ω-3 and ω-6 Fatty Acids Modulate Conventional and Atypical Protein Kinase C Activities in a Brain Fatty Acid Binding Protein Dependent Manner in Glioblastoma Multiforme
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Reverse Transcription PCR (RT-PCR) Analysis
2.2. Preparation of Fatty Acids
2.3. Protein Kinase C Activity
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Batash, R.; Asna, N.; Schaffer, P.; Francis, N.; Schaffer, M. Glioblastoma multiforme, diagnosis and treatment; recent literature review. Curr. Med. Chem. 2017, 24, 3002–3009. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.Y.; Kesari, S. Malignant gliomas in adults. New Engl. J. Med. 2008, 359, 492–507. [Google Scholar] [CrossRef] [PubMed]
- Eyüpoglu, I.Y.; Hore, N.; Savaskan, N.E.; Grummich, P.; Roessler, K.; Buchfelder, M.; Ganslandt, O. Improving the extent of malignant glioma resection by dual intraoperative visualization approach. PLoS ONE 2012, 7, e44885. [Google Scholar] [CrossRef] [PubMed]
- Shibahara, I.; Sonoda, Y.; Shoji, T.; Kanamori, M.; Saito, R.; Inoue, T.; Kawaguchi, T.; Yamashita, Y.; Watanabe, T.; Kumabe, T.; et al. Malignant clinical features of anaplastic gliomas without idh mutation. Neuro-Oncol. 2015, 17, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.G.; Wang, X.F.; Zhou, J.S.; Wang, F.C.; Li, X.W.; Lu, H.Z. Activation of pkc-alpha is required for migration of c6 glioma cells. Acta Neurobiol. Exp. (Wars) 2010, 70, 239–245. [Google Scholar] [PubMed]
- Mita, R.; Beaulieu, M.J.; Field, C.; Godbout, R. Brain fatty acid-binding protein and omega-3/omega-6 fatty acids: Mechanistic insight into malignant glioma cell migration. J. Biol. Chem. 2010, 285, 37005–37015. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, A.; Pellegatta, S.; Rossi, M.; Tunici, P.; Magnoni, L.; Speranza, M.C.; Malusa, F.; Miragliotta, V.; Mori, E.; Finocchiaro, G.; et al. A radial glia gene marker, fatty acid binding protein 7 (fabp7), is involved in proliferation and invasion of glioblastoma cells. PLoS ONE 2012, 7, e52113. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.Z.; Mita, R.; Beaulieu, M.; Gao, Z.; Godbout, R. Fatty acid binding proteins in brain development and disease. Int. J. Dev. Biol. 2010, 54, 1229–1239. [Google Scholar] [CrossRef] [PubMed]
- Glatz, J.F.; Storch, J. Unravelling the significance of cellular fatty acid-binding proteins. Curr. Opin. Lipidol. 2001, 12, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Hatten, M.E.; Heintz, N. Brain lipid-binding protein (BLBP): A novel signaling system in the developing mammalian CNS. Neuron 1994, 12, 895–908. [Google Scholar] [CrossRef]
- Elsherbiny, M.E.; Goruk, S.; Monckton, E.A.; Richard, C.; Brun, M.; Emara, M.; Field, C.J.; Godbout, R. Long-term effect of docosahexaenoic acid feeding on lipid composition and brain fatty acid-binding protein expression in rats. Nutrients 2015, 7, 8802–8817. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Diehn, M.; Watson, N.; Bollen, A.W.; Aldape, K.D.; Nicholas, M.K.; Lamborn, K.R.; Berger, M.S.; Botstein, D.; Brown, P.O.; et al. Gene expression profiling reveals molecularly and clinically distinct subtypes of glioblastoma multiforme. Proc. Natl. Acad. Sci. USA 2005, 102, 5814–5819. [Google Scholar] [CrossRef] [PubMed]
- Godbout, R.; Bisgrove, D.A.; Shkolny, D.; Day, R.S., 3rd. Correlation of b-fabp and gfap expression in malignant glioma. Oncogene 1998, 16, 1955–1962. [Google Scholar] [CrossRef] [PubMed]
- Balendiran, G.K.; Schnutgen, F.; Scapin, G.; Borchers, T.; Xhong, N.; Lim, K.; Godbout, R.; Spener, F.; Sacchettini, J.C. Crystal structure and thermodynamic analysis of human brain fatty acid-binding protein. J. Biol. Chem. 2000, 275, 27045–27054. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, A.; Zimmer, A.; Schnutgen, F.; Bruning, G.; Spener, F.; Muller, T. The expression pattern of a novel gene encoding brain-fatty acid binding protein correlates with neuronal and glial cell development. Development 1994, 120, 2637–2649. [Google Scholar] [PubMed]
- Elsherbiny, M.E.; Emara, M.; Godbout, R. Interaction of brain fatty acid-binding protein with the polyunsaturated fatty acid environment as a potential determinant of poor prognosis in malignant glioma. Prog. Lipid. Res. 2013, 52, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Palmantier, R.; George, M.D.; Akiyama, S.K.; Wolber, F.M.; Olden, K.; Roberts, J.D. Cis-polyunsaturated fatty acids stimulate beta1 integrin-mediated adhesion of human breast carcinoma cells to type iv collagen by activating protein kinases c-epsilon and -mu. Cancer Res. 2001, 61, 2445–2452. [Google Scholar] [PubMed]
- Garg, R.; Benedetti, L.G.; Abera, M.B.; Wang, H.; Abba, M.; Kazanietz, M.G. Protein kinase c and cancer: What we know and what we do not. Oncogene 2014, 33, 5225–5237. [Google Scholar] [CrossRef] [PubMed]
- Mochly-Rosen, D.; Das, K.; Grimes, K.V. Protein kinase c, an elusive therapeutic target? Nat. Rev. Drug Discov. 2012, 11, 937–957. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, R.M.; Barrett, G.M.; Parolin, D.A.; Gillies, J.K.; Paget, J.A.; Lavictoire, S.J.; Gray, D.A.; Lorimer, I.A. Coordination of glioblastoma cell motility by pkciota. Mol. Cancer 2010, 9, 233. [Google Scholar] [CrossRef] [PubMed]
- Seto, K.K.; Andrulis, I.L. Atypical protein kinase c zeta: Potential player in cell survival and cell migration of ovarian cancer. PLoS ONE 2015, 10, e0123528. [Google Scholar] [CrossRef] [PubMed]
- Jane, E.P.; Premkumar, D.R.; Pollack, I.F. Coadministration of sorafenib with rottlerin potently inhibits cell proliferation and migration in human malignant glioma cells. J. Pharmacol. Exp. Ther. 2006, 319, 1070–1080. [Google Scholar] [CrossRef] [PubMed]
- Tabatabai, G.; Frank, B.; Wick, A.; Lemke, D.; von Kurthy, G.; Obermuller, U.; Heckl, S.; Christ, G.; Weller, M.; Wick, W. Synergistic antiglioma activity of radiotherapy and enzastaurin. Ann. Neurol. 2007, 61, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Tso, C.L.; Shintaku, P.; Chen, J.; Liu, Q.; Liu, J.; Chen, Z.; Yoshimoto, K.; Mischel, P.S.; Cloughesy, T.F.; Liau, L.M.; et al. Primary glioblastomas express mesenchymal stem-like properties. Mol. Cancer Res. 2006, 4, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Bollen, A.W.; Aldape, K.D.; Gupta, N. Nuclear fabp7 immunoreactivity is preferentially expressed in infiltrative glioma and is associated with poor prognosis in EGFR-overexpressing glioblastoma. BMC Cancer 2006, 6, 97. [Google Scholar] [CrossRef] [PubMed]
- Kaloshi, G.; Mokhtari, K.; Carpentier, C.; Taillibert, S.; Lejeune, J.; Marie, Y.; Delattre, J.Y.; Godbout, R.; Sanson, M. Fabp7 expression in glioblastomas: Relation to prognosis, invasion and EGFR status. J. Neurooncol. 2007, 84, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Phillips, E.; Lang, V.; Bohlen, J.; Bethke, F.; Puccio, L.; Tichy, D.; Herold-Mende, C.; Hielscher, T.; Lichter, P.; Goidts, V. Targeting atypical protein kinase c iota reduces viability in glioblastoma stem-like cells via a notch signaling mechanism. Int. J. Cancer 2016, 139, 1776–1787. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.; Yoo, K.C.; Kang, S.G.; Kim, R.K.; Cui, Y.H.; Lee, H.J.; Kim, M.J.; Lee, J.S.; Kim, I.G.; Suh, Y.; et al. Pkcdelta activated by c-met enhances infiltration of human glioblastoma cells through notch2 signaling. Oncotarget 2016, 7, 4890–4902. [Google Scholar] [CrossRef] [PubMed]
- Kusne, Y.; Carrera-Silva, E.A.; Perry, A.S.; Rushing, E.J.; Mandell, E.K.; Dietrich, J.D.; Errasti, A.E.; Gibbs, D.; Berens, M.E.; Loftus, J.C.; et al. Targeting APKC disables oncogenic signaling by both the EGFR and the proinflammatory cytokine tnfalpha in glioblastoma. Sci. Signal. 2014, 7, ra75. [Google Scholar] [CrossRef] [PubMed]
- Do Carmo, A.; Balca-Silva, J.; Matias, D.; Lopes, M.C. PKC signaling in glioblastoma. Cancer Biol. Ther. 2013, 14, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Mita, R.; Coles, J.E.; Glubrecht, D.D.; Sung, R.; Sun, X.; Godbout, R. B-fabp-expressing radial glial cells: The malignant glioma cell of origin? Neoplasia 2007, 9, 734–744. [Google Scholar] [CrossRef] [PubMed]
- Bisgrove, D.A.; Monckton, E.A.; Packer, M.; Godbout, R. Regulation of brain fatty acid-binding protein expression by differential phosphorylation of nuclear factor i in malignant glioma cell lines. J. Biol. Chem. 2000, 275, 30668–30676. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.Z.; Li, S.; Garcia, E.; Glubrecht, D.D.; Poon, H.Y.; Easaw, J.C.; Godbout, R. Association between cytoplasmic crabp2, altered retinoic acid signaling, and poor prognosis in glioblastoma. Glia 2016, 64, 963–976. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, D.L.; Ly, L.H.; Fan, Y.Y.; Chapkin, R.S.; McMurray, D.N. Incorporation of a dietary omega 3 fatty acid impairs murine macrophage responses to mycobacterium tuberculosis. PLoS ONE 2010, 5, e10878. [Google Scholar] [CrossRef] [PubMed]
- Ausubel, F.M.; Roger, B.; Robert, E.K.; David, D.M.; Seidman, J.G.; John, A.S.; Struhl, K. Current Protocols in Molecular Biology; John Wiley & Sons: New York, NY, USA, 2003. [Google Scholar]
- Baltuch, G.H.; Dooley, N.P.; Rostworowski, K.M.; Villemure, J.G.; Yong, V.W. Protein kinase c isoform alpha overexpression in c6 glioma cells and its role in cell proliferation. J. Neurooncol. 1995, 24, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Couldwell, W.T.; Uhm, J.H.; Antel, J.P.; Yong, V.W. Enhanced protein kinase c activity correlates with the growth rate of malignant gliomas in vitro. Neurosurgery 1991, 29, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Gu, F.; Li, W.; Zhang, B.; Niu, R.; Fu, L.; Zhang, N.; Ma, Y. Reduction of protein kinase c zeta inhibits migration and invasion of human glioblastoma cells. J. Neurochem. 2009, 109, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Jude, S.; Martel, E.; Vincent, F.; Besson, P.; Couet, C.; Ogilvie, G.K.; Pinault, M.; De Chalendar, C.; Bougnoux, P.; Richard, S.; et al. Dietary long-chain n-3 fatty acids modify blood and cardiac phospholipids and reduce protein kinase-c-delta and protein kinase-c-epsilon translocation. Br. J. Nutr. 2007, 98, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Comba, A.; Lin, Y.H.; Eynard, A.R.; Valentich, M.A.; Fernandez-Zapico, M.E.; Pasqualini, M.E. Basic aspects of tumor cell fatty acid-regulated signaling and transcription factors. Cancer Metastasis Rev. 2011, 30, 325–342. [Google Scholar] [CrossRef] [PubMed]
- Tolle, A.; Krause, H.; Miller, K.; Jung, K.; Stephan, C. Importance of braintype fatty acid binding protein for cell-biological processes in human renal carcinoma cells. Oncol. Rep. 2011, 25, 1307–1312. [Google Scholar] [CrossRef] [PubMed]
- Besson, A.; Davy, A.; Robbins, S.M.; Yong, V.W. Differential activation of erks to focal adhesions by pkc epsilon is required for pma-induced adhesion and migration of human glioma cells. Oncogene 2001, 20, 7398–7407. [Google Scholar] [CrossRef] [PubMed]
- Besson, A.; Wilson, T.L.; Yong, V.W. The anchoring protein rack1 links protein kinase cepsilon to integrin beta chains. Requirements for adhesion and motility. J. Biol. Chem. 2002, 277, 22073–22084. [Google Scholar] [CrossRef] [PubMed]
- Uht, R.M.; Amos, S.; Martin, P.M.; Riggan, A.E.; Hussaini, I.M. The protein kinase c-eta isoform induces proliferation in glioblastoma cell lines through an erk/elk-1 pathway. Oncogene 2007, 26, 2885–2893. [Google Scholar] [CrossRef] [PubMed]
- Acevedo-Duncan, M.; Russell, C.; Patel, S.; Patel, R. Aloe-emodin modulates pkc isozymes, inhibits proliferation, and induces apoptosis in u-373mg glioma cells. Int. Immunopharmacol. 2004, 4, 1775–1784. [Google Scholar] [CrossRef] [PubMed]
- Van Kolen, K.; Slegers, H. Atypical pkczeta is involved in rhoa-dependent mitogenic signaling by the p2y(12) receptor in c6 cells. FEBS J. 2006, 273, 1843–1854. [Google Scholar] [CrossRef] [PubMed]
- Muller, G.; Ayoub, M.; Storz, P.; Rennecke, J.; Fabbro, D.; Pfizenmaier, K. Pkc zeta is a molecular switch in signal transduction of tnf-alpha, bifunctionally regulated by ceramide and arachidonic acid. EMBO J. 1995, 14, 1961–1969. [Google Scholar] [PubMed]
- Gailly, P.; Gong, M.C.; Somlyo, A.V.; Somlyo, A.P. Possible role of atypical protein kinase c activated by arachidonic acid in ca2+ sensitization of rabbit smooth muscle. J. Physiol. 1997, 500, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Mani, I.; Iversen, L.; Ziboh, V.A. Evidence of nuclear pkc/map-kinase cascade in guinea pig model of epidermal hyperproliferation. J. Investig. Dermatol. 1999, 112, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Mani, I.; Iversen, L.; Ziboh, V.A. Upregulation of nuclear pkc and map-kinase during hyperproliferation of guinea pig epidermis: Modulation by 13-(s)-hydroxyoctadecadienoic acid (13-hode). Cell. Signal. 1998, 10, 143–149. [Google Scholar] [CrossRef]
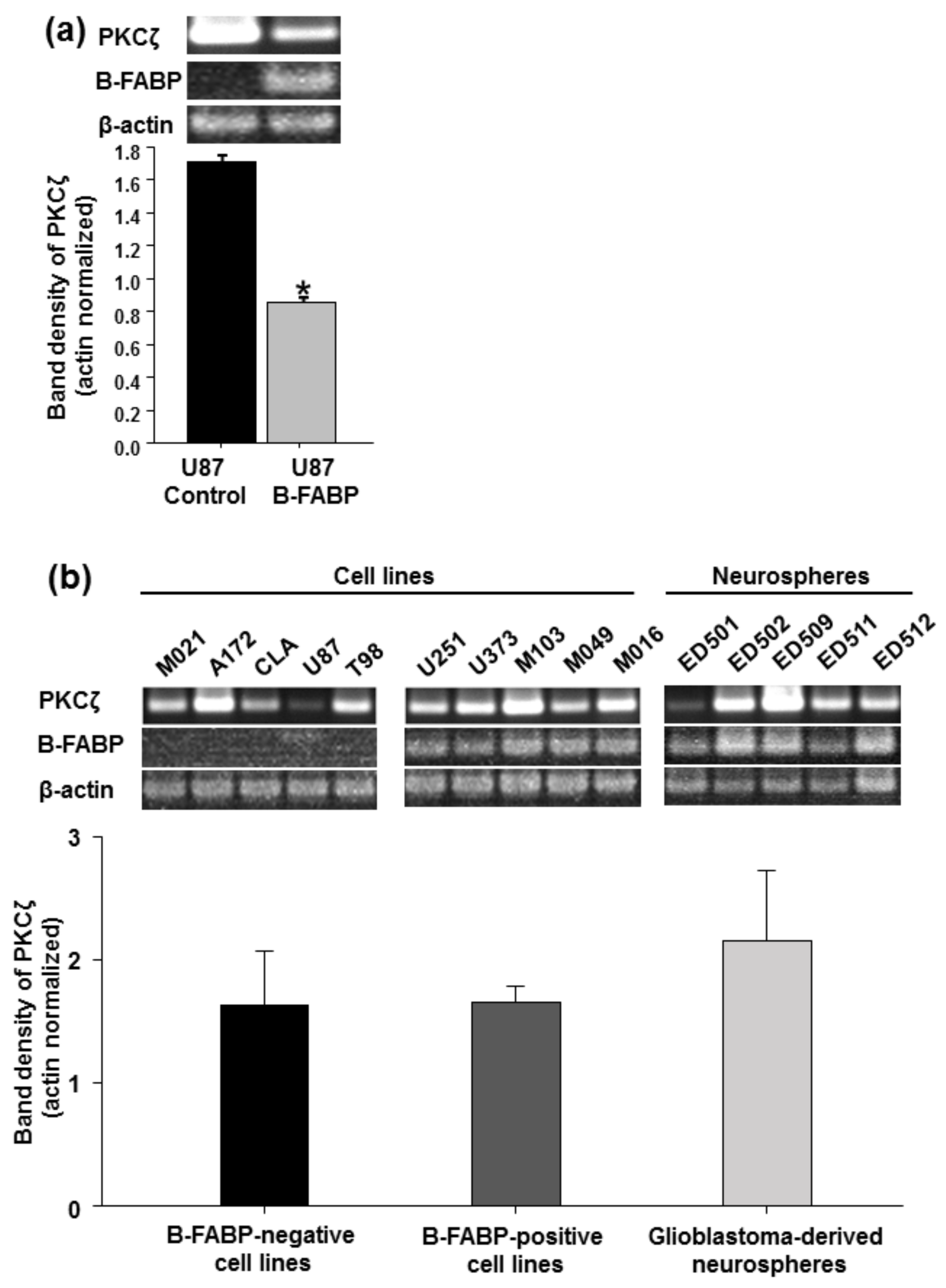
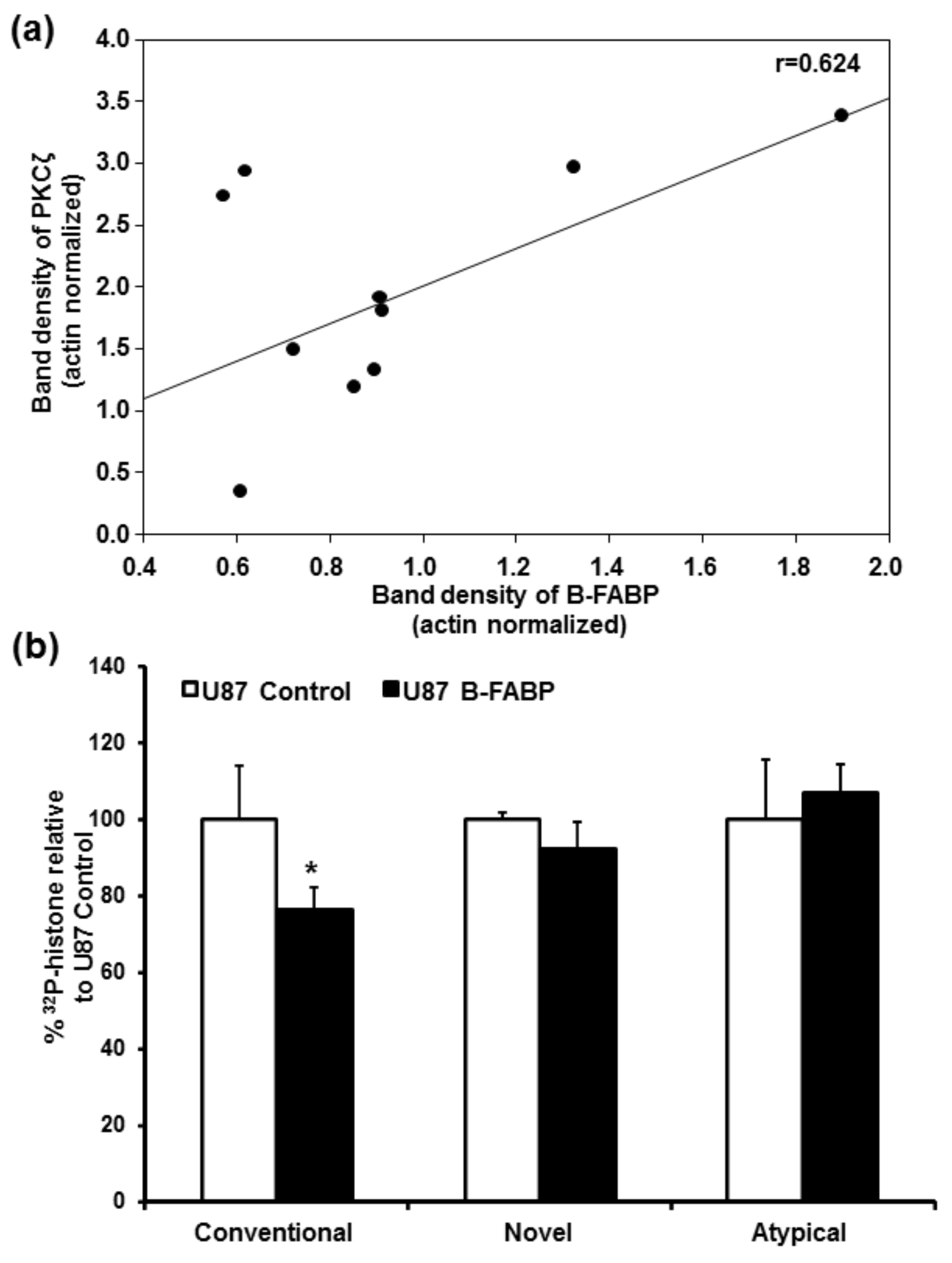
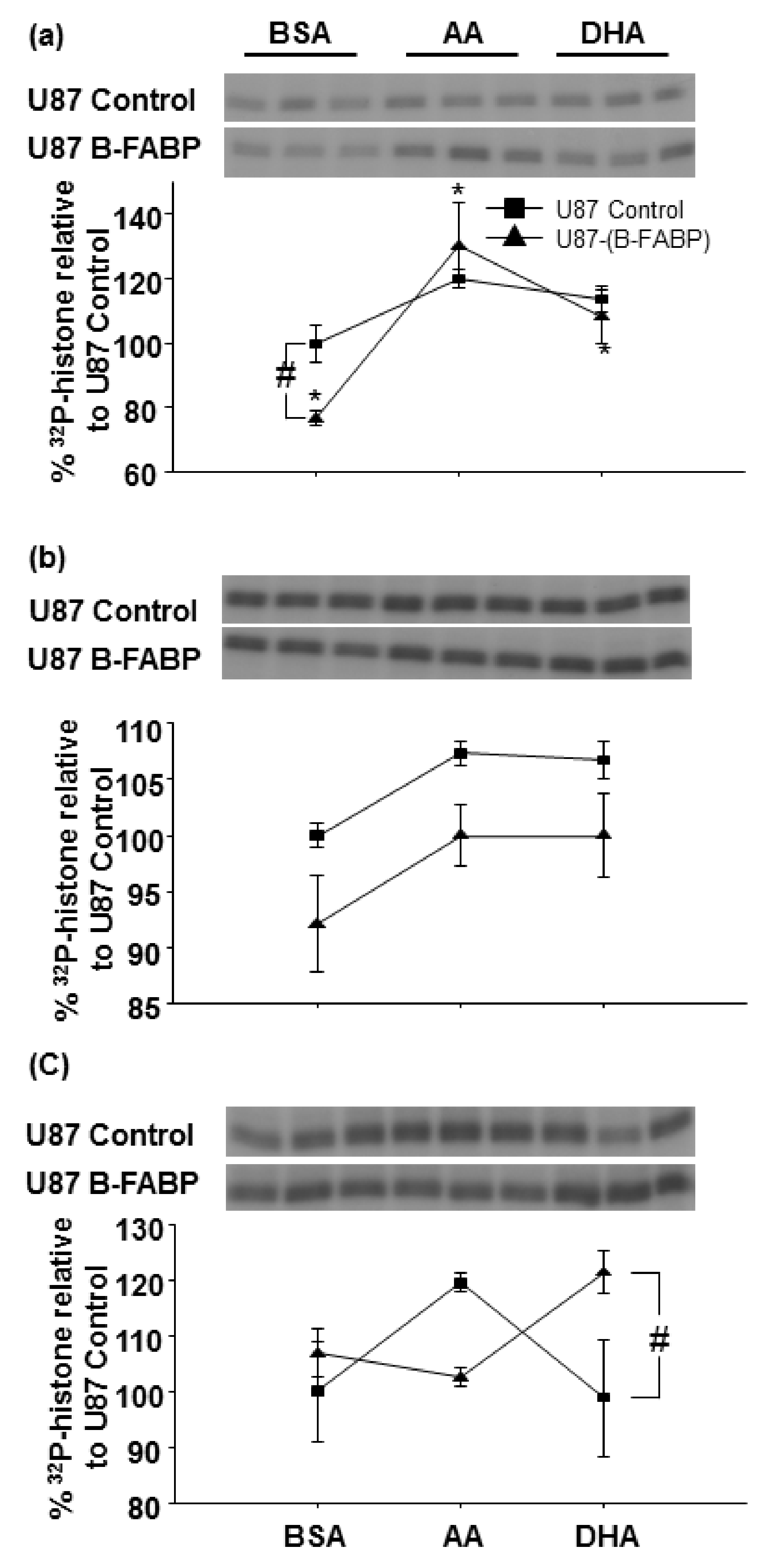
| Gene | Fold Change with B-FABP Expression | p-value b, n = 6 |
|---|---|---|
| Conventional PKCs | ||
| PKCα | +1.63 | 0.001 |
| PKCβ | NC a | 0.138 |
| PKCγ | NC a | 0.502 |
| Novel PKCs | ||
| PKCδ | +1.6 | 0.0001 |
| PKCε | −3.0 | <0.0000001 |
| PKCη | +1.89 | 0.015 |
| Atypical PKCs | ||
| PKCι | −1.8 | <0.0000001 |
| PKCζ | −25 | <0.0000001 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elsherbiny, M.E.; Chen, H.; Emara, M.; Godbout, R. ω-3 and ω-6 Fatty Acids Modulate Conventional and Atypical Protein Kinase C Activities in a Brain Fatty Acid Binding Protein Dependent Manner in Glioblastoma Multiforme. Nutrients 2018, 10, 454. https://doi.org/10.3390/nu10040454
Elsherbiny ME, Chen H, Emara M, Godbout R. ω-3 and ω-6 Fatty Acids Modulate Conventional and Atypical Protein Kinase C Activities in a Brain Fatty Acid Binding Protein Dependent Manner in Glioblastoma Multiforme. Nutrients. 2018; 10(4):454. https://doi.org/10.3390/nu10040454
Chicago/Turabian StyleElsherbiny, Marwa E., Hua Chen, Marwan Emara, and Roseline Godbout. 2018. "ω-3 and ω-6 Fatty Acids Modulate Conventional and Atypical Protein Kinase C Activities in a Brain Fatty Acid Binding Protein Dependent Manner in Glioblastoma Multiforme" Nutrients 10, no. 4: 454. https://doi.org/10.3390/nu10040454





