Preterm Infant Feeding: A Mechanistic Comparison between a Vacuum Triggered Novel Teat and Breastfeeding
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Novel Teat
2.3. Ultrasound Imaging and Measurement of Intra-Oral Pressure
2.4. Ultrasound and Intra-Oral Vacuum Measurement
2.5. Infant Milk Intake
2.6. Bradycardia and Desaturation Events
2.7. Statistical Analysis
2.8. Tongue Movement
2.9. Intra-Oral Vacuum
2.10. Milk Intake
3. Results
3.1. Participant Characteristics
3.2. Characteristics of Monitored Feeds
3.3. Tongue Movement
3.4. Intra-Oral Vacuum
3.5. Bradycardia and Desaturation Events
3.6. Milk Intake
4. Discussion
4.1. Tongue Movement
4.2. Intra-Oral Vacuum
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lewandowski, A.J.; Lamata, P.; Francis, J.M.; Piechnik, S.K.; Ferreira, V.M.; Boardman, H.; Neubauer, S.; Singhal, A.; Leeson, P.; Lucas, A. Breast milk consumption in preterm neonates and cardiac shape in adulthood. Pediatrics 2016, 138, e20160050. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, E.B.; Gadian, D.G.; Sabatini, S.; Chong, W.K.; Quinn, B.T.; Fischl, B.R.; Lucas, A. The effect of early human diet on caudate volumes and IQ. Pediatr. Res. 2008, 63, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, E.B.; Fischl, B.R.; Quinn, B.T.; Chong, W.K.; Gadian, D.G.; Lucas, A. Impact of breast milk on intelligence quotient, brain size, and white matter development. Pediatr. Res. 2010, 67, 357–362. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, D.L.; Jacobs, J.; Hall, R.; Adamkin, D.; Auestad, N.; Castillo, M.; Connor, W.E.; Connor, S.L.; Fitzgerald, K. Growth and development of premature infants fed predominantly human milk, predominantly premature infant formula, or a combination of human milk and premature formula. J. Pediatr. Gastroenterol. Nutr. 2003, 37, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Belfort, M.B.; Anderson, P.J.; Nowak, V.A.; Lee, K.J.; Molesworth, C.; Thompson, D.K.; Doyle, L.W.; Inder, T.E. Breast milk feeding, brain development, and neurocognitive outcomes: A 7-year longitudinal study in infants born at less than 30 weeks’ gestation. J. Pediatr. 2016, 177, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Bronfin, D.R. Misshapen heads in babies: Position or pathology? Ochsner J. 2001, 3, 191–199. [Google Scholar] [PubMed]
- Morris, K.M.; Burns, Y.R. Reduction of craniofacial and palatal narrowing in very low birthweight infants. J. Paediatr. Child Health 1994, 30, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Ash, S.P.; Moss, J.P. An investigation of the features of the preterm infant palate and the effect of prolonged orotracheal intubation with and without protective appliances. Br. J. Orthod. 1987, 14, 253–261. [Google Scholar] [CrossRef] [PubMed]
- De Curtis, M.; Rigo, J. Extrauterine growth restriction in very low-birthweight infants. Acta Paediatr. 2004, 93, 1563–1568. [Google Scholar] [CrossRef] [PubMed]
- Geddes, D.T.; Prescott, S.L. Developmental origins of health and disease: The role of human milk in preventing disease in the 21st century. J. Hum. Lact. 2013, 29, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Callen, J.; Pinelli, J. A review of the literature examining the benefits and challenges, incidence and duration, and barriers to breastfeeding in preterm infants. Adv. Neonatal Care 2005, 5, 72–88. [Google Scholar] [CrossRef] [PubMed]
- Garber, J. Oral-motor function and feeding intervention. Phys. Occup. Ther. Pediatr. 2013, 33, 111–138. [Google Scholar] [CrossRef] [PubMed]
- Casiro, O.G.; McKenzie, M.E.; McFadyen, L.; Shapiro, C.; Seshia, M.M.; MacDonald, N.; Moffatt, M.; Cheang, M.S. Earlier discharge with community-based intervention for low birth weight infants: A randomized trial. Pediatrics 1993, 92, 128–134. [Google Scholar] [PubMed]
- Bertoncelli, N.; Cuomo, G.; Cattani, S.; Mazzi, C.; Pugliese, M.; Coccolini, E.; Zagni, P.; Mordini, B.; Ferrari, F. Oral feeding competences of healthy preterm infants: A review. Int. J. Pediatr. 2012, 2012, 896257. [Google Scholar] [CrossRef] [PubMed]
- Mathew, O.P. Breathing patterns of preterm infants during bottle feeding: Role of milk flow. J. Pediatr. 1991, 119, 960–965. [Google Scholar] [CrossRef]
- Al-Sayed, L.E.; Schrank, W.I.; Thach, B.T. Ventilatory sparing strategies and swallowing pattern during bottle feeding in human infants. J. Appl. Physiol. 1994, 77, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Sakalidis, V.S.; McClellan, H.L.; Hepworth, A.R.; Kent, J.C.; Lai, C.T.; Hartmann, P.E.; Geddes, D.T. Oxygen saturation and suck-swallow-breathe coordination of term infants during breastfeeding and feeding from a teat releasing milk only with vacuum. Int. J. Pediatr. 2012. [Google Scholar] [CrossRef] [PubMed]
- Simmer, K.; Kok, C.; Nancarrow, K.; Hepworth, A.R.; Geddes, D.T. Novel feeding system to promote establishment of breastfeeds after preterm birth: A randomized controlled trial. J. Perinatol. 2016, 36, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Trial Registered on ANZCTR. Available online: http://www.ANZCTR.org.au/ACTRN12614000875606.aspx (accessed on 19 March 2018).
- Geddes, D.T.; Chooi, K.; Nancarrow, K.; Hepworth, A.R.; Gardner, H.; Simmer, K. Characterisation of sucking dynamics of breastfeeding preterm infants: A cross sectional study. BMC 2017, 17, 386. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.; Alagugurusamy, R.; Schanler, R.J.; Smith, E.O.; Shulman, R.J. Characterization of the developmental stages of sucking in preterm infants during bottle feeding. Acta Paediatr. 2000, 89, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.; Schanler, R.J. Oral feeding in premature infants: Advantage of a self-paced milk flow. Acta Paediatr. 2000, 89, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Weber, F.; Woolridge, M.; Baum, J. An ultrasonographic study of the organisation of sucking and swallowing by newborn infants. Dev. Med. Child Neurol. 1986, 28, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Ardran, G.; Kemp, F. A correlation between suckling pressures and the movements of the tongue. Acta Paediatr. 1959, 48, 261–272. [Google Scholar] [PubMed]
- Prieto, C.; Cárdenas, H.; Salvatierra, A.; Boza, C.; Montes, C.; Croxatto, H. Sucking pressure and its relationship to milk transfer during breastfeeding in humans. J. Reprod. Fertil. 1996, 108, 69–74. [Google Scholar] [CrossRef] [PubMed]
- McClellan, H.L.; Sakalidis, V.S.; Hepworth, A.R.; Hartmann, P.E.; Geddes, D.T. Validation of nipple diameter and tongue movement measurements with B-mode ultrasound during breastfeeding. Ultrasound Med. Biol. 2010, 36, 1797–1807. [Google Scholar] [CrossRef] [PubMed]
- Kent, J.C.; Mitoulas, L.R.; Cregan, M.D.; Ramsay, D.T.; Doherty, D.A.; Hartmann, P.E. Volume and frequency of breastfeedings and fat content of breast milk throughout the day. Pediatrics 2006, 117, e387–e395. [Google Scholar] [CrossRef] [PubMed]
- Bagnall, A. Feeding problems. In Feeding and Nutrition in the Preterm Infant; Jones, E., King, C., Eds.; Elsevier Churchill Livingstone: Edinburgh, UK, 2005; pp. 165–183. [Google Scholar]
- Zimmerman, E.; Thompson, K. Clarifying nipple confusion. J. Perinatol. 2015, 35, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Geddes, D.T.; Sakalidis, V.S.; Hepworth, A.R.; McClellan, H.L.; Kent, J.C.; Lai, C.T.; Hartmann, P.E. Tongue movement and intra-oral vacuum of term infants during breastfeeding and feeding from an experimental teat that released milk under vacuum only. Early Hum. Dev. 2012, 88, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.L.; Kang, S.M. Preliminary ultrasound observation of lingual movement patterns during nutritive versus non-nutritive sucking in a premature infant. Dysphagia 2007, 22, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Eishima, K. The analysis of sucking behaviour in newborn infants. Early Hum. Dev. 1991, 27, 163–173. [Google Scholar] [CrossRef]
- Meier, P.P.; Brown, L.P.; Hurst, N.M.; Spatz, D.L.; Engstrom, J.L.; Borucki, L.C.; Krouse, A.M. Nipple shields for preterm infants: Effect on milk transfer and duration of breastfeeding. J. Hum. Lact. 2000, 16, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Maastrup, R.; Hansen, B.M.; Kronborg, H.; Bojesen, S.N.; Hallum, K.; Frandsen, A.; Kyhnaeb, A.; Svarer, I.; Hallström, I. Breastfeeding progression in preterm infants is influenced by factors in infants, mothers and clinical practice: The results of a national cohort study with high breastfeeding initiation rates. PLoS ONE 2014, 9, e108208. [Google Scholar] [CrossRef] [PubMed]
- Maastrup, R.; Hansen, B.M.; Kronborg, H.; Bojesen, S.N.; Hallum, K.; Frandsen, A.; Kyhnaeb, A.; Svarer, I.; Hallström, I. Factors associated with exclusive breastfeeding of preterm infants. Results from a prospective national cohort study. PLoS ONE 2014, 9, e89077. [Google Scholar] [CrossRef] [PubMed]
- Geddes, D.T.; Kent, J.C.; Mitoulas, L.R.; Hartmann, P.E. Tongue movement and intra-oral vacuum in breastfeeding infants. Early Hum. Dev. 2008, 84, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Sakalidis, V.S.; Williams, T.M.; Garbin, C.P.; Hepworth, A.R.; Hartmann, P.E.; Paech, M.J.; Geddes, D.T. Ultrasound imaging of infant sucking dynamics during the establishment of lactation. J. Hum. Lact. 2013, 29, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Elad, D.; Kozlovsky, P.; Blum, O.; Laine, A.F.; Po, M.J.; Botzer, E.; Dollberg, S.; Zelicovich, M.; Ben Sira, L. Biomechanics of milk extraction during breast-feeding. Proc. Natl. Acad. Sci. USA 2014, 111, 5230–5235. [Google Scholar] [CrossRef] [PubMed]
- Nowak, A.J.; Smith, W.L.; Erenberg, A. Imaging evaluation of breast-feeding and bottle-feeding systems. J. Pediatr. 1995, 126, S130–S134. [Google Scholar] [CrossRef]
- Hohoff, A.; Rabe, H.; Ehmer, U.; Harms, E. Palatal development of preterm and low birthweight infants compared to term infants—What do we know? Part 1: The palate of the term newborn. Head Face Med. 2005, 1, 8. [Google Scholar] [CrossRef] [PubMed]
- Hohoff, A.; Rabe, H.; Ehmer, U.; Harms, E. Palatal development of preterm and low birthweight infants compared to term infants—What do we know? Part 2: The palate of the preterm/low birthweight infant. Head Face Med. 2005, 1, 9. [Google Scholar] [CrossRef] [PubMed]
- Victora, C.G.; Bahl, R.; Barros, A.J.; França, G.V.; Horton, S.; Krasevec, J.; Murch, S.; Sankar, M.J.; Walker, N.; Rollins, N.C.; et al. Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. Lancet 2016, 387, 475–490. [Google Scholar] [CrossRef]
- Palmer, B. The influence of breastfeeding on the development of the oral cavity: A commentary. J. Hum. Lact. 1998, 14, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Arvedson, J.C. Assessment of pediatric dysphagia and feeding disorders: Clinical and instrumental approaches. Dev. Disabil. Res. Rev. 2008, 14, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Delaney, A.; Arvedson, J.C. Development of swallowing and feeding: Prenatal through first year of life. Dev. Disabil. Res. Rev. 2008, 14, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Barlow, S.M. Oral and respiratory control for preterm feeding. Curr. Opin. Otolaryngol. Head Neck Surg. 2009, 17, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Lau, C. Development of suck and swallow mechanisms in infants. Ann. Nutr. Metab. 2015, 66, 7–14. [Google Scholar] [CrossRef] [PubMed]
- McClellan, H.; Geddes, D.; Kent, J.; Garbin, C.; Mitoulas, L.; Hartmann, P. Infants of mothers with persistent nipple pain exert strong sucking vacuums. Acta Paediatr. 2008, 97, 1205–1209. [Google Scholar] [CrossRef] [PubMed]
- Sakalidis, V.S.; Kent, J.C.; Garbin, C.P.; Hepworth, A.R.; Hartmann, P.E.; Geddes, D.T. Longitudinal changes in suck-swallow-breathe, oxygen saturation, and heart rate patterns in term breastfeeding infants. J. Hum. Lact. 2013, 29, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Schrank, W.; Al-Sayed, L.E.; Beahm, P.H.; Thach, B.T. Feeding responses to free-flow formula in term and preterm infants. J. Pediatr. 1998, 132, 426–430. [Google Scholar] [CrossRef]
- Chen, C.H.; Wang, T.M.; Chang, H.M.; Chi, C.S. The effect of breast- and bottle-feeding on oxygen saturation and body temperature in preterm infants. J. Hum. Lact. 2000, 16, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, C.C.; Tsai, M.L.; Chen, C.C.; Lin, H.C. Early optimal nutrition improves neurodevelopmental outcomes for very preterm infants. Nutr. Rev. 2014, 72, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Cannon, A.M.; Sakalidis, V.S.; Lai, C.T.; Perrella, S.L.; Geddes, D.T. Vacuum characteristics of the sucking cycle and relationships with milk removal from the breast in term infants. Early Hum. Dev. 2016, 96, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Paulsson, L.; Soderfeldt, B.; Bondemark, L. Malocclusion traits and orthodontic treatment needs in prematurely born children. Angle Orthod. 2008, 78, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, I.; Thayath, M.N.; Singh, S.; Sinha, A. Preterm birth: A primary etiological factor for delayed oral growth and development. Int. J. Clin. Pediatr. Dent. 2015, 8, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.S.; Guilleminault, C. Pediatric obstructive sleep apnea and the critical role of oral-facial growth: Evidences. Front. Neurol. 2012, 3, 184. [Google Scholar] [CrossRef] [PubMed]

| Infant and Feed Characteristics | Breast | Novel Teat | Difference | p-Value |
|---|---|---|---|---|
| Feed duration (min) | 10.6 (9.0, 20.3) | 12.3 (9.1, 16.6) | 1.9 (−4.0, 9.4) | 0.28 |
| Time sucking (min) | 3.4 (2.4, 7.8) | 2.7 (1.8, 3.7) | 1.4 (0.0, 4.6) | 0.027 |
| Time Pausing (min) | 7.9 (5.2, 11.7) | 9.5 (5.8, 11.4) | 0.3 (−3.6, 2.4) | 0.078 |
| Mean vacuum (mmHg) | −30.7 (−50.0, −21.2) | −6.0 (−14.0, −3.4) | −24.6 (−46.4, −9.2) | <0.001 |
| Proportion of feed spent sucking | 0.36 ± 0.16 | 0.27 ± 0.15 | 0.14 (−0.07, 0.09) | 0.072 |
| Number of suck bursts | 35 (29, 66) | 34 (25, 51) | 6 (−5, 29) | 0.28 |
| Prescribed volume (mL) | 46.1 ± 8.2 | 45.7 ± 7.1 | 0 (0, 0) | 0.36 |
| Milk intake (mL) | 12 (3, 15.5) | 33 (22.5, 42.5) | −19 (−35, −4) | 0.007 |
| Milk transfer (mL/min) | 2.2 (1.2, 3.2) | 9.2 (7.0, 13.2) | −7.9 (−12.6, −3.4) | <0.001 |
| Age at monitored feed PMA (weeks) | 36.3 ± 1.8 | 36.4 ± 1.5 | −0.14 (−0.3, 0) | 0.74 |
| Post-natal (weeks) | 7.1 ± 4.2 | 7.2 ± 3.9 | ||
| Weight at monitored feed (g) | 2120 ± 421.7 | 2146 ± 294.8 | −65 (−129, 0) | 0.50 |
| Infant Intra-Oral and Nipple Diameter Measures | Breast | Novel Teat | ||
|---|---|---|---|---|
| Tongue Up | Tongue Down | Tongue Up | Tongue Down | |
| N-HSPJ distance (mm) | 7.1 ± 2.9 | 5.2 ± 2.6 | 5.6 ± 1.4 | 4.7 ± 1.4 |
| Intra-oral depth (mm) | 0.3 ± 0.5 | 4.2 ± 2.0 | 0.1 ± 0.2 | 4.1 ± 1.3 |
| Nipple diameters (mm) | ||||
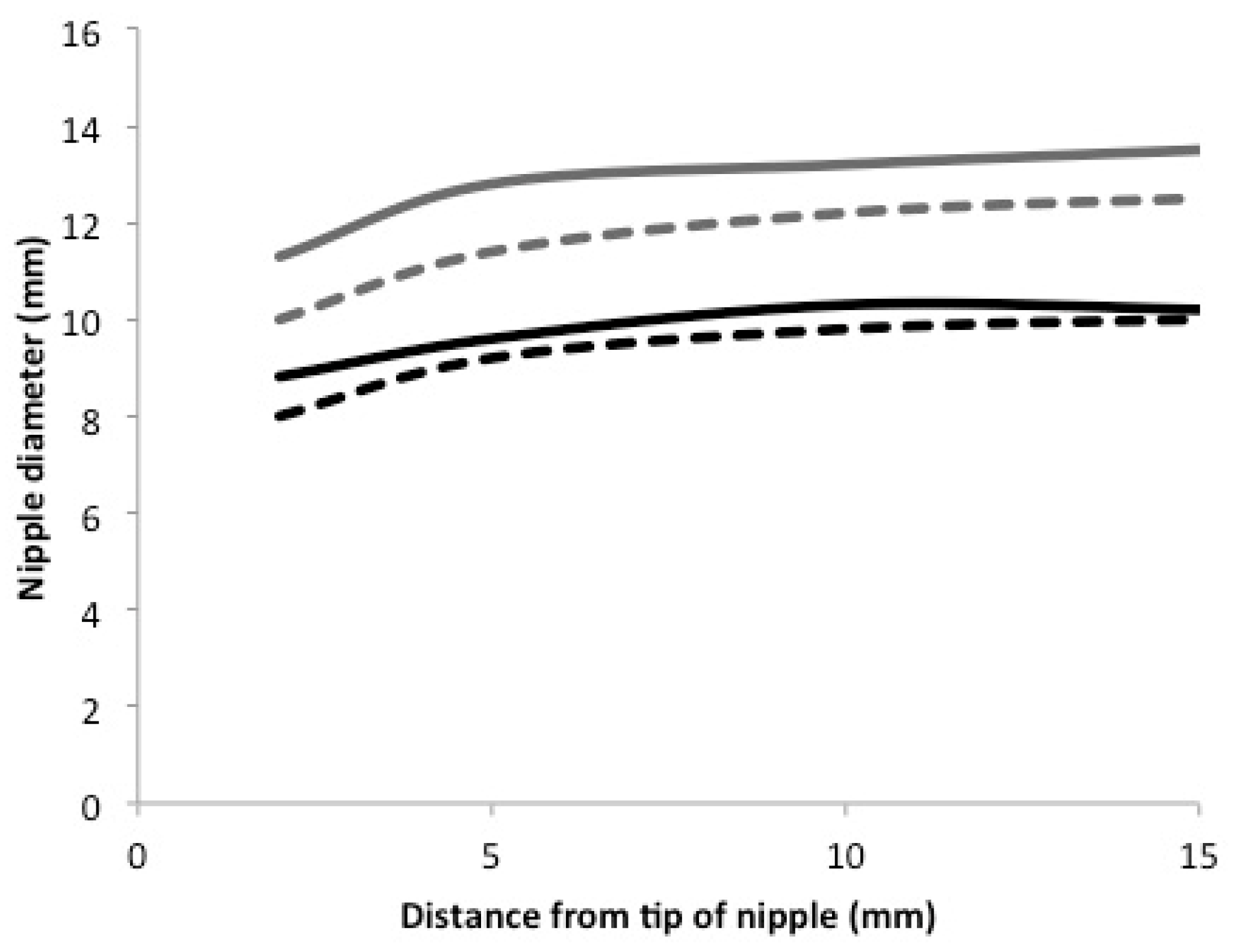
| 2 mm | 10.0 ± 3.0 | 11.3 ± 2.3 | 8.0 ± 1.1 | 8.8 ± 1.1 |
| 5 mm | 11.4 ± 2.6 | 12.8 ± 2.5 | 9.2 ± 0.9 | 9.6 ± 1.3 |
| 10 mm | 12.2 ± 2.5 | 13.2 ± 2.4 | 9.8 ± 1.0 | 10.3 ± 1.3 |
| 15 mm | 12.5 ± 2.7 | 13.5 ± 2.6 | 10.0 ± 1.0 | 10.2 ± 1.5 |
| Random Effects | N-HSPJ | IOD | Nipple Diameters | |||
|---|---|---|---|---|---|---|
| Coeff (95% CI) | p-Value | Coeff (95% CI) | p-Value | Coeff (95% CI) | p-Value | |
| Feed within Infant | Tongue Position within Feed within Infant | Tongue Position within Feed within Infant | ||||
| Main effects models | ||||||
| Reference a | 5.2 (4.1, 6.2) | - | 0.3 (−0.2, 0.7) | 0.30 | 11.2 (10.3, 12.2) | - |
| Tongue down | 1.6 (1.3, 1.8) | <0.001 | 3.9 (3.4, 4.5) | <0.001 | 1.0 (0.7, 1.4) | <0.001 |
| Novel Teat | −0.5 (−1.9, 0.8) | 0.42 | −0.1 (−0.7, 0.5) | 0.72 | −2.3 (−3.6, −1.0) | 0.002 |
| Location | <0.001 b | |||||
| 2 mm | - | - | - | - | −1.2 (−1.4, −1.0) | <0.001 |
| 10 mm | - | - | - | - | 0.6 (0.4, 0.8) | <0.001 |
| 15 mm | - | - | - | - | 0.8 (0.6, 1.0) | <0.001 |
| Interaction models | ||||||
| Reference a | 5.1 (4.0, 6.1) | - | 0.3 (−0.5, 0.8) | 0.28 | 11.1 (10.1, 12.1) | |
| Tongue down | 1.7 (1.3, 2.1) | <0.001 | 3.8 (3.1, 4.6) | <0.001 | 1.3 (0.9, 1.8) | <0.0001 |
| Novel Teat feed | −0.4 (−1.8, 1.1) | 0.60 | −0.2 (−1.0, 0.6) | 0.61 | −2.0 (−3.4, −0.6) | 0.007 |
| Location | <0.0001 b | |||||
| 2 | - | - | - | - | −1.4 (−1.7, −1.1) | <0.0001 |
| 10 | - | - | - | - | 0.6 (0.3, 0.9) | <0.0001 |
| 15 | - | - | - | - | 0.9 (0.6, 1.2) | <0.0001 |
| Tongue down Teat | −0.4 (−0.9, 0.2) | 0.21 | 0.2 (−0.9, 1.3) | 0.72 | −0.6 (−1.3, 0.1) | 0.069 |
| Location Teat | 0.010 b | |||||
| 2 mm | - | - | - | - | 0.4 (0.002, 0.8) | 0.039 |
| 10 mm | - | - | - | - | −0.1 (−0.5, 0.3) | 0.56 |
| 15 mm | - | - | - | - | 0.3 (−0.7, 0.2) | 0.26 |
| Breast | Novel Teat | |||
|---|---|---|---|---|
| Sucks (n = 912) | Pauses (n = 895) | Sucks (n = 729) | Pauses (n = 712) | |
| Duration * (s) | 4.5 (2.7, 7.4) | 5.4 (2.7, 9.0) | 2.4 (1.3, 4.6) | 5.2 (2.4, 11.6) |
| (0.5, 52.4) | (0.5, 712.6) | (0.4, 132.8) | (0.6, 379.7) | |
| Sucking Events * (n) | 6 (3.8, 9) | - | 3 (1, 5) | - |
| (1, 54) | (1, 147) | |||
| Sucking Rate (n/min) | 88.0 (73.7, 102.6) | - | 77.6 (62.4, 96.6) | - |
| (19.0, 194.4) | (14.2, 191.2) | |||
| Vacuum (mmHg) | ||||
| Baseline | −31.1 (−60.0, −12.7) | - | −5.8 (−11.0, 0.1) | - |
| (−181.0, 12.4) | (−53.3, 14.3) | |||
| Mean * | −53.6 (−89.3, −31.5) | −23.9 (−48.8, −11.7) | −15.5 (−21.8, −8.9) | −5.1 (−11.0, −0.6) |
| (−199.3, 0.4) | (−185.1, 1.9) | (−77.6, 2.2) | (−79.6, 5.3) | |
| Peak | −106.2 (−153.0, −65.5) | - | −40.0 (−54.6, −27.1) | - |
| (−325.7, −9.6) | (−116.7, −3.1) | |||
| Vacuum Variability | ||||
| Baseline | 23.4 (17.3, 37.0) | - | 5.5 (4.4, 6.1) | - |
| (5.3, 47.3) | (1.4, 12.0) | |||
| Mean * | 26.2 (25.0, 41.6) | - | 9.3 (7.8, 10.5) | - |
| (11.2, 73.9) | (3.6, 18.9) | |||
| Peak | 50.0 (33.0, 60.7) | - | 25.4 (20.6, 29.3) | - |
| (18.9, 154.3) | (3.9, 39.4) | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geddes, D.; Kok, C.; Nancarrow, K.; Hepworth, A.; Simmer, K. Preterm Infant Feeding: A Mechanistic Comparison between a Vacuum Triggered Novel Teat and Breastfeeding. Nutrients 2018, 10, 376. https://doi.org/10.3390/nu10030376
Geddes D, Kok C, Nancarrow K, Hepworth A, Simmer K. Preterm Infant Feeding: A Mechanistic Comparison between a Vacuum Triggered Novel Teat and Breastfeeding. Nutrients. 2018; 10(3):376. https://doi.org/10.3390/nu10030376
Chicago/Turabian StyleGeddes, Donna, Chooi Kok, Kathryn Nancarrow, Anna Hepworth, and Karen Simmer. 2018. "Preterm Infant Feeding: A Mechanistic Comparison between a Vacuum Triggered Novel Teat and Breastfeeding" Nutrients 10, no. 3: 376. https://doi.org/10.3390/nu10030376




