
- Kapadia, S.P.; Pudakalkatti, P.S.; Shivanaikar, S. Detection of antimicrobial activity of banana peel (Musa paradisiaca L.) on Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans: An in vitro study. Contemp. Clin. Dent. 2015, 6, 496–499. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, M.; Venkatesalu, V. Antibacterial and antifungal activity of Syzygium jambolanum seeds. J. Ethnopharmacol. 2004, 91, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Studer, R.K.; Decker, K.; Melhem, S.; Georgescu, H. Nitric oxide inhibition of IGF-1 stimulated proteoglycan synthesis: Role of cGMP. J. Orthop. Res. 2003, 21, 914–921. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Hatipoglu, H.; Yaylak, F.; Gungor, Y. A brief review on the periodontal health in metabolic syndrome patients. Diabetes Metab. Syndr. 2015, 9, 124–126. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.; Lo, M.T.; Wang, P.E.; Wang, T.T.; Chen, T.H.; Wu, G.H. A community-based epidemiological study of periodontal disease in Keelung, Taiwan: A model from Keelung community-based integrated screening programme (KCIS No. 18). J. Clin. Periodontol. 2007, 34, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Huttunen, E.; Noro, K.; Yang, Z. Purification and identification of antimicrobial substances produced by two Lactobacillus casei strains. Int. Dairy J. 1995, 5, 503–513. [Google Scholar] [CrossRef]
- Pangsomboon, K.; Kaewnopparat, S.; Pitakpornpreecha, T.; Srichana, T. Antibacterial activity of a bacteriocin from Lactobacillus paracasei HL32 against Porphyromonas gingivalis. Arch. Oral Biol. 2006, 51, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Goldin, B.R. Health benefits of probiotics. Br. J. Nutr. 1998, 80, 203–207. [Google Scholar]
- Cotter, P.D.; Hill, C.; Ross, R.P. Bacteriocins: Developing innate immunity for food. Nat. Rev. Microbiol. 2005, 3, 777–788. [Google Scholar] [CrossRef] [PubMed]
- MacMicking, J.; Xie, Q.W.; Nathan, C. Nitric oxide and macrophage function. Annu. Rev. Immunol. 1997, 15, 323–350. [Google Scholar] [CrossRef] [PubMed]
- Moncada, S. Nitric oxide: Discovery and impact on clinical medicine. J. R. Soc. Med. 1999, 92, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.H.; Huang, R.Y.; Chou, T.C. Magnolol ameliorates ligature-induced periodontitis in rats and osteoclastogenesis: In vivo and in vitro study. Evid. Based Complement. Altern. Med. 2013, 2013, 634095. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Wei, C.; Zhou, L.; Qin, A.; Yang, M.; Tickner, J.; Huang, Y.; Zhao, J.; Xu, J. Luteoloside prevents lipopolysaccharide-induced osteolysis and suppresses RANKL-induced osteoclastogenesis through attenuating RANKL signaling cascades. J. Cell. Physiol. 2018, 233, 1723–1735. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhi, X.; Pan, P.; Cui, J.; Cao, L.; Weng, W.; Zhou, Q.; Wang, L.; Zhai, X.; Zhao, Q.; et al. Matrine prevents bone loss in ovariectomized mice by inhibiting RANKL-induced osteoclastogenesis. FASEB J. 2017, 31, 4855–4865. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.A.; Park, M.; Kim, Y.H.; Ryu, K.H.; Woo, S.Y. Mesenchymal stem cells inhibit RANKL-RANKL interactions between osteoclasts and TH17 cells via osteoprotegerin activity. Oncotarget 2017, 8, 83419–83431. [Google Scholar] [CrossRef] [PubMed]
- Ciucci, T.; Ibáñez, L.; Boucoiran, A.; Birgy-Barelli, E.; Pène, J.; Abou-Ezzi, G.; Arab, N.; Rouleau, M.; Hébuterne, X.; Yssel, H.; et al. Bone marrow TH17 TNFα cells induce osteoclast differentiation, and link bone destruction to IBD. Gut 2015, 64, 1072–1081. [Google Scholar] [CrossRef] [PubMed]
| Concentration (mg/mL) | DPPH Eliminating Effect (%) | Reducing Power (OD700) | ||||||
|---|---|---|---|---|---|---|---|---|
| UF EE | UF WE | NTU 101 EE | NTU 101 WE | UF EE | UF WE | NTU 101 EE | NTU 101 WE | |
| 20.0 | 50.94 ± 2.06 Ac | 45.11 ± 4.24 Ac | 66.34 ± 3.57 Aa | 59.58 ± 4.17 Ab | 0.451 ± 0.03 Ac | 0.215 ± 0.01 Ad | 0.604 ± 0.02 Aa | 0.350 ± 0.01 Ab |
| 10.0 | 42.55 ± 1.51 Bc | 37.36 ± 1.03 Bd | 59.66 ± 1.64 Ba | 54.15 ± 1.55 Bb | 0.389 ± 0.00 Bb | 0.207 ± 0.00 ABd | 0.507 ± 0.02 Ba | 0.288 ± 0.01 Bc |
| 5.0 | 35.58 ± 0.57 Cc | 20.37 ± 0.92 Cd | 54.67 ± 1.65 Ca | 41.26 ± 1.70 Cb | 0.343 ± 0.00 Cb | 0.187 ± 0.00 Bd | 0.381 ± 0.00 Ca | 0.261 ± 0.01 BCc |
| 1.0 | 27.11 ± 2.49 Dc | N.D. | 45.36 ± 1.98 Da | 35.24 ± 1.01 Db | 0.276 ± 0.00 Da | 0.121 ± 0.01 Cb | 0.275 ± 0.01 Da | 0.251 ± 0.04 CDa |
| 0.1 | 16.06 ± 1.54 Ec | N.D. | 40.95 ± 2.09 Ea | 28.11 ± 2.21 Eb | 0.207 ± 0.00 Eb | 0.070 ± 0.02 Dc | 0.236 ± 0.01 Ea | 0.225 ± 0.01 Dab |
| Concentrations (mg/mL) | Inhibition Zone (Diameter, mm) a,b | |
|---|---|---|
| P. gingivalis BCRC 14417 | A. actinomycetemcomitans BCRC 14405 | |
| 200 | 16.50 ± 0.20 | 22.75 ± 0.35 |
| 100 | - | 19.50 ± 1.41 |
| 50 | - | 18.50 ± 0.71 |
| 25 | - | 11.75 ± 1.06 |
| Periodontal Pathogens | MIC (mg/mL) | MBC (mg/mL) |
|---|---|---|
| P. gingivalis BCRC 14417 | 30.00 | 30.00 |
| A. actinomycetemcomitans BCRC 14405 | 1.00 | 2.50 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.-H.; Tsai, T.-Y.; Pan, T.-M. The Anti-Periodontitis Effects of Ethanol Extract Prepared Using Lactobacillus paracasei subsp. paracasei NTU 101. Nutrients 2018, 10, 472. https://doi.org/10.3390/nu10040472
Liu T-H, Tsai T-Y, Pan T-M. The Anti-Periodontitis Effects of Ethanol Extract Prepared Using Lactobacillus paracasei subsp. paracasei NTU 101. Nutrients. 2018; 10(4):472. https://doi.org/10.3390/nu10040472
Chicago/Turabian StyleLiu, Te-Hua, Tsung-Yu Tsai, and Tzu-Ming Pan. 2018. "The Anti-Periodontitis Effects of Ethanol Extract Prepared Using Lactobacillus paracasei subsp. paracasei NTU 101" Nutrients 10, no. 4: 472. https://doi.org/10.3390/nu10040472




