Chemopreventive Effect of Aster glehni on Inflammation-Induced Colorectal Carcinogenesis in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Standardization of the Extract of A. glehni
2.3. Experimental Animals
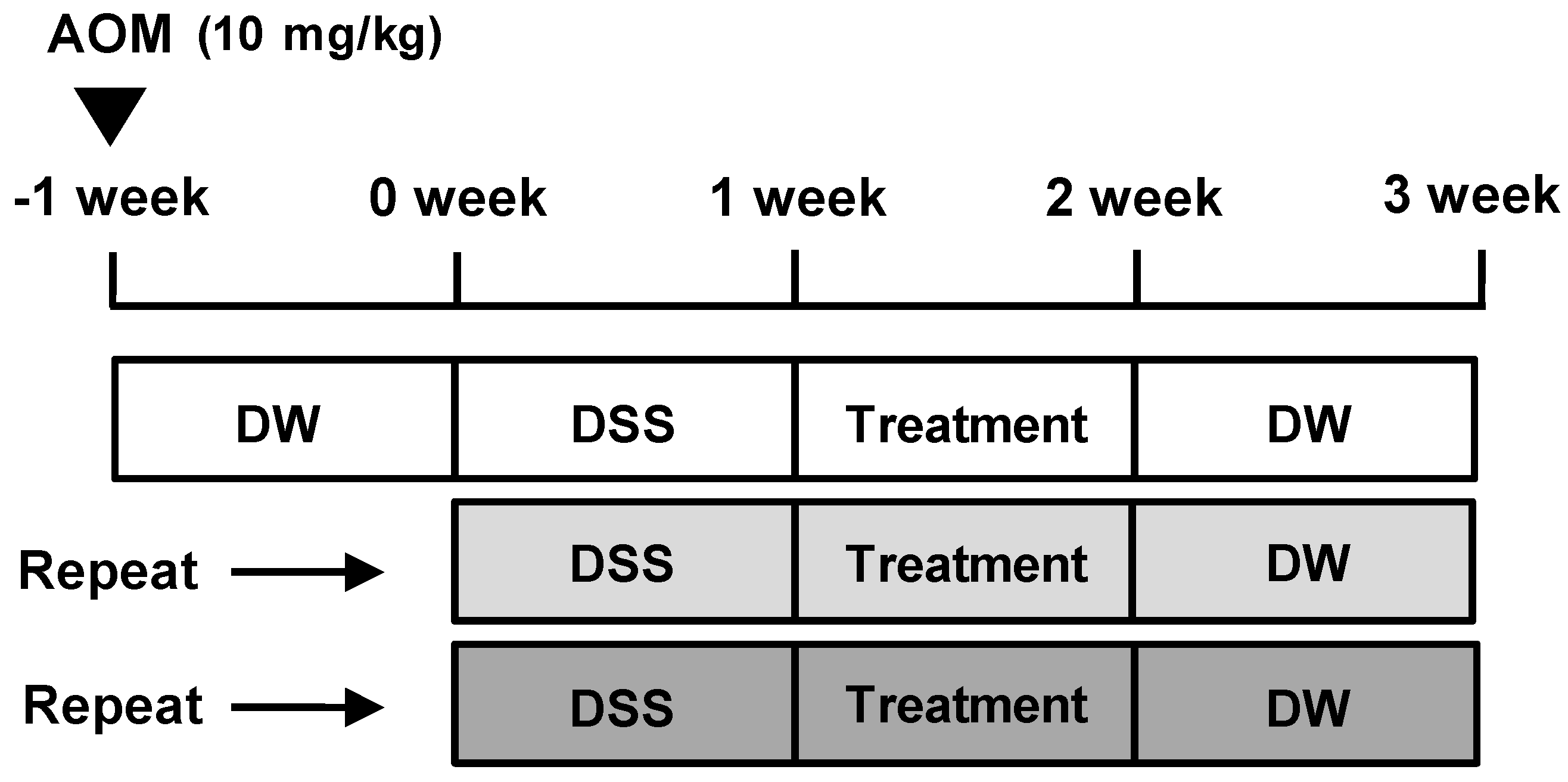
2.4. AOM/DSS-Induced CAC Model and Treatment
2.5. Histopathological Analysis
2.6. Measurement of Cytokine Production
2.7. Western Blot Analysis
2.8. Nuclear Extraction
2.9. Statistical Analyses
3. Results
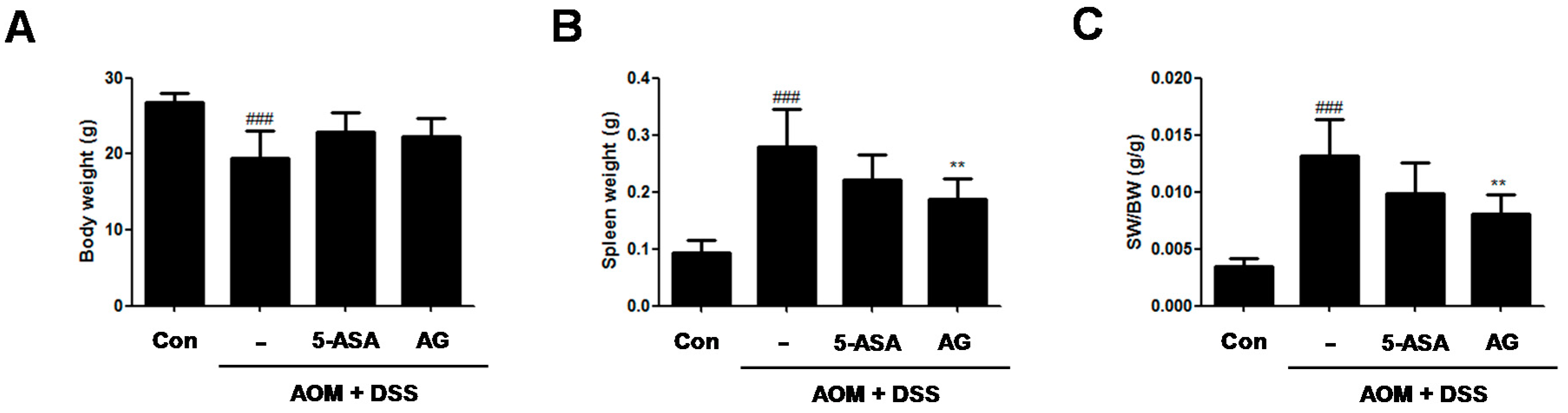
3.1. AG Improved AOM/DSS-Induced Spleen Enlargement
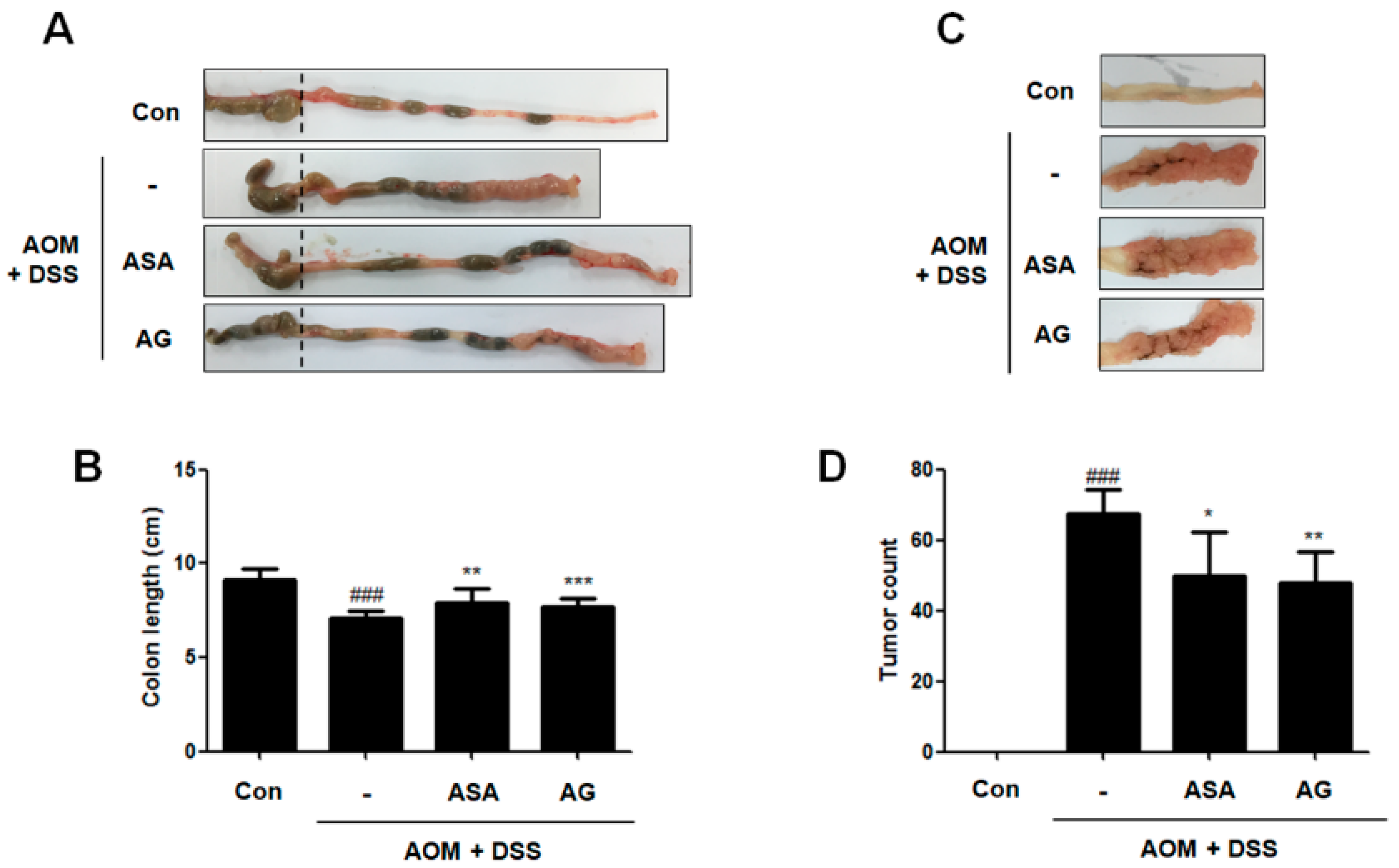
3.2. AG Prevented AOM/DSS-Induced Colon Shortening and Tumor Formation
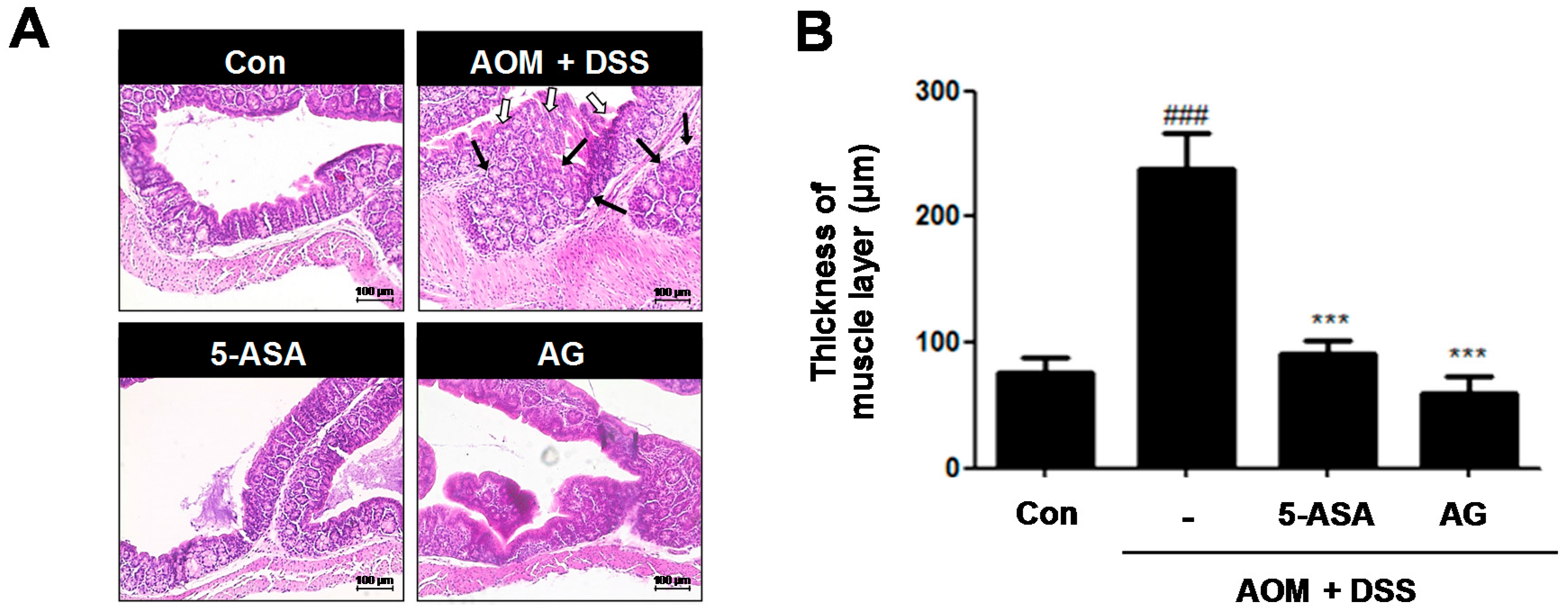
3.3. AG Ameliorated AOM/DSS-Induced Histopathological Signs and Thickness of Muscle Layer in the Colon
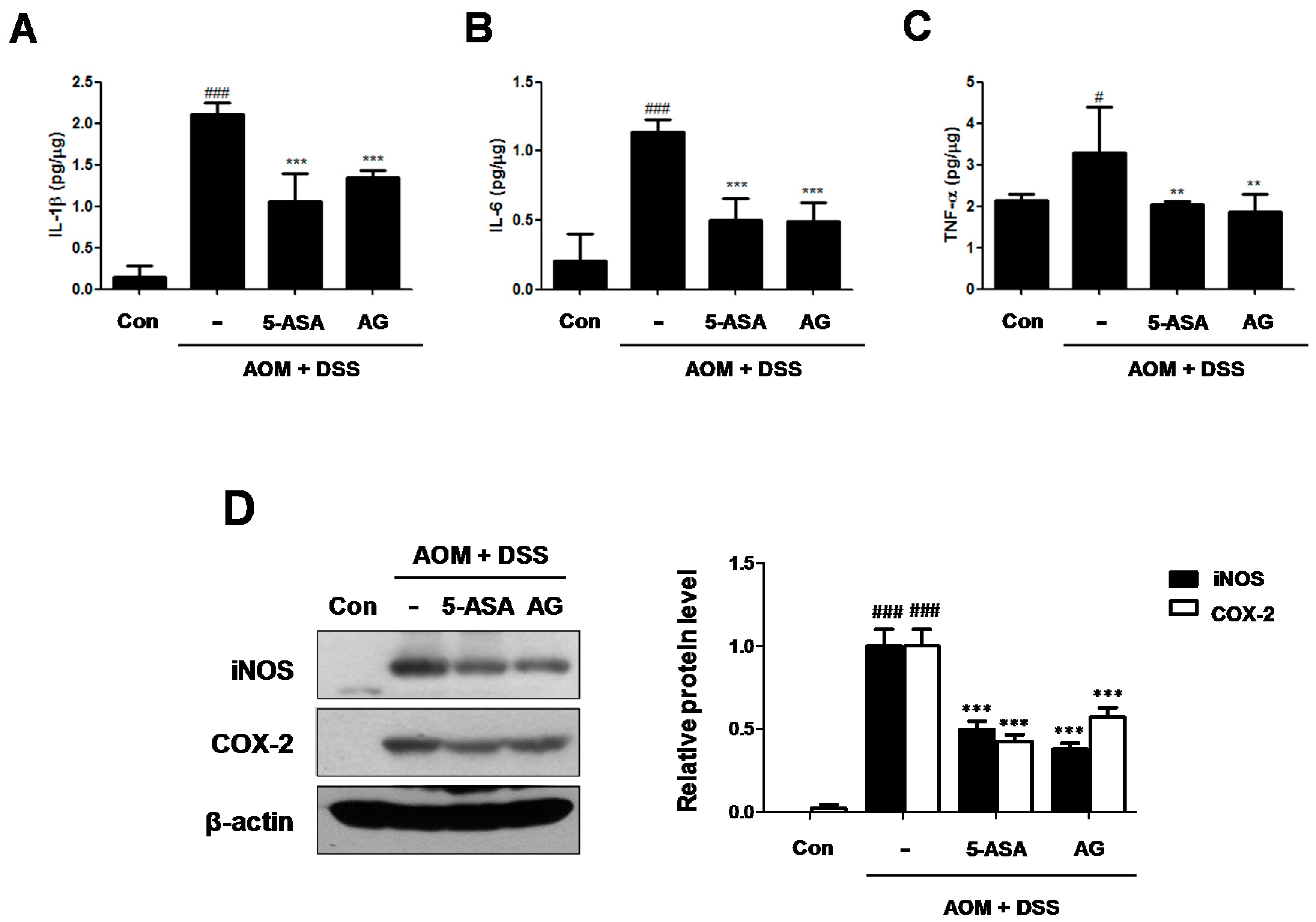
3.4. AG Attenuated the Production of Inflammatory Cytokines and the Expression of Inflammatory Proteins in AOM/DSS-Treated Mice
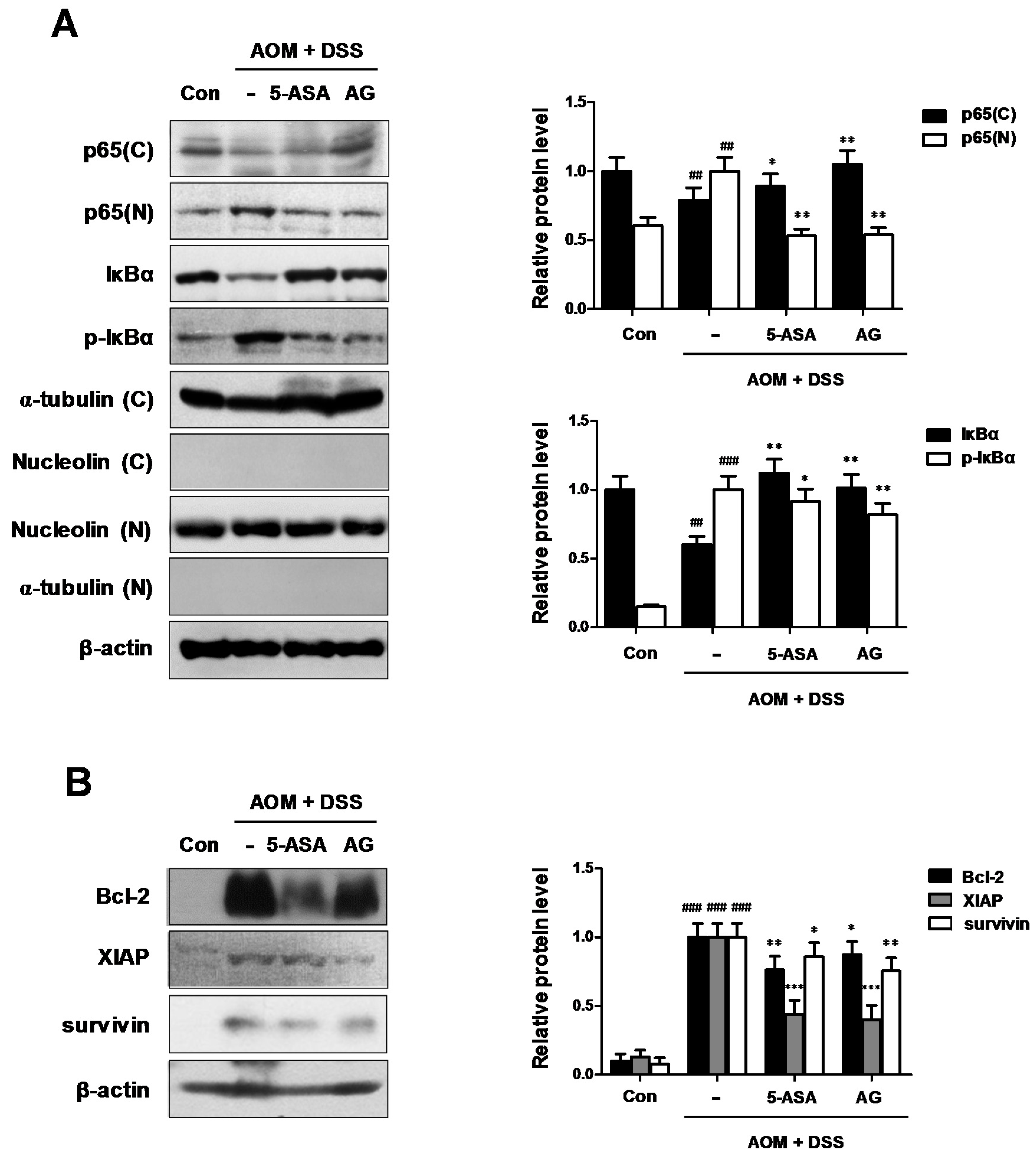
3.5. AG Downregulated NF-κB Activation Involving NF-κB-Associated Protein Expression in AOM/DSS-Treated Mice
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflict of Interest
References
- McCarthy, N. Tumorigenesis: All together now. Nat. Rev. Cancer 2013, 13, 148. [Google Scholar] [CrossRef] [PubMed]
- Feagins, L.A.; Souza, R.F.; Spechler, S.J. Carcinogenesis in IBD: Potential targets for the prevention of colorectal cancer. Nat. Rev. Gastroenterol. Hepatol. 2009, 6, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Lakatos, P.L.; Lakatos, L. Risk for colorectal cancer in ulcerative colitis: Changes, causes and management strategies. World J. Gastroenterol. 2008, 14, 3937–3947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terzic, J.; Grivennikov, S.; Karin, E.; Karin, M. Inflammation and colon cancer. Gastroenterology 2010, 138, 2101–2114. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.G.; Murillo, G.; Naithani, R.; Peng, X. Cancer chemoprevention by natural products: How far have we come? Pharm. Res. 2010, 27, 950–961. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.H.; Lai, C.S.; Wu, J.C.; Ho, C.T. Molecular mechanisms for chemoprevention of colorectal cancer by natural dietary compounds. Mol. Nutr. Food Res. 2011, 55, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Chung, K.S.; Jin, B.R.; Cheon, S.Y.; Nugroho, A.; Roh, S.S.; An, H.J. Anti-inflammatory effects of an ethanol extract of Aster glehni via inhibition of NF-kB activation in mice with DSS-induced colitis. Food Funct. 2017, 8, 2611–2620. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.M.; Yang, G.; Ahn, T.G.; Kim, M.D.; Nugroho, A.; Park, H.J.; Lee, K.T.; Park, W.; An, H.J. Antiadipogenic effects of Aster glehni extract: In vivo and in vitro effects. Evid. Based Complement. Alternat. Med. 2013, 2013, 859624. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.S.; Choi, H.E.; Shin, J.S.; Cho, E.J.; Cho, Y.W.; Choi, J.H.; Baek, N.I.; Lee, K.T. Chemopreventive effects of standardized ethanol extract from the aerial parts of Artemisia princeps Pampanini cv. Sajabal via NF-κB inactivation on colitis-associated colon tumorigenesis in mice. Food Chem. Toxicol. 2015, 75, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr. Protoc. Immunol. 2014, 104, 15–25. [Google Scholar]
- Blennerhassett, M.G.; Bovell, F.M.; Lourenssen, S.; McHugh, K.M. Characteristics of inflammation-induced hypertrophy of rat intestinal smooth muscle cell. Dig. Dis. Sci. 1999, 44, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Burstein, E.; Fearon, E.R. Colitis and cancer: A tale of inflammatory cells and their cytokines. J. Clin. Investig. 2008, 118, 464–467. [Google Scholar] [CrossRef] [PubMed]
- Karin, M. Nuclear factor-κB in cancer development and progression. Nature 2006, 441, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Shin, H.R.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D.M. Estimates of worldwide burden of cancer in 2008: Globocan 2008. Int. J. Cancer 2010, 127, 2893–2917. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.D.; Hord, N.G. Nutritional “omics” technologies for elucidating the role(s) of bioactive food components in colon cancer prevention. J. Nutr. 2005, 135, 2694–2697. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.I. Inflammation and colorectal cancer: Colitis-associated neoplasia. Semin. Immunopathol. 2013, 35, 229–244. [Google Scholar] [CrossRef] [PubMed]
- Fantini, M.C.; Pallone, F. Cytokines: From gut inflammation to colorectal cancer. Curr. Drug Targets 2008, 9, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, M.; LaRusso, N.F.; Gores, G.J. Nitric oxide in gastrointestinal epithelial cell carcinogenesis: Linking inflammation to oncogenesis. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 281, G626–G634. [Google Scholar] [CrossRef] [PubMed]
- Villegas, I.; Sanchez-Fidalgo, S.; de la Lastra, C.A. Chemopreventive effect of dietary curcumin on inflammation-induced colorectal carcinogenesis in mice. Mol. Nutr. Food Res. 2011, 55, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Viennois, E.; Chen, F.; Merlin, D. NF-κB pathway in colitis-associated cancers. Transl. Gastrointest. Cancer 2013, 2, 21–29. [Google Scholar] [PubMed]
- Ahmed, M.Z.D.; Dewan, M.Z.; Xu, R. Nuclear factor-κB in inflammatory bowel disease and colorectal cancer. Am. J. Dig. Dis. 2014, 1, 84–96. [Google Scholar]
- Gewirtz, A.T.; Rao, A.S.; Simon, P.O., Jr.; Merlin, D.; Carnes, D.; Madara, J.L.; Neish, A.S. Salmonella typhimurium induces epithelial IL-8 expression via Ca2+-mediated activation of the NF-κB pathway. J. Clin. Investig. 2000, 105, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Kucharczak, J.; Simmons, M.J.; Fan, Y.; Gelinas, C. To be, or not to be: NF-κB is the answer—Role of Rel/NF-kB in the regulation of apoptosis. Oncogene 2003, 22, 8961–8982. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, M.; Metcalf, D.; Merryfull, J.; Beg, A.; Baltimore, D.; Gerondakis, S. The combined absence of the transcription factors Rel and RelA leads to multiple hemopoietic cell defects. Proc. Natl. Acad. Sci. USA 1999, 96, 11848–11853. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Minemoto, Y.; Dibling, B.; Purcell, N.H.; Li, Z.; Karin, M.; Lin, A. Inhibition of JNK activation through NF-κB target genes. Nature 2001, 414, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Altieri, D.C. Survivin—The inconvenient IAP. Semin. Cell Dev. Biol. 2015, 39, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Nigam, J.; Chandra, A.; Kazmi, H.R.; Parmar, D.; Singh, D.; Gupta, V.; Noushif, M. Expression of survivin mRNA in gallbladder cancer: A diagnostic and prognostic marker? Tumour Biol. 2014, 35, 9241–9246. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, K.-S.; Cheon, S.-Y.; Roh, S.-S.; Lee, M.; An, H.-J. Chemopreventive Effect of Aster glehni on Inflammation-Induced Colorectal Carcinogenesis in Mice. Nutrients 2018, 10, 202. https://doi.org/10.3390/nu10020202
Chung K-S, Cheon S-Y, Roh S-S, Lee M, An H-J. Chemopreventive Effect of Aster glehni on Inflammation-Induced Colorectal Carcinogenesis in Mice. Nutrients. 2018; 10(2):202. https://doi.org/10.3390/nu10020202
Chicago/Turabian StyleChung, Kyung-Sook, Se-Yun Cheon, Seong-Soo Roh, Minho Lee, and Hyo-Jin An. 2018. "Chemopreventive Effect of Aster glehni on Inflammation-Induced Colorectal Carcinogenesis in Mice" Nutrients 10, no. 2: 202. https://doi.org/10.3390/nu10020202





