1. Introduction
Secondary hyperparathyroidism (SHPT) is a major complication among dialysis patients which can have renal osteodystrophy and cardiovascular consequences [
1,
2]. Dietary control, phosphate binders and active vitamin D analogs are used in earlier SHPT whereas calcimimetic agent, cinacalcet, is indicated in later stages of SHPT cases who have markedly elevated parathyroid hormone (PTH) levels or failed to respond earlier treatment. The doses of active vitamin D analogs are reduced or cinacalcet is added when the patients have progressively high calcium, phosphate and Ca × P products to prevent cardiovascular and soft-tissue calcification [
3,
4]. Many studies demonstrated that cinacalcet improves PTH control and achieves recommended serum calcium and phosphorus values when used in combination with active vitamin D analogs and phosphate binders [
5,
6,
7,
8,
9]. In our previous study, we revealed additional PTH lowering and anti-inflammatory effects of nutritional vitamin D (cholecalciferol) supplementation to active vitamin D analogs in SHPT patients [
10]. However, data regarding the addition of cholecalciferol supplementation to combined cinacalcet and active vitamin D analogs in severe SHPT patients is still lacking.
Circulating PTH levels were found to be inversely correlated with serum 25(OH) D
3, a circulating vitamin D metabolite which indicated the vitamin D status [
11,
12]. 25(OH)D
3 was an important substrate for the local generation of 1,25(OH)
2D with the help of local 1α-hydroxylase (1α-OHase) activity [
13,
14]. Most end-stage renal disease (ESRD) patients had low serum 25(OH)D
3 levels [
15,
16]. Segersten et al. [
17] revealed 1α-OHase expression in parathyroid glands which presumably suppressed the PTH gland hyperplasia in an autocrine/paracrine manner. Previous research revealed coincident increased expression of 1α-OHase (approximately increased in 10 folds) and reduced 24-hydroxylase in most SHPT glands [
18] and highlighted the requirement of more 25(OH)D
3 in these patients. CS Ritter et al. [
19] proved that the local effect of 25(OH)D
3 on PTH suppression possibly occurs through direct activation of the vitamin D receptor (VDR) in parathyroid glands. Furthermore, 25(OH)D
3 played less role in systemic hypercalcemia and related complications. These findings explain the possible additive role of nutritional vitamin D (cholecalciferol) supplementation in SHPT patients.
Among two types of parathyroid cells (chief cells (CC) and oxyphil cells (OC)), OC markedly increased in chronic kidney disease (CKD) [
20]. Lomonte C et al. revealed calcitriol therapy significantly increases the OC content in parathyroid glands [
21]. Cinacalcet acts through CaR in the CC of parathyroid glands and exerts antiproliferative and proapoptotic action [
22,
23]. Studies revealed that cinacalcet significantly increases the OC/CC ratio (approx. increase 3.42 times) [
24] and increases the oxyphil area [
25]. These OC excessively express 1α OHase enzyme and increase local calcitriol production [
26], which further carries out autocrine/paracrine regulation of PTH synthesis and release. Thus, cinacalcet use further increases the requirement of substrate chocalciferol for 1α OHase to produce local calcitriol production in OC. Calcimimetics also up-regulate decreased parathyroid CaR and VDR in both in vitro and in vivo studies [
27,
28] which further mediates parathyroid proliferation.
Therefore, we speculate that combining cinacalcet to calcitriol therapy increases the parathyroid OC/CC ratio, increases local 1αOHase activity, and increases VDR expression which additively needs more 25(OH)D3 for local calcitriol synthesis. We hypothesize that the cholecalciferol supplementation in SHPT patients together with calcitriol and cinacalcet therapy increase local calcitriol production, which further suppresses intact parathyroid hormone (iPTH) secretion. We further describe the changes in bone turnover markers and bone densities with or without cholecalciferol supplementation in SHPT hemodialysis patients.
2. Materials and Methods
2.1. Study Design
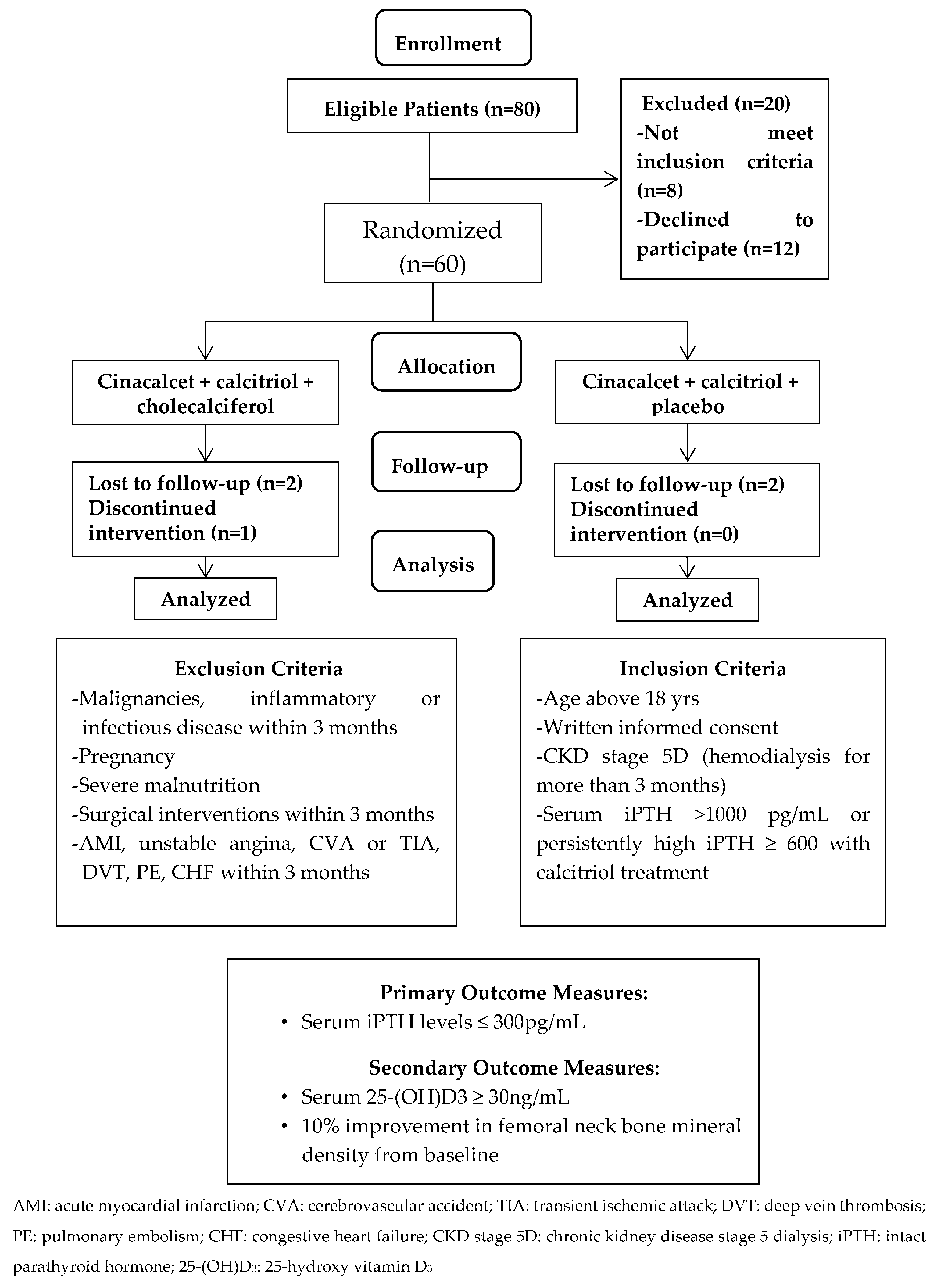
The trial was designed as a randomized, controlled open-label study. A total of 80 patients were eligible and agreed to participate. Group matching with gender, age (within 5 years), and duration of hemodialysis (within 1 year) was conducted for every pair group and they were then randomly assigned to either treatment with cinacalcet, calcitriol and cholecalciferol (CCC, study group) or control group, treated with cinacalcet, calcitriol and placebo (CCP, control group). G*power was used to calculate the required sample size [
29] and effects were detected in a two-sided test with a power of (1 − β) = 80% at a significance level of 0.05. Other calculation settings were as follows: (1) the randomization process was based on 1:1 proportion of this study; (2) the effect size was set as 0.8. The required sample size for calculating was at least 25 subjects in the CCC group and 25 subjects in the CCP group.
2.2. Patient Eligibility and Randomization
Patients aged above 18 years treated with maintenance hemodialysis three times per week for at least 3 months before screening were eligible. Patients with severe SHPT (serum iPTH > 1000 pg/mL or persistantly high serum iPTH ≥ 600 pg/mL even after more than 3 months of calcitriol treatment) were enrolled. All patients needed to stop receiving an active vitamin D at least 30 days before entering the study. Patients using calcimimetic agents and/or native vitamin D analogs were excluded. The patients were excluded if they were pregnant, breastfeeding or of childbearing potential and not practicing birth control. Those with malignancies, severe malnutrition, and inflammatory or infectious disorders diagnosed for more than 3 months before the study were also excluded. Other exclusion criteria included surgical interventions and vascular diseases, including acute coronary syndrome, unstable angina, cerebrovascular accident, transient ischemic attack, deep vein thrombosis, pulmonary embolism, or congestive heart failure within 3 months of the study period. The patients were also excluded if they had a history of allergy to medications (
Figure 1). Sixty hemodialysis (HD) patients fulfilled the criteria and were randomized into the CCC study group (
N = 30) and CCP control group (
N = 30). Patients were well matched by treatment allocation (
Figure 1). Protocol and informed consent were approved by the authorities of the Institutional Review Board of Cardinal Tien Hospital and Taipei Medical University (CTH106A-2B01 and CTH-104-3-5-022). All the patients provided written consent before study enrollment.
2.3. Treatment Intervention
All patients were given oral cinacalcet (30 mg/day) from the start of the study. Patients in the study group were given an oral form of cholecalciferol 5000 IU per day (Healthy Origens, Pittsburgh, PA 15241, USA); and cholecalciferol placebo (olive oil) was given in the control group. During the treatment, the calcitriol dose could be decreased when iPTH was ≤ 300 pg/mL and Ca × P was >55 mg2/dl2. The doses of Ca-based and other phosphate binders could be adjusted throughout the study. Ca-based phosphate binders could be increased when the serum Ca was <8.4 mg/dL or the patient had symptoms of hypocalcemia. Cinacalcet was given in a fixed low dose (30 mg/day) during the whole study period, and was withdrawn if the serum iPTH was persistently low (iPTH ≤ 300 pg/mL) for 4 weeks after the reduction of calcitriol or serum calcium <8.4 mg/dl or the patient had symptoms of hypocalcemia. All patients used low dialysate Ca 2.5 mEq/L and adjusted according to serum Ca levels throughout the study. A baseline visit was performed just before the start of the study, and further study visits were performed at 4, 8, 12, 16, 20 and 24 weeks after the medication. Serum biochemical parameters and bone turnover markers were assessed at baseline and during follow-up. The femoral neck (FN) and lumbar spine (LS) bone mineral density (BMD) were determined by dual X-ray absorptiometry (DXA) before and at the end of the study period. Hospital records were obtained and examined by two practicing nephrologists.
2.4. Serum Biochemical and Bone Metabolism Parameters
Blood samples were collected and serum was separated within 1 h of collection and immediately frozen until analysis. Serum levels of iPTH, 25(OH)D3, phosphorus, calcium and other bone metabolism parameters were determined. Serum iPTH levels were measured in an immunoradiometric assay (Nichols Institute Diagnostics, San Juan Capistrano, CA, USA). Serum 25(OH)D3 was determined by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions (Immundiagnostik AG, Bensheim, Germany). Serum bone specific alkaline phosphatase (BAP), a marker of bone formation was determined by ELISA (Quidel, Inc., San Diego, CA, USA); whereas tartrate-resistant acid phosphatase (TRACP)-5b, a marker of bone resorption was measured by ELISA (Quidel®, Tecomedical Group, Sissach, Switzerland). The doses of calcitriol, cinacalcet and phosphate binders were recorded at each visit. Adverse events were collected from patients’ reports and in response to non-directed questioning at each study visit.
2.5. Objectives and Outcomes and Measures
Our study used the Kidney Disease Outcomes Quality Initiative (K/DOQI) [
30,
31] targeted bone metabolism levels; serum Ca 8.4–9.5 mg/dL, P 3.5–5.5 mg/dL, iPTH 150–300 pg/mL. Interpretation of DXA scans using lumbar spine BMD was not precise in our patients since it might have been interfered with by aorta calcification and degenerative joint diseases. Thus, we used 10% improvement in femoral BMD instead of lumbar spine BMD as our outcome.
Primary Outcome: The primary outcome measure was serum iPTH ≤ 300 pg/mL.
Secondary Outcome: The secondary outcome measures were serum 25-(OH)D3 ≥ 30 ng/mL and 10% improvement in femoral neck BMD from baseline.
2.6. Data Collection and Statistical Analysis
The results were expressed as mean ± standard deviation or median (interquartile range). Parametric or non-parametric tests were used for analysis; for paired data, the Student t or Wilcoxon tests, respectively, and for between-group comparisons, the Student t, one-way ANOVA or Mann–Whitney U tests were used. Unilateral correlation analysis was performed using Pearson (r) or Spearman correlation (rs), as appropriate. All the tests were two-sided, and p < 0.05 was considered statistically significant. Statistica (Version 11, Stat Soft, Inc., 2300 East 14th Street, Tulsa, OK 74104, USA) was used for calculations.
4. Discussion
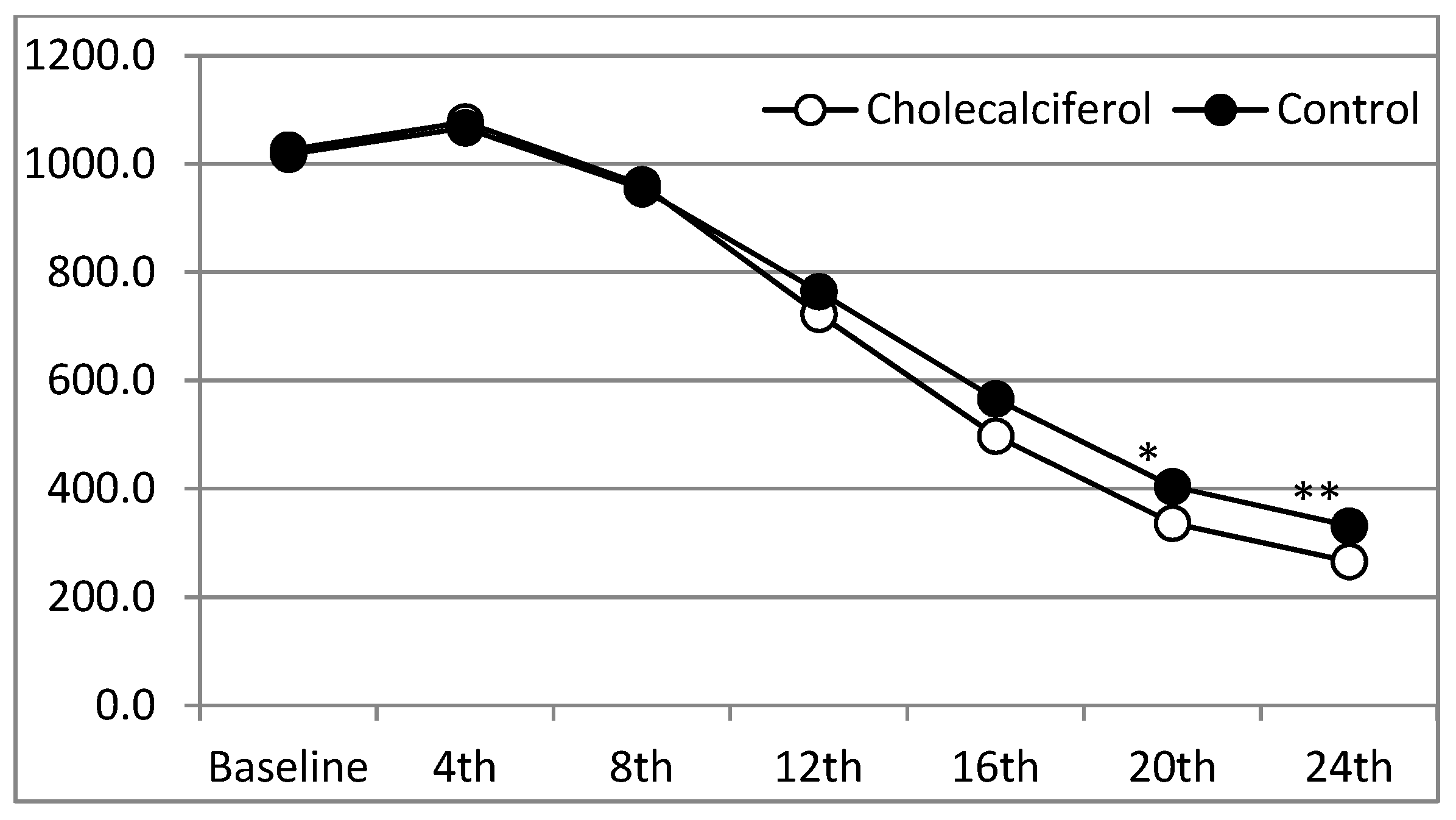
Our results demonstrated that more than 80% of patients with severe SHPT achieved the primary target serum iPTH ≤300 pg/ml level with cholecalciferol supplementation. Nearly 90% of patients achieved the target serum 25-(OH)D3 (≥30 ng/mL) with high-dose cholecalciferol supplementation (5000 IU/day). About 40% of patients receiving cholecalciferol achieved a 10% increase in FN BMD by the end of the study. To our knowledge, this is the first study to reveal the beneficial effects of cholecalciferol supplementation in further lowering iPTH and bone density improvement when combined with cinacalcet and calcitriol among severe SHPT patients.
As previously described, cinacalcet significantly increases the oxyphil cells (OC) and chief cells (CC) OC/CC ratio (approx. increase 3.42 times) [
24], further increases local calcitriol production [
26], and regulates local PTH secretion in a paracrine/autocrine manner. Thus, the need for local 25(OH)D
3 substrate increases with cinacalcet treatment. We proved in our study that addition of nutritional vitamin D (cholecalciferol) improves the 25(OH)D
3 requirement by cinacalcet treatment, and further inhibits PTH secretion among those patients with severe SHPT when used in combination with cinacalcet/active vitamin D analogs. About 55% of the patients achieved the iPTH target (iPTH ≤ 300 pg/mL) at the 20th week and almost 81% of patients achieved that target after the 24th week of cholecalciferol supplementation. This effect was also seen among those patients with severely deficient vitamin D levels (serum 25(OH)D
3 < 12.5 ng/mL). Furthermore, a higher percentage of patients achieved the primary endpoint in the CCC group, irrespective of the prior use of active vitamin D, calcitriol.
Although the CCP group also significantly improved iPTH levels, the target iPTH level was not achieved in most patients by the end of the study. Previous studies also proved that cinacalcet therapy improved the targets of bone metabolism (PTH, Ca, P, and the Ca-P product (Ca-P)) when used alone [
32] or in combination of low-dose active vitamin D analogs [
7]. However, all these studies used titrated doses of cinacalcet (30–180 mg per day) according to biochemical measures. We used a single daily dose of cinacalcet (30 mg per day) throughout our study, since the drug is not covered by health insurance in Taiwan and patients took the drug by self-payment. We also found that lower doses of calcitriol were needed among our study group with time (
Table 5). Most of the CCC group patients achieved KDOQI targets [
33] (serum PTH 150–300 pg/mL, Ca 8.4–9.5 mg/dL, P 3.5–5.5 mg/dL, CaxP product < 55 mg
2/dL
2) and none had hypocalcemia. Since hypocalcemia is the major event prohibiting the use of cinacalcet, and hypercalcemia is a major complication of active vitamin D analogs, the supplementation of nutritional vitamin D avoids both hypo- and hypercalcemic events in such combination therapy.
We found that up to 70% of CCC patients achieved the target 25(OH)D
3 level (≥30 ng/mL) from the 12th week of supplementation and nearly 90% had the target level by the end of the study. However, no such improvement was seen in the CCP group. Clinical studies reported that the combined use of nutritional vitamin D might reduce the active vitamin D doses [
3,
34] and improve adverse effects. Accordingly, we found our CCC group patients used progressively lower doses of calcitriol compared to the CCP group. Studies regarding the effective dosage of vitamin D supplementation are still controversial [
35,
36]. Our previous study used low-dose cholecalciferol (5000 IU/week) and about 60% of patients achieved the target 25(OH)D
3 level (≥30 ng/dL) at 16 weeks [
10]. Growing evidence shows that active vitamin D analogs may aggravate 25(OH)D
3 deficiency because of the feedback inhibition of hepatic 1α-OHase and 25α-OHase [
37,
38] and induction of the 24-OHase enzyme [
39]. Thus, we used quite a high dose of cholecalciferol (5000 IU/day) in combination with calcitriol in our study. We closely monitored 25(OH)D
3 levels to avoid toxicity, and all of our patients tolerate high-dose cholecalciferol supplementation (5000 IU/day) well.
The next major observation of our study was that about 40% in the CCC group gained >10% improvement in femoral BMD compared with the CCP group (~7%). Other clinical studies also revealed that calcimimetic agents reduced the elevated bone formation rate/tissue area with improvement in high turnover bone disorders [
40,
41]. In a recent Indian study among CKD patients, researchers found a significant reduction in iPTH and bone turnover markers, bone-specific alkaline phosphatase (BAP) and serum C-terminal cross-linked collagen type I telopeptides (CTX-1) with cholecalciferol supplementation [
42]. Previous studies [
43,
44] also proved that cinacalcet treatment improves the BMD of the FN without affecting the LS BMD. How and whether the combination of these agents interacts to improve bone parameters needs to be explored in future studies.
Our study has several limitations. Firstly, we cannot apply our findings or draw definitive conclusions in the general HD population due to the relatively small sample size; however, the results were indeed promising and clinically important. We used fixed low doses of cinacalcet in both study and control arms, which needed to be followed longer for efficiency. Furthermore, we excluded patients with severe malnutrition and inflammatory or infectious disorders who might have benefited the most from the intervention due to vitamin D deficiency. However, our cohort had significantly low vitamin D levels in both arms of the study. Next, we did not determine the morbidity or mortality benefits of combination therapy. Although we performed some questionnaires on various bone fractures, the results were inconsistent and non-significant due to shorter follow-up duration. The optimal serum 25(OH)D3 target level in dialysis patients remains unknown; however, we supposed some additive PTH lowering effects with 25(OH)D3 ≥ 30 ng/dL. Although no toxicity levels and signs were noted in our patients, the beneficial role and possible side effects of high-dose cholecalciferol in dialysis patients still requires a multifaceted long-term approach and needs further study in larger clinical trials.