The Effect of Vitamin D Supplementation on Glycemic Control in Type 2 Diabetes Patients: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Methods
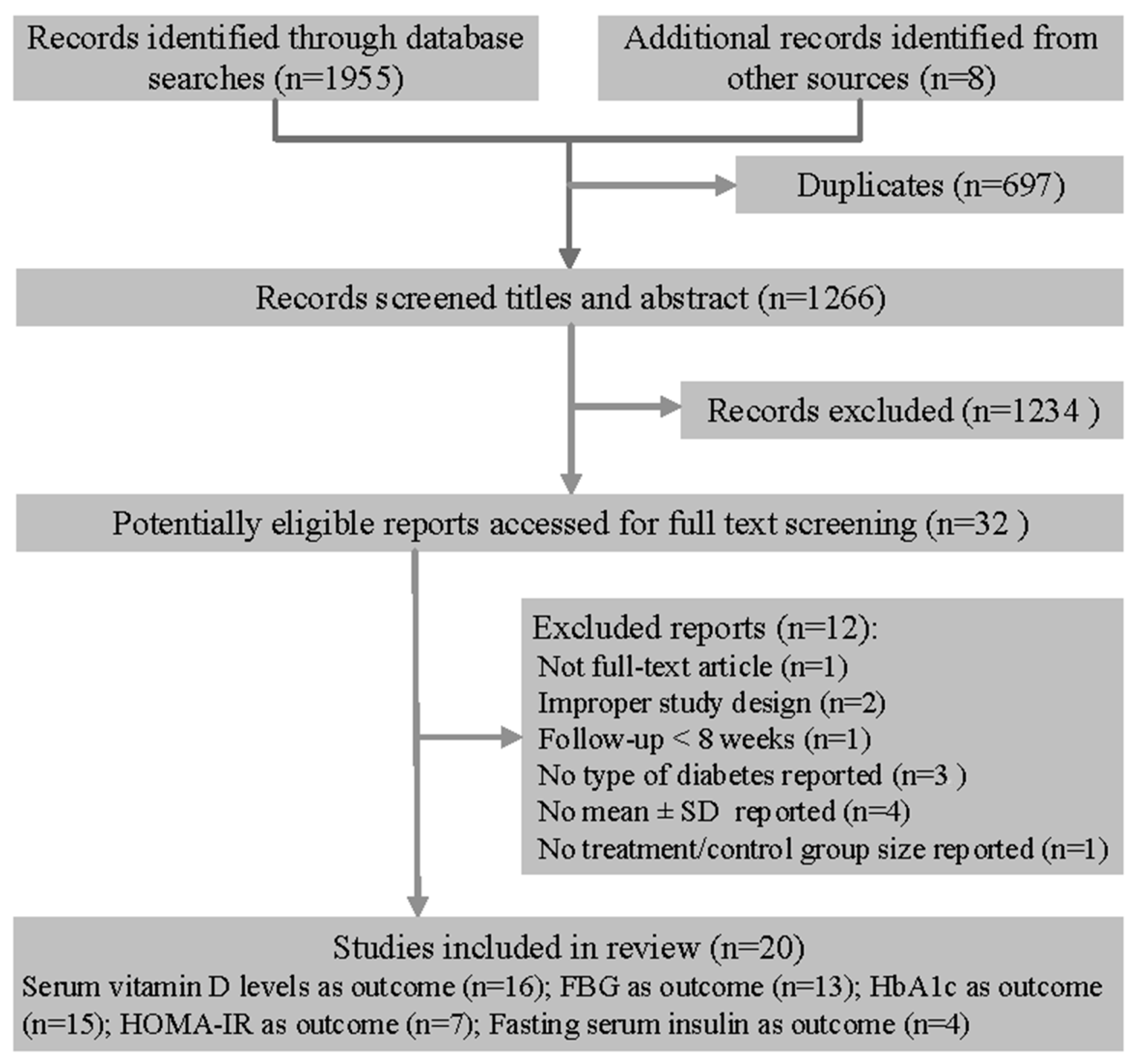
2.1. Selection of Studies
2.2. Data Sources and Search Strategy
2.3. Data Extraction and Risk of Bias Assessment
2.4. Data Analysis and Rating Quality of Evidence
3. Results
3.1. Study Characteristics
3.2. Risk of Bias
3.3. The Effect on Serum Vitamin D Level
3.4. The Effect on FBG
3.5. The Effect on HbA1c
3.6. The Effect on HOMA-IR
3.7. The Effect of Fasting Insulin
3.8. Meta-Regression
3.9. Sensitivity Analyses
3.10. Publication Bias
3.11. Quality of Evidence
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar]
- Joergensen, C.; Gall, M.A.; Schmedes, A.; Tarnow, L.; Parving, H.H.; Rossing, P. Vitamin D levels and mortality in type 2 diabetes. Diabetes Care 2010, 33, 2238–2243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitri, J.; Muraru, M.D.; Pittas, A.G. Vitamin D and type 2 diabetes: A systematic review. Eur. J. Clin. Nutr. 2011, 65, 1005–1015. [Google Scholar] [CrossRef] [PubMed]
- Pittas, A.G.; Lau, J.; Hu, F.B.; Dawsonhughes, B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2007, 92, 2017–2029. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, L.; Pittas, A.G.; Del Gobbo, L.C.; Zhang, C.; Manson, J.E.; Hu, F.B. Blood 25-Hydroxy Vitamin D Levels and Incident Type 2 Diabetes. Diabetes Care 2013, 36, 1422–1428. [Google Scholar] [CrossRef] [PubMed]
- Grimnes, G.; Emaus, N.; Joakimsen, R.M.; Jorde, R. Baseline serum 25-hydroxyvitamin D concentrations in the Troms Study 1994-95 and risk of developing type 2 diabetes mellitus during 11 years of follow-up: Vitamin D and diabetes—A prospective study. Diabet. Med. 2010, 27, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Gysemans, C.A.; Cardozo, A.K.; Callewaert, H.; Giulietti, A.; Hulshagen, L.; Bouillon, R.; Eizirik, D.L.; Mathieu, C. 1,25-Dihydroxyvitamin D3 modulates expression of chemokines and cytokines in pancreatic islets: implications for prevention of diabetes in nonobese diabetic mice. Endocrinology 2005, 146, 1956–1964. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, D.S.; Kang, S. Vitamin D deficiency impairs glucose-stimulated insulin secretion and increases insulin resistance by reducing PPAR-gamma expression in nonobese Type 2 diabetic rats. J. Nutr. Biochem. 2016, 27, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.G.; Hou, F.F.; Guo, Z.J.; Liang, M.; Wang, G.B.; Zhang, X. 1,25-Dihydroxyvitamin D improved the free fatty-acid-induced insulin resistance in cultured C2C12 cells. Diabetes Metab. Res. Rev. 2008, 24, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Krul-Poel, Y.H.; Ter, W.M.; Lips, P.; Simsek, S. MANAGEMENT OF ENDOCRINE DISEASE: The effect of vitamin D supplementation on glycaemic control in patients with Type 2 Diabetes Mellitus: A systematic review and meta-analysis. Eur. J. Endocrinol. 2016, 176, R1–R14. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.J.; Iyer, G.; Liu, Y.; Kalyani, R.R.; Bamba, N.; Liqon, C.B.; Varma, S.; Mathioudakis, N. The effect of vitamin D supplementation on glucose metabolism in type 2 diabetes mellitus: A systematic review and meta-analysis of intervention studies. J. Diabetes Complicat. 2017, 31, 1115–1126. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Qiu, S.; Zhu, X.; Li, L. Vitamin D supplementation and glycemic control in type 2 diabetes patients: A systematic review and meta-analysis. Metabolism 2017, 73, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Mirhosseini, N.; Vatanparast, H.; Mazidi, M.; Kimball, S.M. The Effect of Improved Serum 25-Hydroxyvitamin D Status on Glycemic Control in Diabetic Patients: A Meta-Analysis. J. Clin. Endocrinol. Metab. 2017, 102, 3097–3110. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, J.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; Version 5.1.0; The Cochrane Collaboration: London, UK, 2013. [Google Scholar]
- WHO (World Health Organization). The Asia-Pacific Perspective: Redefining Obesity and Its Treatment; WHO: Geneva, Switzerland, 2000. [Google Scholar]
- Holick, M.F.; Binkley, N.C.; Bischoffferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [PubMed]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schunemann, H.J.; GRADE Working Group. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [PubMed]
- Anyanwu, A.C.; Fasanmade, O.A.; Odeniyi, I.A.; Iwuala, S.; Coker, H.B.; Ohwovoriole, A.E. Effect of Vitamin D supplementation on glycemic control in Type 2 diabetes subjects in Lagos, Nigeria. Indian J. Endocrinol. Metab. 2016, 20, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Baziar, N.; Jafarian, K.; Shadman, Z.; Qorbani, M.; Khoshniat, N.M.; Abd, M.M. Effect of therapeutic dose of vitamin d on serum adiponectin and glycemia in vitamin D-insufficient or deficient type 2 diabetic patients. Iran. Red Crescent Med. J. 2014, 16, e21458. [Google Scholar] [CrossRef] [PubMed]
- Eftekhari, M.H.; Akbarzadeh, M.; Dabbaghmanesh, M.H.; Hasanzadeh, J. Impact of treatment with oral calcitriol on glucose indices in type 2 diabetes mellitus patients. Asia Pac. J. Clin. Nutr. 2011, 20, 521–526. [Google Scholar] [PubMed]
- Ghavamzadeh, S.; Mobasseri, M.; Mahdavi, R. The effect of vitamin D supplementation on adiposity, blood glycated hemoglobin, serum leptin and TNF-α in type 2 diabetic patients. Int. J. Prev. Med. 2014, 5, 1091–1098. [Google Scholar] [PubMed]
- Jorde, R.; Figenschau, Y. Supplementation with cholecalciferol does not improve glycaemic control in diabetic subjects with normal serum 25-hydroxyvitamin D levels. Eur. J. Nutr. 2009, 48, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Kampmann, U.; Mosekilde, L.; Juhl, C.; Moller, N.; Christensen, B.; Rejnmark, L.; Wamberq, L.; Orskov, L. Effects of 12 weeks high dose vitamin D3 treatment on insulin sensitivity, beta cell function, and metabolic markers in patients with type 2 diabetes and vitamin D insufficiency—A double-blind, randomized, placebo-controlled trial. Metabolism 2014, 63, 1115–1124. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kang, C.K.; Park, H.; Lee, M.G. Effects of vitamin D supplementation and circuit training on indices of obesity and insulin resistance in T2D and vitamin D deficient elderly women. J. Exerc. Nutr. Biochem. 2014, 18, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Krul-Poel, Y.H.; Westra, S.; Ten, B.E.; Ter Wee, M.M.; Van Schoor, N.M.; Van Wijland, H.; Stam, F.; Lips, P.T.; Simsek, S. Effect of Vitamin D Supplementation on Glycemic Control in Patients with Type 2 Diabetes (SUNNY Trial): A Randomized Placebo-Controlled Trial. Diabetes Care 2015, 38, 1420–1426. [Google Scholar] [CrossRef] [PubMed]
- Maggi, S.; Siviero, P.; Brocco, E.; Albertin, M.; Romanato, G.; Crepaldi, G. Vitamin D deficiency, serum leptin and osteoprotegerin levels in older diabetic patients: an input to new research avenues. Acta Diabetol. 2014, 51, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Nasri, H.; Behradmanesh, S.; Maghsoudi, A.R.; Ahmadi, A.; Nasri, P.; Rafieian-Kopaei, M. Efficacy of supplementary vitamin D on improvement of glycemic parameters in patients with type 2 diabetes mellitus; a randomized double blind clinical trial. J. Renal Inj. Prev. 2014, 3, 31–34. [Google Scholar] [PubMed]
- Punthakee, Z.; Bosch, J.; Dagenais, G.; Diaz, R.; Holman, R.; Probstfield, J.; Ramachandran, A.; Riddle, M.; Ryden, L.E.; Zinman, B.; et al. Design, history and results of the Thiazolidinedione Intervention with vitamin D Evaluation (TIDE) randomised controlled trial. Diabetologia 2012, 55, 36–45. [Google Scholar] [PubMed]
- Rashidi, H.; Ghaderian, S.B.; Shirinpour, Z.; Yazdanpanah, L.; Kaykhaei, M.A.; Aleali, A.M.; Latifi, S.M.; Bazdar, M. The effect of vitamin D supplementation on insulin resistance and glycemic control in patients with type 2 diabetes. Int. J. Pharm. Technol. 2016, 8, 11665–11674. [Google Scholar]
- Ryu, O.H.; Chung, W.; Lee, S.; Hong, K.S.; Choi, M.G.; Yoo, H.J. The effect of high-dose vitamin D supplementation on insulin resistance and arterial stiffness in patients with type 2 diabetes. Korean. J. Intern. Med. 2014, 29, 620–629. [Google Scholar] [CrossRef] [PubMed]
- Ryu, O.H.; Lee, S.; Yu, J.; Choi, M.G.; Yoo, H.J.; Mantero, F. A prospective randomized controlled trial of the effects of vitamin D supplementation on long-term glycemic control in type 2 diabetes mellitus of Korea. Endocr. J. 2014, 61, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Sadiya, A.; Ahmed, S.M.; Carlsson, M.; Ali, S.H.; Siddieq, H.H.; Abusnana, S. Vitamin D supplementation in obese type 2 diabetes subjects in Ajman, UAE: A randomized controlled double-blinded clinical trial. Eur. J. Clin. Nutr. 2015, 69, 707–711. [Google Scholar] [CrossRef] [PubMed]
- Sugden, J.A.; Davies, J.I.; Witham, M.D.; Morris, A.D.; Struthers, A.D. Vitamin D improves endothelial function in patients with Type 2 diabetes mellitus and low vitamin D levels. Diabet. Med. 2008, 25, 320. [Google Scholar] [CrossRef] [PubMed]
- Tabesh, M.; Azadbakht, L.; Faghihimani, E.; Tabesh, M.; Esmaillzadeh, A. Effects of calcium-vitamin D co-supplementation on metabolic profiles in vitamin D insufficient people with type 2 diabetes: A randomised controlled clinical trial. Diabetologia 2014, 57, 2038–2047. [Google Scholar] [CrossRef] [PubMed]
- Witham, M.D.; Dove, F.J.; Dryburgh, M.; Sugden, J.A.; Morris, A.D.; Struthers, A.D. The effect of different doses of vitamin D(3) on markers of vascular health in patients with type 2 diabetes: A randomised controlled trial. Diabetologia 2010, 53, 2112. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, R.E.; Djalali, M.; Koohdani, F.; Saboor-Yaraqhi, A.A.; Eshraqhian, M.R.; Javanbakht, M.H.; Saboori, S.; Zarei, M.; Hosseinzadeh-After, M.J. The Effects of Vitamin D Supplementation on Glucose Control and Insulin Resistance in Patients with Diabetes Type 2: A Randomized Clinical Trial Study. Iran. J. Public Health 2014, 43, 1651–1656. [Google Scholar]
- Zhou, W.; Ye, S.D. Relationship between Serum 25-Hydroxyvitamin D and Lower Extremity Arterial Disease in Type 2 Diabetes Mellitus Patients and the Analysis of the Intervention of Vitamin D. J. Diabetes Res. 2015, 2015, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Nigil, H.N.; Anton, A.; John, J.; Mittal, M. Effect of vitamin D supplementation on glycemic control in patients with type 2 diabetes: A systematic review of interventional studies. J. Diabetes Metab. Disord. 2015, 14, 3. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, Y.; Li, L.; Yu, F.; Cui, L.; Ba, Y.; Li, W.; Wang, C. Association of the vitamin D binding protein polymorphisms with the risk of type 2 diabetes mellitus: A meta-analysis. BMJ Open 2014, 4, e5617. [Google Scholar] [CrossRef] [PubMed]
- Lips, P. Worldwide status of vitamin D nutrition. J. Steroid Biochem. Mol. Biol. 2010, 121, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Rajan, S.; Weishaar, T.; Keller, B. Weight and skin colour as predictors of vitamin D status: Results of an epidemiological investigation using nationally representative data. Public Health Nutr. 2016, 20, 1857–1864. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Hu, B.; Liu, J.F.; Zha, L.X.; Zhu, X.L. Effects of Vitamin D Supplementation on Glycaemic Control, Insulin Resistance and β Cell Function in Type 2 Diabetes Mellitus: A Meta-analysis. Chin. J. Evid.-Based Med. 2016, 16, 1080–1089. (In Chinese) [Google Scholar]
- Soric, M.M.; Renner, E.T.; Smith, S.R. Effect of daily vitamin D supplementation on HbA1c in patients with uncontrolled type 2 diabetes mellitus: a pilot study. J. Diabetes 2012, 4, 104–105. [Google Scholar] [CrossRef] [PubMed]



| Author Year | Country | Participants (T/C 1) | Female (%) | Attrition Rate (%) | Age(y) (Mean ± SD) | BMI (kg/m2) 1 (Mean ± SD) | Baseline Vitamin D Level (nmol/L) (Mean ± SD) | Study Duration | Supplementation | Vitamin D Type | Dose & Frequency | Outcomes 2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baziar 2014 [21] | Iran | 41/40 | 33.3 | 6.9 | T: 50.34 ± 6.71 C: 52.75 ± 6.34 | T: 27.33 ± 1.64 C: 27.25 ± 1.35 | T: 37.26 ± 15.21 C: 40.30 ± 14.43 | 8 weeks | Vit D | VD3 | 50,000 IU/week | ①②③④ |
| Maggi 2014 [28] | Italy | 14/16 | 23.3 | 0.0 | 69 | 29 | T: 27.79 ± 10.81 C: 33.73 ± 17.13 | 24 weeks | Vit D | VD3 | 300,000 IU once | ①② |
| Anyanwu 2016 [20] | Nigeria | 17/16 | 57.6 | 21.4 | T: 52.5 ± 2.2 C: 51.1 ± 1.9 | NR 1 | T: 17.9 ± 2.3 C: 19.2 ± 5.5 | 12 weeks | Vit D | VD3 | 3000 IU/day | ①② |
| Eftekhari 2011 [22] | Iran | 35/35 | 50.0 | 0.0 | T: 53.8 ± 8.9 C: 52.4 ± 7.8 | T: 28.3 ±4.4 C: 27.0 ± 3.4 | T: 112.6 ± 83.5 C: 100.1 ± 77.7 | 12 weeks | Vit D | VD3 | 20 IU/day | ①②③④ |
| Ghavamzadeh 2014 [23] | Iran | 26/25 | 58.8 | 57.5 | T: 52.26 ± 2.09 C: 49.28 ± 2.00 | T: 28.9 ± 0.86 C: 27.9 ± 0.93 | T: 21.46 ± 4.65 C: 22.16 ± 5.32 | 14 weeks | Vit D | VD3 | 400 IU/day | ①③ |
| Jorde 2009 [24] | Norway | 16/16 | 43.8 | 11.1 | T: 57.7 ± 9.7 C: 54.8 ± 5.9 | T: 32.8 ± 6.8 C: 31.3 ± 6.3 | T: 60.0 ± 14.0 C: 58.5 ± 21.0 | 6 months | Vit D | VD3 | 40,000 IU/week | ①②③④ |
| Kampmann 2014 [25] | Denmark | 7/8 | 53.3 | 6.3 | T: 61.6 ± 4.4 C: 57 ± 4.5 | T: 35.3 ± 2.9 C: 32.4 ± 2.0 | T: 31.0 ± 4.9 C: 34.8 ± 3.8 | 12 weeks | Vit D | VD3 | 11,200 IU/day × 2 weeks, then 5600 IU/day × 10 weeks | ①②③⑤ |
| Krul-Poel 2015 [27] | The Netherlands | 129/132 | 34.9 | 5.1 | T: 67 ± 8 C: 67 ± 9 | T: 28.7 ± 4.6 C: 28.5 ± 4.5 | T: 60.6 ± 23.3 C: 59.1 ± 23.2 | 6 months | Vit D | VD3 | 50,000 IU/month | ①②③ |
| Nasri 2014 [29] | Iran | 30/30 | 71.7 | 0.0 | 55 ± 10.7 | NR | T: 83.9 ± 52 C: 105.7 ± 64 | 12 weeks | Vit D | VD3 | 50,000 IU/week | ①③ |
| Ryu 2014 a [32] | Korea | 32/30 | NR | 23.5 | T: 54.5 ± 7.4 C: 56.7 ± 7.9 | T: 24.4 ± 5.0 C: 25.3 ± 3.4 | T: 32.0 ± 7.8 C: 27.8 ± 6.8 | 6 months | Vit D + Ca | VD3 | 2000 IU/day | ①②③ |
| Sadiya 2015 [34] | UAE1 | 43/39 | 81.6 | 5.7 | T: 49 ± 8 C: 48 ± 8 | T: 37.9 ± 6.1 C: 37.6± 7.7 | T: 28.5 ± 9.2 C: 30.5 ± 11.3 | 6 months | Vit D | VD3 | 6000 IU/day × 3 months, then 3000 IU/day × 3 months | ①②③ |
| Yousefi 2014 [38] | Iran | 28/30 | 37.9 | 10.8 | T: 50.03 C: 49.90 | T: 27.94 ± 0.92 C: 28.75 ± 0.95 | T: 40.43 ± 4.97 C: 38.06 ± 5.77 | 2 months | Vit D | NR | 4000 IU/day | ①②③④⑤ |
| Tabesh 2014 [36] | Iran | 29/30 | 50.0 | 1.7 | T: 50.2 ± 6.6 C: 51.0 ± 6.1 | T: 30.5 ± 5.3 C: 30.3 ± 3.8 | T: 28.0 ± 13.9 C: 45.7 ± 16.4 | 2 months | Vit D | VD3 | 50,000 U/week | ④ |
| Witham 2010 [37] | UK 1 | 19/21 | 32.8 | 2.4 | T: 65.3 ± 11.1 C: 66.7 ± 9.7 | T: 31.1 ± 6.7 C: 33.3 ± 7.1 | T: 41 ± 14 C: 45 ± 17 | 4 months | Vit D | VD3 | 100,000 IU once | ①③④ |
| Ryu 2014 b [33] | Korea | 64/65 | 50.0 | 18.4 | T:54.8 ± 7.6 C:55.9 ± 8.1 | T: 25.0 ± 3.3 C: 25.6 ± 3.6 | T: 28.08 ± 13.26 C: 26.26 ± 10.14 | 6 months | Vit D +Ca | VD3 | 2000 IU/day | ①②③ |
| Punthakee 2012 [30] | 33 countries | 607/614 | 40.9 | 0.9 | T: 66.7 ± 6.7 C: 66.6 ± 6.3 | T: 30.6 ± 5.3 C: 30.7 ± 5.3 | NR | 4 months | Vit D | VD3 | 1000 IU/day | ②③ |
| Sugden 2008 [35] | UK 1 | 17/17 | 47.1 | 21.0 | T: 64.9 ± 10.3 C: 63.5 ± 9.5 | T: 31.7 ±6.4 C: 31.7 ± 6.5 | T: 40.2 ± 10.3 C: 36.4 ± 8.5 | 2 months | Vit D | VD2 | 100,000 IU once | ①③ |
| Rashidi 2016 [31] | Iran | 48/46 | 41.7 | 13.0 | 47 | T: 28.08 ± 3.46 C: 28.65 ± 2.9 | NR | 3 months | Vit D | NR | 50,000 IU/2 weeks | ③ |
| Kim 2014 [26] | Korea | 11/13 | 100.0 | 13.3 | T: 73.27 ± 2.06 C: 70.08 ± 1.37 | T: 24.08 ± 0.73 C: 23.72 ± 0.68 | T: 27.14 ± 4.68 C: 30.32 ± 7.28 | 3 months | Vit D | VD3 | 1200 IU/day | ③④⑤ |
| Zhou 2015 [39] | China | 31/31 | 38.7 | 9.7 | 58.85 ± 6.18 | T: 25.05 ± 3.30 C: 24.09 ± 3.77 | T: 32.21 ± 21.76 C: 34.58 ± 20.18 | 3 months | Vit D | VD3 | 1000 IU/day | ①③ |
| Subgroups | Serum Vitamin D | FBG 1 | HbA1c 1 | HOMA-IR 1 | Fasting Insulin | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WMD 1 | 95%CI | p | WMD | 95%CI | p | WMD | 95%CI | p | SMD | 95%CI | p | SMD | 95%CI | p | ||
| Ethnicity | ||||||||||||||||
| Middle Easterners | 40.03 | (27.71, 52.34) | <0.001 | −10.43 | (−14.80, −6.06) | <0.001 | −0.36 | (−0.87, 0.15) | 0.170 | −0.65 | (−1.37, 0.08) | 0.081 | −1.48 | (−3.59, 0.63) | 0.170 | |
| Other Asians | 32.23 | (22.72, 41.73) | <0.001 | −1.83 | (−7.80, 4.15) | 0.549 | −0.18 | (−0.77, 0.42) | 0.565 | −1.59 | (−2.42, −0.76) | <0.001 | −1.80 | (−2.66, −0.95) | <0.001 | |
| Other ethnicities | 30.72 | (12.25, 49.19) | 0.001 | 1.67 | (−1.50, 4.85) | 0.301 | 0.17 | (0.00, 0.33) | 0.044 | 0.01 | (−0.45, 0.47) | 0.961 | 1.67 | (0.47, 2.87) | 0.006 | |
| BMI 1 | ||||||||||||||||
| Normal 2 | 38.20 | (27.89, 48.51) | <0.001 | −5.21 | (−11.76, 1.34) | 0.119 | 0.16 | (−0.13, 0.45) | 0.274 | −1.59 | (−2.42, −0.76) | <0.001 | −1.80 | (−2.66, −0.95) | <0.001 | |
| Overweight 2 | 33.01 | (23.94, 42.09) | <0.001 | −5.71 | (−14.09, 2.66) | 0.181 | −0.32 | (−0.70, 0.06) | 0.102 | −0.84 | (−1.78, 0.11) | 0.084 | −1.48 | (−3.59, 0.63) | 0.170 | |
| Obese 2 | 39.34 | (18.71, 59.97) | <0.001 | 0.64 | (−3.32, 4.60) | 0.751 | 0.22 | (0.05, 0.38) | 0.010 | −0.04 | (−0.38, 0.30) | 0.825 | 1.67 | (0.47, 2.87) | 0.006 | |
| Dose | ||||||||||||||||
| ≤2000 IU/day | 25.16 | (18.16, 32.16) | <0.001 | 0.35 | (−3.18, 3.89) | 0.844 | −0.21 | (−0.53, 0.11) | 0.189 | −0.50 | (−1.35, 0.35) | 0.249 | −1.80 | (−2.66, −0.95) | <0.001 | |
| >2000 IU/day | 48.45 | (29.94, 66.97) | <0.001 | −8.70 | (−12.96, −4.44) | <0.001 | 0.05 | (−0.41, 0.51) | 0.832 | −0.64 | (−1.42, 0.14) | 0.107 | −0.50 | (−2.44, 1.45) | 0.617 | |
| Duration | ||||||||||||||||
| ≤3 m | 35.51 | (18.84, 52.18) | <0.001 | −8.44 | (−12.72, −4.15) | <0.001 | −0.11 | (−0.42, 0.21) | 0.590 | −0.81 | (−1.49, −0.13) | 0.019 | ---- | ---- | ---- | |
| >3 m | 32.61 | (24.951, 40.271) | <0.001 | 2.04 | (-0.94, 5.02) | 0.180 | −0.12 | (−0.54, 0.31) | 0.509 | 0.01 | (−0.45, 0.47) | 0.961 | ---- | ---- | ---- | |
| Baseline 25(OH)D | ||||||||||||||||
| <50 nmol/L | 31.65 | (21.31, 41.99) | <0.001 | −5.77 | (−10.48, −1.05) | 0.017 | −0.11 | (−0.47, 0.26) | 0.563 | −0.81 | (−1.51, −0.11) | 0.024 | ---- | ---- | ---- | |
| 50–75 nmol/L | 48.33 | (29.46, 67.21) | <0.001 | 3.37 | (−1.81, 8.54) | 0.202 | −0.00 | (−0.14, 0.14) | 1.000 | 0.03 | (−0.67, 0.72) | 0.941 | ---- | ---- | ---- | |
| >75 nmol/L | 32.98 | (−39.64, 105.06) | 0.373 | −18.00 | (−43.24, 7.24) | 0.162 | −0.39 | (−0.78, 0.00) | 0.052 | −0.08 | (−0.55, 0.39) | 0.737 | ---- | ---- | ---- | |
| Baseline HbA1c 1 | ||||||||||||||||
| ≤7% | 45.27 | (25.39, 65.15) | <0.001 | −4.09 | (−15.44, 7.27) | 0.481 | −0.17 | (−0.86, 0.52) | 0.635 | −0.27 | (−0.65, 0.11) | 0.160 | 0.55 | (−1.49,2.59) | 0.53 | |
| >7% | 29.86 | (18.22, 41.49) | <0.001 | −3.53 | (−9.42, 2.35) | 0.240 | −0.08 | (−0.34, 0.18) | 0.548 | −0.65 | (−1.83, 0.54) | 0.286 | −2.57 | (−3.27, −1.87) | <0.001 | |
| Overall | 33.98 | (24.60, 43.37) | <0.001 | −3.59 | (−7.94, 0.76) | 0.105 | −0.11 | (−0.35, 0.13) | 0.381 | −0.58 | (−1.11, −0.05) | 0.033 | −0.83 | (−2.31, 0.64) | 0.268 | |
| Outcomes | No. of Studies | Limitations | Inconsistency | Indirectness | Imprecision | Publication Bias | Evidence Quality | |
|---|---|---|---|---|---|---|---|---|
| Serum vitamin D levels | 16 | Serious 2,3,4 | Serious 5 | Not serious | Not serious | Not found | ⊕⊕⊝⊝ | Low |
| FBG 1 | 13 | Serious 2,3,4 | Serious 5 | Not serious | Serious 6 | Not found | ⊕⊝⊝⊝ | Very low |
| HbA1c 1 | 15 | Serious 3,4 | Serious 5 | Not serious | Serious 6 | Not found | ⊕⊝⊝⊝ | Very low |
| HOMA-IR 1 | 7 | Serious 3,4 | Serious 5 | Not serious | Not serious | Not assessed 7 | ⊕⊕⊝⊝ | Low |
| Fasting serum insulin | 4 | Serious 4 | Serious 5 | Not serious | Serious 6 | Not assessed 7 | ⊕⊝⊝⊝ | Very low |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Liu, Y.; Zheng, Y.; Wang, P.; Zhang, Y. The Effect of Vitamin D Supplementation on Glycemic Control in Type 2 Diabetes Patients: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 375. https://doi.org/10.3390/nu10030375
Li X, Liu Y, Zheng Y, Wang P, Zhang Y. The Effect of Vitamin D Supplementation on Glycemic Control in Type 2 Diabetes Patients: A Systematic Review and Meta-Analysis. Nutrients. 2018; 10(3):375. https://doi.org/10.3390/nu10030375
Chicago/Turabian StyleLi, Xinyi, Yan Liu, Yingdong Zheng, Peiyu Wang, and Yumei Zhang. 2018. "The Effect of Vitamin D Supplementation on Glycemic Control in Type 2 Diabetes Patients: A Systematic Review and Meta-Analysis" Nutrients 10, no. 3: 375. https://doi.org/10.3390/nu10030375





