A Role for Exercise in Attenuating Unhealthy Food Consumption in Response to Stress
Abstract
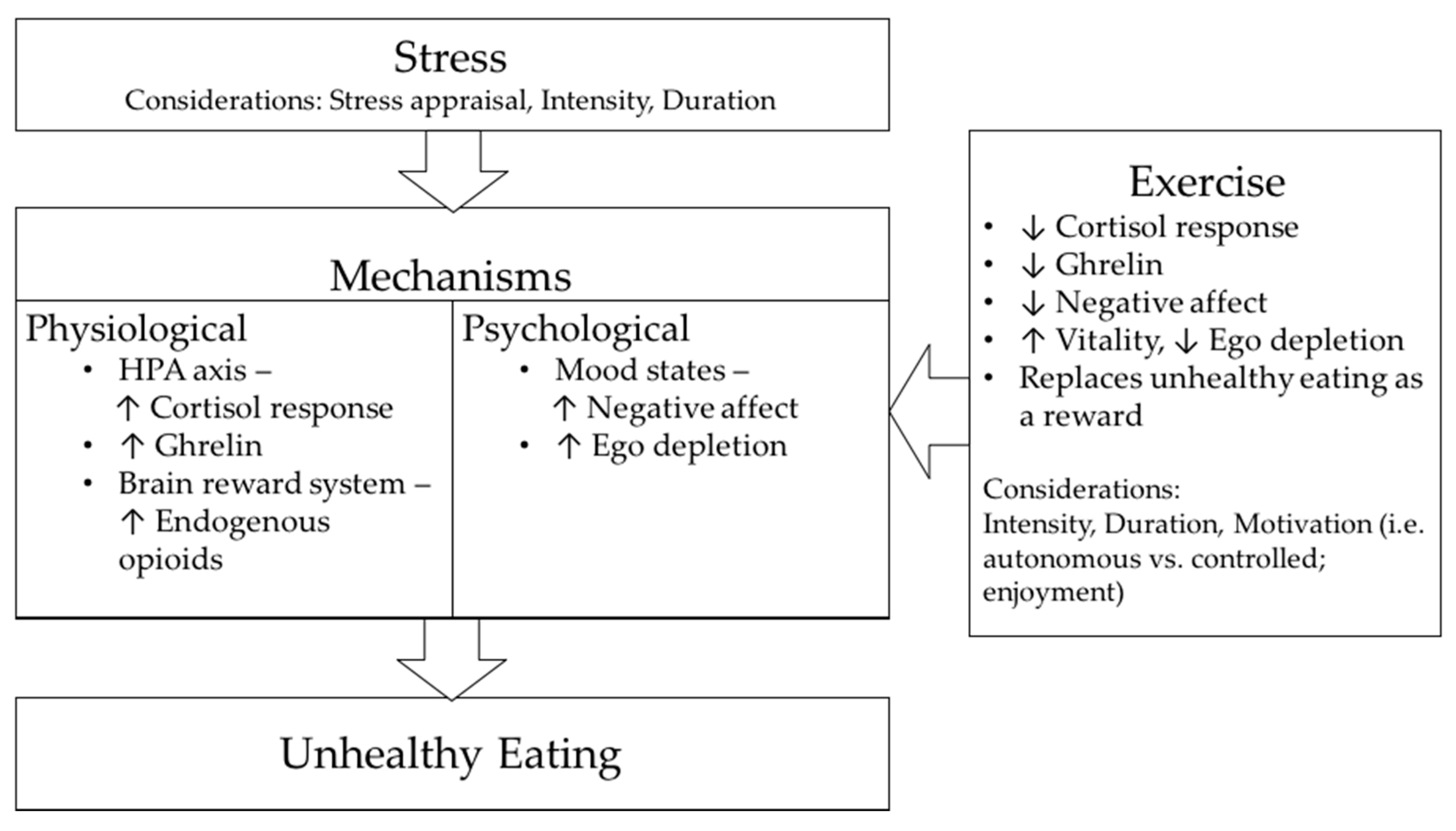
:1. Introduction
2. Understanding “Stress”
3. The Problem—Distress
4. Effect of Stress on Eating Behaviour and Food Choices
4.1. Stress-Induced Eating: Potential Physiological Mechanisms
4.2. Stress-Induced Eating: Potential Psychological Mechanisms
5. Stress Management
6. Effect of Physical Exercise on Stress
7. Effect of Physical Exercise on Stress-Induced Eating
7.1. Evidence for an Effect of Physical Exercise on Stress-Induced Eating
7.2. Potential Physiological Mechanisms through Which Exercise May Attenuate Stress-Induced Eating
7.3. Potential Psychological Mechanisms through Which Exercise May Attenuate Stress-Induced Eating
8. Conclusions
Author Contributions
Conflicts of Interest
References
- Black, P.H.; Garbutt, L.D. Stress, inflammation and cardiovascular disease. J. Psychosom. Res. 2002, 52, 1–23. [Google Scholar]
- Vale, S. Psychosocial stress and cardiovascular diseases. Postgrad. Med. J. 2005, 81, 429–435. [Google Scholar]
- Matthews, K.A.; Katholi, C.R.; McCreath, H.; Whooley, M.A.; Williams, D.R.; Zhu, S.; Markovitz, J.H. Blood pressure reactivity to psychological stress predicts hypertension in the cardia study. Circulation 2004, 110, 74–78. [Google Scholar]
- Born, J.M.; Lemmens, S.G.; Rutters, F.; Nieuwenhuizen, A.G.; Formisano, E.; Goebel, R.; Westerterp-Plantenga, M.S. Acute stress and food-related reward activation in the brain during food choice during eating in the absence of hunger. Int. J. Obes. 2010, 34, 172–181. [Google Scholar]
- Dallman, M.F. Stress-induced obesity and the emotional nervous system. Trends Endocrinol. Metab. 2010, 21, 159–165. [Google Scholar]
- Kandiah, J.; Yake, M.; Jones, J.; Meyer, M. Stress influences appetite and comfort food preferences in college women. Nutr. Res. 2006, 26, 118–123. [Google Scholar]
- Steptoe, A.; Kivimäki, M. Stress and cardiovascular disease. Nat. Rev. Cardiol. 2012, 9, 360–370. [Google Scholar]
- Bunker, S.J.; Colquhoun, D.M.; Esler, M.D.; Hickie, I.B.; Hunt, D.; Jelinek, V.M.; Oldenburg, B.F.; Peach, H.G.; Ruth, D.; Tennant, C.C. “Stress” and coronary heart disease: Psychosocial risk factors. Med. J. Aust. 2003, 178, 272–276. [Google Scholar]
- Dimsdale, J.E. Psychological stress and cardiovascular disease. J. Am. Coll. Cardiol. 2008, 51, 1237–1246. [Google Scholar]
- Barlow, D.H. Principles and Practice of Stress Management; Guilford Press: New York, NY, USA, 2007. [Google Scholar]
- Scully, D.; Kremer, J.; Meade, M.M.; Graham, R.; Dudgeon, K. Physical exercise and psychological well being: A critical review. Br. J. Sports Med. 1998, 32, 111–120. [Google Scholar]
- Salmon, P. Effects of physical exercise on anxiety, depression and sensitivity to stress: A unifying theory. Clin. Psychol. Rev. 2001, 21, 33–61. [Google Scholar]
- Hobson, M.L.; Rejeski, W.J. Does the dose of acute exercise mediate psychophysiological responses to mental stress? J. Sport Exerc. Psychol. 1993, 15, 77–87. [Google Scholar]
- Rejeski, W.J.; Thompson, A.; Brubaker, P.H.; Miller, H.S. Acute exercise: Buffering psychosocial stress responses in women. Health Psychol. 1992, 11, 355–362. [Google Scholar]
- Roy, M.; Steptoe, A. The inhibition of cardiovascular responses to mental stress following aerobic exercise. Psychophysiology 1991, 28, 689–700. [Google Scholar]
- Zschucke, E.; Renneberg, B.; Dimeo, F.; Wüstenberg, T.; Ströhle, A. The stress-buffering effect of acute exercise: Evidence for HPA axis negative feedback. Psychoneuroendocrinology 2015, 51, 414–425. [Google Scholar]
- Selye, H. Stress without Distress; J. B. Lippencott Comp.: New York, NY, USA, 1974; pp. 26–39. [Google Scholar]
- Holmes, T.H.; Rahe, R.H. The social readjustment rating scale. J. Psychosom. Res. 1967, 11, 213–218. [Google Scholar]
- Werner, J. Stressors and health outcomes: Synthesis of nursing research, 1980–1990. In Stress and Coping: State of the Science and Implications for Nursing Theory, Research and Practice; Center for Nursing Press of Sigma Theta Tau International: Indianapolis, Indiana, 1993; pp. 11–38. [Google Scholar]
- Lazarus, R.S.; Folkman, S. Stress, Appraisal and Coping; Springer: New York, NY, USA, 1984. [Google Scholar]
- Cannon, W.B. Bodily Changes in Pain, Hunger, Fear and Rage; D. Appleton and Company: New York, NY, USA, 1929. [Google Scholar]
- Lyon, B.L. Stress, coping and health. In Handbook of Stress, Coping and Health: Implications for Nursing Research, Theory and Practice; SAGE Publications: Thousand Oaks, CA, USA, 2000; pp. 3–23. [Google Scholar]
- Folkman, S.; Lazarus, R.S. If it changes it must be a process: Study of emotion and coping during three stages of a college examination. J. Personal. Soc. Psychol. 1985, 48, 150–170. [Google Scholar]
- Lazarus, R.S. From psychological stress to the emotions: A history of changing outlooks. Ann. Rev. Psychol. 1993, 44, 1–22. [Google Scholar]
- MacDonald, W. The impact of job demands and workload on stress and fatigue. Aust. Psychol. 2003, 38, 102–117. [Google Scholar]
- Lazarus, R.S. Psychological Stress and the Coping Process; McGraw-Hill: New York, NY, USA, 1966. [Google Scholar]
- Yerkes, R.M.; Dodson, J.D. The relation of strength of stimulus to rapidity of habit-formation. J. Comp. Neurol. 1908, 18, 459–482. [Google Scholar]
- Bunker, L.; Rotella, R. Achievement and stress in sport: Research findings and practical suggestion. In Sport Psychology An analysis of Athlete Behaviour, 2nd ed.; Movement Publications: Ithaca, NY, USA, 1980. [Google Scholar]
- Hanton, S.; Neil, R.; Mellalieu, S.D. Recent developments in competitive anxiety direction and competition stress research. Int. Rev. Sport Exerc. Psychol. 2008, 1, 45–57. [Google Scholar]
- Brooks, A.W. Get excited: Reappraising pre-performance anxiety as excitement. J. Exp. Psychol. Gen. 2014, 143, 1144–1158. [Google Scholar]
- Beltzer, M.L.; Nock, M.K.; Peters, B.J.; Jamieson, J.P. Rethinking butterflies: The affective, physiological and performance effects of reappraising arousal during social evaluation. Emotion 2014, 14, 761–768. [Google Scholar]
- Kajantie, E.; Räikkönen, K. Early life predictors of the physiological stress response later in life. Neurosci. Biobehav. Rev. 2010, 35, 23–32. [Google Scholar]
- Anderson, N.B.; Anderson, P. Emotional Longevity: What Really Determines How Long You Live; Viking: New York, NY, USA, 2003. [Google Scholar]
- Baum, A.; Posluszny, D.M. Health psychology: Mapping biobehavioral contributions to health and illness. Ann. Rev. Psychol. 1999, 50, 137–163. [Google Scholar]
- Pervanidou, P.; Chrousos, G.P. Metabolic consequences of stress during childhood and adolescence. Metab. Clin. Exp. 2012, 61, 611–619. [Google Scholar]
- McEwen, B.S.; Gianaros, P.J. Central role of the brain in stress and adaptation: Links to socioeconomic status, health and disease. Ann. N. Y. Acad. Sci. 2010, 1186, 190–222. [Google Scholar]
- Australian Psychological Society. Stress & Wellbeing: How Australians Are Coping with Life: The Findings of the Australian Psychological Society Stress and Wellbeing in Australia Survey 2015; Australian Psychological Society: Melbourne, Australia, 2015. [Google Scholar]
- Anderson, N.B.; Belar, C.D.; Breckler, S.J.; Nordal, K.C.; Ballard, D.W.; Bufka, L.F.; Bossolo, L.; Bethune, S.; Brownawell, A.; Wiggins, K. Stress in America: Paying with Our Health; American Psychological Association: Washington, DC, USA, 2015; Available online: http://www.apa.org/news/press/releases/stress/2014/stress-report.pdf (accessed on 4 February 2018).
- Anderson, N.B.; Belar, C.D.; Breckler, S.J.; Nordal, K.C.; Ballard, D.W.; Bufka, L.F.; Bossolo, L.; Bethune, S.; Brownawell, A.; Wiggins, K. Stress in America: Are Teens Adopting Adults’ Stress Habits? American Psychological Association: Washington, DC, USA, 2014; Available online: http://www.apa.org/news/press/releases/stress/2013/stress-report.pdf (accessed on 4 February 2018).
- Adler, N.; Matthews, K. Health psychology: Why do some people get sick and some stay well? Ann. Rev. Psychol. 1994, 45, 229–259. [Google Scholar]
- Steptoe, A. Invited review: The links between stress and illness. J. Psychosom. Res. 1991, 35, 633–644. [Google Scholar]
- Anderson, N.B.; Belar, C.D.; Breckler, S.J.; Nordal, K.C.; Ballard, D.W.; Bufka, L.F.; Bossolo, L.; Bethune, S.; Brownawell, A.; Wiggins, K. Stress in America: Our Health at Risk; American Psychological Association: Washington, DC, USA, 2012; Available online: https://www.apa.org/news/press/releases/stress/2011/final-2011.pdf (accessed on 4 February 2018).
- Brady, K.T.; Sonne, S.C. The role of stress in alcohol use, alcoholism treatment and relapse. Alcohol Res. Health 1999, 23, 263. [Google Scholar]
- Cooper, M.L.; Russell, M.; Skinner, J.B.; Frone, M.R.; Mudar, P. Stress and alcohol use: Moderating effects of gender, coping and alcohol expectancies. J. Abnorm. Psychol. 1992, 101, 139–152. [Google Scholar]
- Cole, G.; Tucker, L.; Friedman, G.M. Relationships among measures of alcohol drinking behaviour, life-events and perceived stress. Psychol. Rep. 1990, 67, 587–591. [Google Scholar]
- Cartwright, M.; Wardle, J.; Steggles, N.; Simon, A.E.; Croker, H.; Jarvis, M.J. Stress and dietary practices in adolescents. Health Psychol. 2003, 22, 362–369. [Google Scholar]
- Oliver, G.; Wardle, J. Perceived effects of stress on food choice. Physiol. Behav. 1999, 66, 511–515. [Google Scholar]
- Steptoe, A.; Lipsey, Z.; Wardle, J. Stress, hassles and variations in alcohol consumption, food choice and physical exercise: A diary study. Br. J. Health Psychol. 1998, 3, 51–63. [Google Scholar]
- Wardle, J.; Steptoe, A.; Oliver, G.; Lipsey, Z. Stress, dietary restraint and food intake. J. Psychosom. Res. 2000, 48, 195–202. [Google Scholar]
- Pollard, T.M.; Steptoe, A.; Canaan, L.; Davies, G.J.; Wardle, J. Effects of academic examination stress on eating behavior and blood lipid levels. Int. J. Behav. Med. 1995, 2, 299–320. [Google Scholar]
- Stone, A.A.; Brownell, K.D. The stress-eating paradox: Multiple daily measurements in adult males and females. Psychol. Health 1994, 9, 425–436. [Google Scholar]
- Geliebter, A.; Carnell, S.; Gluck, M.E. Cortisol and ghrelin concentrations following a cold pressor stress test in overweight individuals with and without night eating. Int. J. Obes. 2013, 37, 1104–1108. [Google Scholar]
- Popper, R.; Smits, G.; Meiselman, H.L.; Hirsch, E. Eating in combat: A survey of us marines. Mil. Med. 1989, 154, 619–623. [Google Scholar]
- Côté, M.; Gagnon-Girouard, M.-P.; Provencher, V.; Bégin, C. Induced dyadic stress and food intake: Examination of the moderating roles of body mass index and restraint. Eat. Behav. 2016, 23, 86–90. [Google Scholar]
- Huh, J.; Shiyko, M.; Keller, S.; Dunton, G.; Schembre, S.M. The time-varying association between perceived stress and hunger within and between days. Appetite 2015, 89, 145–151. [Google Scholar]
- Hansen, M.J.; Schiöth, H.B.; Morris, M.J. Feeding responses to a melanocortin agonist and antagonist in obesity induced by a palatable high-fat diet. Brain Res. 2005, 1039, 137–145. [Google Scholar]
- Rowland, N.E.; Antelman, S.M. Stress-induced hyperphagia and obesity in rats: A possible model for understanding human obesity. Science 1976, 191, 310–312. [Google Scholar]
- Rozen, R.; Brigant, L.; Apfelbaum, M. Effects of cycles of food restriction followed by ad libitum refeeding on body composition and energy expenditure in obese rats. Am. J. Clin. Nutr. 1994, 59, 560–565. [Google Scholar]
- Adam, T.C.; Epel, E.S. Stress, eating and the reward system. Physiol. Behav. 2007, 91, 449–458. [Google Scholar]
- Dallman, M.F.; Pecoraro, N.C.; La Fleur, S.E.; Warne, J.P.; Ginsberg, A.B.; Akana, S.F.; Laugero, K.C.; Houshyar, H.; Strack, A.M.; Bhatnagar, S. Glucocorticoids, chronic stress and obesity. Prog. Brain Res. 2006, 153, 75–105. [Google Scholar]
- Pecoraro, N.C.; Reyes, F.; Gomez, F.; Bhargava, A.; Dallman, M.F. Chronic stress promotes palatable feeding, which reduces signs of stress: Feedforward and feedback effects of chronic stress. Endocrinology 2004, 145, 3754–3762. [Google Scholar]
- Tomiyama, A.J.; Dallman, M.F.; Epel, E.S. Comfort food is comforting to those most stressed: Evidence of the chronic stress response network in high stress women. Psychoneuroendocrinology 2011, 36, 1513–1519. [Google Scholar]
- Dallman, M.F.; Pecoraro, N.C.; Akana, S.F.; La Fleur, S.E.; Gomez, F.; Houshyar, H.; Bell, M.E.; Bhatnagar, S.; Laugero, K.D.; Manalo, S. Chronic stress and obesity: A new view of “comfort food”. Proc. Natl. Acad. Sci. USA 2003, 100, 11696–11701. [Google Scholar]
- Foster, M.T.; Warne, J.P.; Ginsberg, A.B.; Horneman, H.F.; Pecoraro, N.C.; Akana, S.F.; Dallman, M.F. Palatable foods, stress and energy stores sculpt corticotropin-releasing factor, adrenocorticotropin and corticosterone concentrations after restraint. Endocrinology 2009, 150, 2325–2333. [Google Scholar]
- La Fleur, S.E.; Houshyar, H.; Roy, M.; Dallman, M.F. Choice of lard but not total lard calories, damps adrenocorticotropin responses to restraint. Endocrinology 2005, 146, 2193–2199. [Google Scholar]
- Gibson, E.L. Emotional influences on food choice: Sensory, physiological and psychological pathways. Physiol. Behav. 2006, 89, 53–61. [Google Scholar]
- Newman, E.; O’Connor, D.B.; Conner, M. Daily hassles and eating behaviour: The role of cortisol reactivity status. Psychoneuroendocrinology 2007, 32, 125–132. [Google Scholar]
- O’Connor, D.B.; Jones, F.; Conner, M.; McMillan, B.; Ferguson, E. Effects of daily hassles and eating style on eating behavior. Health Psychol. 2008, 27, S20. [Google Scholar]
- Pecoraro, N.C.; Dallman, M.F.; Warne, J.P.; Ginsberg, A.B.; Laugero, K.D.; la Fleur, S.E.; Houshyar, H.; Gomez, F.; Bhargava, A.; Akana, S.F. From Malthus to motive: How the HPA axis engineers the phenotype, yoking needs to wants. Prog. Neurobiol. 2006, 79, 247–340. [Google Scholar]
- Zellner, D.A.; Loaiza, S.; Gonzalez, Z.; Pita, J.; Morales, J.; Pecora, D.; Wolf, A. Food selection changes under stress. Physiol. Behav. 2006, 87, 789–793. [Google Scholar]
- Epel, E.S.; Lapidus, R.; McEwen, B.; Brownell, K. Stress may add bite to appetite in women: A laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology 2001, 26, 37–49. [Google Scholar]
- Garg, N.; Wansink, B.; Inman, J.J. The influence of incidental affect on consumers’ food intake. J. Mark. 2007, 71, 194–206. [Google Scholar]
- Gluck, M.E.; Geliebter, A.; Hung, J.; Yahav, E. Cortisol, hunger and desire to binge eat following a cold stress test in obese women with binge eating disorder. Psychosom. Med. 2004, 66, 876–881. [Google Scholar]
- Oliver, G.; Wardle, J.; Gibson, E.L. Stress and food choice: A laboratory study. Psychosom. Med. 2000, 62, 853–865. [Google Scholar]
- Rutters, F.; Nieuwenhuizen, A.G.; Lemmens, S.G.T.; Born, J.M.; Westerterp-Plantenga, M.S. Acute stress-related changes in eating in the absence of hunger. Obesity 2009, 17, 72–77. [Google Scholar]
- Takeda, E.; Terao, J.; Nakaya, Y.; Miyamoto, K.I.; Baba, Y.; Chuman, H.; Kaji, R.; Ohmori, T.; Rokutan, K. Stress control and human nutrition. J. Med. Investig. 2004, 51, 139–145. [Google Scholar]
- De Brouwer, S.J.M.; Kraaimaat, F.W.; Sweep, F.C.G.J.; Donders, R.T.; Eijsbouts, A.; van Koulil, S.; van Riel, P.L.C.M.; Evers, A.W.M. Psychophysiological responses to stress after stress management training in patients with rheumatoid arthritis. PLoS ONE 2011, 6, e27432. [Google Scholar]
- Heinrichs, M.; Baumgartner, T.; Kirschbaum, C.; Ehlert, U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol. Psychiatry 2003, 54, 1389–1398. [Google Scholar]
- Lennartsson, A.K.; Sjörs, A.; Währborg, P.; Ljung, T.; Jonsdottir, I.H. Burnout and hypocortisolism—A matter of severity? A study on acth and cortisol responses to acute psychosocial stress. Front. Psychiatry 2015, 6, 8. [Google Scholar] [CrossRef]
- Bayazit, V.; Demir, N.; Tosun, F. Evaluation of relationships among cortisol, stress, autism and exercise. Aust. J. Basic Appl. Sci. 2009, 3, 1013–1021. [Google Scholar]
- Kudielka, B.M.; Schommer, N.C.; Hellhammer, D.H.; Kirschbaum, C. Acute HPA axis responses, heart rate and mood changes to psychosocial stress (TSST) in humans at different times of day. Psychoneuroendocrinology 2004, 29, 983–992. [Google Scholar]
- George, S.A.; Khan, S.; Briggs, H.; Abelson, J.L. CRH-stimulated cortisol release and food intake in healthy, non-obese adults. Psychoneuroendocrinology 2010, 35, 607–612. [Google Scholar]
- Tataranni, P.A.; Larson, D.E.; Snitker, S.; Young, J.B.; Flatt, J.P.; Ravussin, E. Effects of glucocorticoids on energy metabolism and food intake in humans. Am. J. Physiol. Endocrinol. Metab. 1996, 271, E317–E325. [Google Scholar]
- Appelhans, B.M.; Pagoto, S.L.; Peters, E.N.; Spring, B.J. HPA axis response to stress predicts short-term snack intake in obese women. Appetite 2010, 54, 217–220. [Google Scholar]
- Björntorp, P.; Holm, G.; Rosmond, R.; Folkow, B. Hypertension and the metabolic syndrome: Closely related central origin? Blood Press. 2000, 9, 71–82. [Google Scholar]
- Fries, E.; Hesse, J.; Hellhammer, J.; Hellhammer, D.H. A new view on hypocortisolism. Psychoneuroendocrinology 2005, 30, 1010–1016. [Google Scholar]
- Tryon, M.; DeCant, R.; Laugero, K. Having your cake and eating it too: A habit of comfort food may link chronic social stress exposure and acute stress-induced cortisol hyporesponsiveness. Physiol. Behav. 2013, 114, 32–37. [Google Scholar]
- Torres, S.J.; Nowson, C.A. Relationship between stress, eating behavior and obesity. Nutrition 2007, 23, 887–894. [Google Scholar]
- Van Strien, T.; Roelofs, K.; de Weerth, C. Cortisol reactivity and distress-induced emotional eating. Psychoneuroendocrinology 2013, 38, 677–684. [Google Scholar]
- Higgins, S.C.; Gueorguiev, M.; Korbonits, M. Ghrelin, the peripheral hunger hormone. Ann. Med. 2007, 39, 116–136. [Google Scholar]
- Cummings, D.E.; Purnell, J.Q.; Frayo, R.S.; Schmidova, K.; Wisse, B.E.; Weigle, D.S. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 2001, 50, 1714–1719. [Google Scholar]
- Rouach, V.; Bloch, M.; Rosenberg, N.; Gilad, S.; Limor, R.; Stern, N.; Greenman, Y. The acute ghrelin response to a psychological stress challenge does not predict the post-stress urge to eat. Psychoneuroendocrinology 2007, 32, 693–702. [Google Scholar]
- Kirschbaum, C.; Pirke, K.M.; Hellhammer, D.H. The ‘trier social stress test’—A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology 1993, 28, 76–81. [Google Scholar]
- Chuang, J.-C.; Zigman, J.M. Ghrelin’s roles in stress, mood and anxiety regulation. Int. J. Pept. 2010, 2010, 460549. [Google Scholar]
- Jaremka, L.M.; Belury, M.A.; Andridge, R.R.; Malarkey, W.B.; Glaser, R.; Christian, L.; Emery, C.F.; Kiecolt-Glaser, J.K. Interpersonal stressors predict ghrelin and leptin levels in women. Psychoneuroendocrinology 2014, 48, 178–188. [Google Scholar]
- Steptoe, A.; Gibson, E.L.; Hamer, M.; Wardle, J. Neuroendocrine and cardiovascular correlates of positive affect measured by ecological momentary assessment and by questionnaire. Psychoneuroendocrinology 2007, 32, 56–64. [Google Scholar]
- Glaser, R.; Kiecolt-Glaser, J.K. Stress-induced immune dysfunction: Implications for health. Nat. Rev. Immunol. 2005, 5, 243–251. [Google Scholar]
- Feldman, P.J.; Cohen, S.; Lepore, S.J.; Matthews, K.A.; Kamarck, T.W.; Marsland, A.L. Negative emotions and acute physiological responses to stress. Ann. Behav. Med. 1999, 21, 216–222. [Google Scholar]
- Macht, M.; Roth, S.; Ellgring, H. Chocolate eating in healthy men during experimentally induced sadness and joy. Appetite 2002, 39, 147–158. [Google Scholar]
- Wallis, D.J.; Hetherington, M.M. Emotions and eating. Self-reported and experimentally induced changes in food intake under stress. Appetite 2009, 52, 355–362. [Google Scholar]
- Anton, S.D.; Miller, P.M. Do negative emotions predict alcohol consumption, saturated fat intake and physical activity in older adults? Behav. Modif. 2005, 29, 677–688. [Google Scholar]
- Nguyen-Michel, S.T.; Unger, J.B.; Spruijt-Metz, D. Dietary correlates of emotional eating in adolescence. Appetite 2007, 49, 494–499. [Google Scholar]
- Haedt-Matt, A.A.; Keel, P.K.; Racine, S.E.; Burt, S.A.; Hu, J.Y.; Boker, S.; Neale, M.; Klump, K.L. Do emotional eating urges regulate affect? Concurrent and prospective associations and implications for risk models of binge eating. Int. J. Eat. Disord. 2014, 47, 874–877. [Google Scholar]
- Lehman, A.K.; Rodin, J. Styles of self-nurturance and disordered eating. J. Consult. Clin. Psychol. 1989, 57, 117–122. [Google Scholar]
- Yau, Y.H.; Potenza, M.N. Stress and eating behaviors. Minerva Endocrinol. 2013, 38, 255–267. [Google Scholar]
- Muraven, M.; Baumeister, R.F. Self-regulation and depletion of limited resources: Does self-control resemble a muscle? Psychol. Bull. 2000, 126, 247–259. [Google Scholar]
- Vohs, K.D.; Heatherton, T.F. Self-regulatory failure: A resource-depletion approach. Psychol. Sci. 2000, 11, 249–254. [Google Scholar]
- Gailliot, M.T.; Baumeister, R.F. Self-regulation and sexual restraint: Dispositionally and temporarily poor self-regulatory abilities contribute to failures at restraining sexual behavior. Personal. Soc. Psychol. Bull. 2007, 33, 173–186. [Google Scholar]
- Stucke, T.S.; Baumeister, R.F. Ego depletion and aggressive behavior: Is the inhibition of aggression a limited resource? Eur. J. Soc. Psychol. 2006, 36, 1–13. [Google Scholar]
- Muraven, M.; Collins, R.L.; Neinhaus, K. Self-control and alcohol restraint: An initial application of the self-control strength model. Psychol. Addict. Behav. 2002, 16, 113–120. [Google Scholar]
- Baumeister, R.F.; Heatherton, T.F. Self-regulation failure: An overview. Psychol. Inq. 1996, 7, 1–15. [Google Scholar]
- Magaraggia, C.; Dimmock, J.A.; Jackson, B. The effect of learning climate on snack consumption and ego depletion among undergraduate students. Appetite 2013, 69, 174–179. [Google Scholar]
- Kahan, D.; Polivy, J.; Herman, C.P. Conformity and dietary disinhibition: A test of the ego-strength model of self-regulation. Int. J. Eat. Disord. 2003, 33, 165–171. [Google Scholar]
- Hockey, R. Varieties of attentional state: The effects of environment. In Varieties of Attention; Parasuraman, R., Davies, D.R., Eds.; Academic Press: New York, NY, USA, 1984; pp. 449–481. [Google Scholar]
- Wegner, D.M.; Pennebaker, J.W. Changing our minds: An introduction to mental control. In Handbook of Mental Control; Wegner, D.M., Pennebaker, J.W., Eds.; Prentice-Hall, Inc.: Englewood Cliffs, NJ, USA, 1993; Volume IX, pp. 1–12. [Google Scholar]
- Baldacchino, D.; Draper, P. Spiritual coping strategies: A review of the nursing research literature. J. Adv. Nurs. 2001, 34, 833–841. [Google Scholar]
- DeGraff, A.H.; Schaffer, J. Emotion-focused coping: A primary defense against stress for people living with spinal cord injury. J. Rehabil. 2008, 74, 19–24. [Google Scholar]
- Contrada, R.J.; Baum, A.E. The Handbook of Stress Science: Biology, Psychology and Health; Springer: New York, NY, USA, 2011. [Google Scholar]
- Folkman, S.; Lazarus, R.S. Coping as a mediator of emotion. J. Personal. Soc. Psychol. 1988, 54, 466–475. [Google Scholar]
- Barlow, C.E.; LaMonte, M.J.; FitzGerald, S.J.; Kampert, J.B.; Perrin, J.L.; Blair, S.N. Cardiorespiratory fitness is an independent predictor of hypertension incidence among initially normotensive healthy women. Am. J. Epidemiol. 2006, 163, 142–150. [Google Scholar]
- Ketelhut, R.G.; Franz, I.W.; Scholze, J. Regular exercise as an effective approach in antihypertensive therapy. Med. Sci. Sports Exerc. 2004, 36, 4–8. [Google Scholar]
- Hopkins, M.E.; Davis, F.C.; VanTieghem, M.R.; Whalen, P.J.; Bucci, D.J. Differential effects of acute and regular physical exercise on cognition and affect. Neuroscience 2012, 215, 59–68. [Google Scholar]
- Jonsdottir, I.H.; Rödjer, L.; Hadzibajramovic, E.; Börjesson, M.; Ahlborg, G. A prospective study of leisure-time physical activity and mental health in Swedish health care workers and social insurance officers. Prev. Med. 2010, 51, 373–377. [Google Scholar] [Green Version]
- Schnohr, P.; Kristensen, T.S.; Prescott, E.; Scharling, H. Stress and life dissatisfaction are inversely associated with jogging and other types of physical activity in leisure time—The Copenhagen city heart study. Scand. J. Med. Sci. Sports 2005, 15, 107–112. [Google Scholar]
- Atlantis, E.; Chow, C.M.; Kirby, A.; Singh, M.F. An effective exercise-based intervention for improving mental health and quality of life measures: A randomized controlled trial. Prev. Med. 2004, 39, 424–434. [Google Scholar]
- Connell, C.M.; Janevic, M.R. Effects of a telephone-based exercise intervention for dementia caregiving wives: A randomized controlled trial. J. Appl. Gerontol. 2009, 28, 171–194. [Google Scholar]
- Imayama, I.; Alfano, C.M.; Kong, A.; Foster-Schubert, K.E.; Bain, C.E.; Xiao, L.; Duggan, C.; Wang, C.Y.; Campbell, K.L.; Blackburn, G.L.; et al. Dietary weight loss and exercise interventions effects on quality of life in overweight/obese postmenopausal women: A randomized controlled trial. Int. J. Behav. Nutr. Phys. Act. 2011, 8. [Google Scholar] [CrossRef]
- King, A.C.; Baumann, K.; O’Sullivan, P.; Wilcox, S.; Castro, C. Effects of moderate-intensity exercise on physiological, behavioral and emotional responses to family caregiving: A randomized controlled trial. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2002, 57, M26–M36. [Google Scholar]
- Wilcox, S.; Dowda, M.; Leviton, L.C.; Bartlett-Prescott, J.; Bazzarre, T.; Campbell-Voytal, K.; Carpenter, R.A.; Castro, C.M.; Dowdy, D.; Dunn, A.L.; et al. Active for life: Final results from the translation of two physical activity programs. Am. J. Prev. Med. 2008, 35, 340–351. [Google Scholar]
- Aldana, S.G.; Sutton, L.D.; Jacobson, B.H.; Quirk, M.G. Relationships between leisure time physical activity and perceived stress. Percept. Mot. Skills 1996, 82, 315–321. [Google Scholar]
- Hull, E.M.; Young, S.H.; Ziegler, M.G. Aerobic fitness affects cardiovascular and catecholamine responses to stressors. Psychophysiology 1984, 21, 353–360. [Google Scholar]
- Perkins, K.A.; Dubbert, P.M.; Martin, J.E.; Faulstich, M.E.; Harris, J.K. Cardiovascular reactivity to psychological stress in aerobically trained versus untrained mild hypertensives and normotensives. Health Psychol. 1986, 5, 407–421. [Google Scholar]
- Boone, J.B., Jr.; Probst, M.M.; Rogers, M.W.; Berger, R. Postexercise hypotension reduces cardiovascular responses to stress. J. Hypertens. 1993, 11, 449–453. [Google Scholar]
- Probst, M.M.; Bulbulian, R.; Knapp, C. Hemodynamic responses to the stroop and cold pressor tests after submaximal cycling exercise in normotensive males. Physiol. Behav. 1997, 62, 1283–1290. [Google Scholar]
- Rejeski, W.J.; Gregg, E.; Thompson, A.; Berry, M. The effects of varying doses of acute aerobic exercise on psychophysiological stress responses in highly trained cyclists. J. Sport Exerc. Psychol. 1991, 13, 188–199. [Google Scholar]
- Brownley, K.A.; Hinderliter, A.L.; West, S.G.; Girdler, S.S.; Sherwood, A.; Light, K.C. Sympathoadrenergic mechanisms in reduced hemodynamic stress responses after exercise. Med. Sci. Sports Exerc. 2003, 35, 978–986. [Google Scholar]
- Taylor, A.H.; Oliver, A.J. Acute effects of brisk walking on urges to eat chocolate, affect and responses to a stressor and chocolate cue: An experimental study. Appetite 2009, 52, 155–160. [Google Scholar]
- Ledochowski, L.; Ruedl, G.; Taylor, A.H.; Kopp, M. Acute effects of brisk walking on sugary snack cravings in overweight people, affect and responses to a manipulated stress situation and to a sugary snack cue: A crossover study. PLoS ONE 2015, 10, e0119278. [Google Scholar]
- Oh, H.; Taylor, A.H. Brisk walking reduces ad libitum snacking in regular chocolate eaters during a workplace simulation. Appetite 2012, 58, 387–392. [Google Scholar]
- Skoluda, N.; Strahler, J.; Schlotz, W.; Niederberger, L.; Marques, S.; Fischer, S.; Thoma, M.V.; Spoerri, C.; Ehlert, U.; Nater, U.M. Intra-individual psychological and physiological responses to acute laboratory stressors of different intensity. Psychoneuroendocrinology 2015, 51, 227–236. [Google Scholar]
- Horsch, A.; Wobmann, M.; Kriemler, S.; Munsch, S.; Borloz, S.; Balz, A.; Marques-Vidal, P.; Borghini, A.; Puder, J.J. Impact of physical activity on energy balance, food intake and choice in normal weight and obese children in the setting of acute social stress: A randomized controlled trial. BMC Pediatr. 2015, 15, 12–21. [Google Scholar]
- Kudielka, B.M.; Hellhammer, D.H.; Kirschbaum, C.; Harmon-Jones, E.; Winkielman, P. Ten years of research with the trier social stress test—Revisited. In Social Neuroscience: Integrating Biological and Psychological Explanations of Social Behavior; Guilford Press: New York, NY, USA, 2007; pp. 56–83. [Google Scholar]
- Neumeier, W.H.; Goodner, E.; Biasini, F.; Dhurandhar, E.J.; Menear, K.S.; Turan, B.; Hunter, G.R. Exercise following mental work prevented overeating. Med. Sci. Sports Exerc. 2016, 48, 1803–1809. [Google Scholar]
- Duclos, M.; Gouarne, C.; Bonnemaison, D. Acute and chronic effects of exercise on tissue sensitivity to glucocorticoids. J. Appl. Physiol. 2003, 94, 869–875. [Google Scholar]
- Duclos, M.; Corcuff, J.B.; Pehourcq, F.; Tabarin, A. Decreased pituitary sensitivity to glucocorticoids in endurance-trained men. Eur. J. Endocrinol. 2001, 144, 363–368. [Google Scholar]
- Hershberger, A.M.; McCammon, M.R.; Garry, J.P.; Mahar, M.T.; Hickner, R.C. Responses of lipolysis and salivary cortisol to food intake and physical activity in lean and obese children. J. Clin. Endocrinol. Metab. 2004, 89, 4701–4707. [Google Scholar]
- Jürimäe, J.; Purge, P.; Jürimäe, T. Adiponectin and stress hormone responses to maximal sculling after volume-extended training season in elite rowers. Metab. Clin. Exp. 2006, 55, 13–19. [Google Scholar]
- Traustadóttir, T.; Bosch, P.R.; Matt, K.S. The HPA axis response to stress in women: Effects of aging and fitness. Psychoneuroendocrinology 2005, 30, 392–402. [Google Scholar]
- King, J.A.; Wasse, L.K.; Stensel, D.J.; Nimmo, M.A. Exercise and ghrelin. A narrative overview of research. Appetite 2013, 68, 83–91. [Google Scholar]
- Broom, D.R.; Stensel, D.J.; Bishop, N.C.; Burns, S.F.; Miyashita, M. Exercise-induced suppression of acylated ghrelin in humans. J. Appl. Physiol. 2007, 102, 2165–2171. [Google Scholar]
- Holliday, A.; Blannin, A. Appetite, food intake and gut hormone responses to intense aerobic exercise of different duration. J. Endocrinol. 2017, 235, 193–205. [Google Scholar]
- Yeung, R.R. The acute effects of exercise on mood state. J. Psychosom. Res. 1996, 40, 123–141. [Google Scholar]
- Hansen, C.J.; Stevens, L.C.; Coast, J.R. Exercise duration and mood state: How much is enough to feel better? Health Psychol. 2001, 20, 267–275. [Google Scholar]
- Biddle, S. Exercise and psychosocial health. Res. Q. Exerc. Sport 1995, 66, 292–297. [Google Scholar]
- Steptoe, A.; Edwards, S.; Moses, J.; Mathews, A. The effects of exercise training on mood and perceived coping ability in anxious adults from the general population. J. Psychosom. Res. 1989, 33, 537–547. [Google Scholar]
- Crews, D.J.; Landers, D.M. A meta-analytic review of aerobic fitness and reactivity to psychosocial stressors. Med. Sci. Sports Exerc. 1987, 19, S114–S120. [Google Scholar]
- Petruzzello, S.J.; Landers, D.M.; Hatfield, B.D.; Kubitz, K.A.; Salazar, W. A meta-analysis on the anxiety-reducing effects of acute and chronic exercise. Sports Med. 1991, 11, 143–182. [Google Scholar]
- Arias-Carrión, O.; Stamelou, M.; Murillo-Rodríguez, E.; Menéndez-González, M.; Pöppel, E. Dopaminergic reward system: A short integrative review. Int. Arch. Med. 2010, 3, 24. [Google Scholar] [CrossRef]
- Esch, T.; Stefano, G.B. Endogenous reward mechanisms and their importance in stress reduction, exercise and the brain. Arch. Med. Sci. AMS 2010, 6, 447–455. [Google Scholar]
- More, A.; Jackson, B.; Dimmock, J.A.; Thornton, A.L.; Colthart, A.; Furzer, B.J. Exercise in the treatment of youth substance use disorders: Review and recommendations. Front. Psychol. 2017, 8, 1839. [Google Scholar] [CrossRef]
- Taylor, A.H.; Ussher, M.H.; Faulkner, G. The acute effects of exercise on cigarette cravings, withdrawal symptoms, affect and smoking behaviour: A systematic review. Addiction 2007, 102, 534–543. [Google Scholar]
- Lynch, W.J.; Peterson, A.B.; Sanchez, V.; Abel, J.; Smith, M.A. Exercise as a novel treatment for drug addiction: A neurobiological and stage-dependent hypothesis. Neurosci. Biobehav. Rev. 2013, 37, 1622–1644. [Google Scholar]
- Inzlicht, M.; Schmeichel, B.J. What is ego depletion? Toward a mechanistic revision of the resource model of self-control. Perspect. Psychol. Sci. 2012, 7, 450–463. [Google Scholar]
- Legault, L.; Inzlicht, M. Self-determination, self-regulation and the brain: Autonomy improves performance by enhancing neuroaffective responsiveness to self-regulation failure. J. Personal. Soc. Psychol. 2013, 105, 123–138. [Google Scholar]
- Moller, A.C.; Deci, E.L.; Ryan, R.M. Choice and ego-depletion: The moderating role of autonomy. Personal. Soc. Psychol. Bull. 2006, 32, 1024–1036. [Google Scholar]
- Muraven, M. Autonomous self-control is less depleting. J. Res. Personal. 2008, 42, 763–770. [Google Scholar]
- Deci, E.L.; Ryan, R.M. Intrinsic Motivation and Self-Determination in Human Behavior; Plenum Press: New York, NY, USA, 1985. [Google Scholar]
- Deci, E.L.; Ryan, R.M. The “what” and “why” of goal pursuits: Human needs and the self-determination of behavior. Psychol. Inq. 2000, 11, 227–268. [Google Scholar]
- Fenzl, N.; Bartsch, K.; Koenigstorfer, J. Labeling exercise fat-burning increases post-exercise food consumption in self-imposed exercisers. Appetite 2014, 81, 1–7. [Google Scholar]
- Dimmock, J.A.; Guelfi, K.J.; West, J.S.; Masih, T.; Jackson, B. Does motivation for exercise influence post-exercise snacking behavior? Nutrients 2015, 7, 4804–4816. [Google Scholar]
- Pelletier, L.G.; Dion, S.C.; Slovinec-D’Angelo, M.; Reid, R. Why do you regulate what you eat? Relationships between forms of regulation, eating behaviors, sustained dietary behavior change and psychological adjustment. Motiv. Emot. 2004, 28, 245–277. [Google Scholar]
- West, J.; Guelfi, K.J.; Dimmock, J.A.; Jackson, B. “I deserve a treat”: Exercise motivation as a predictor of post-exercise dietary licensing beliefs and implicit associations toward unhealthy snacks. Psychol. Sport Exerc. 2017, 32, 93–101. [Google Scholar]
- Beer, N.J.; Dimmock, J.A.; Jackson, B.; Guelfi, K.J. Providing choice in exercise influences food intake at the subsequent meal. Med. Sci. Sports Exerc. 2017, 49, 2110–2118. [Google Scholar]
| Authors (Year) | Participants | Experimental Design | Intervention | Stressor | Appetite-Related Variable | Results |
|---|---|---|---|---|---|---|
| Taylor & Oliver (2009) [137] | 25 normal weight, regular chocolate eaters | Within subjects | Ex (15 min brisk walking) vs. Con (15 min quiet sitting) (pre-stressor) | Stroop colour-word interference task | Chocolate cravings | Exercise did not significantly reduce cravings (p = 0.06, moderate effect sizes) |
| Ledochowski et al. (2015) [138] | 47 overweight sugary snack eaters | Within subjects | Ex (15 min brisk walking) vs. Con (15 min quiet sitting) (pre-stressor) | Stroop colour-word interference task | Sugary snack cravings | Exercise significantly reduced cravings (p < 0.01) |
| Oh & Taylor (2012) [139] | 78 normal weight, regular chocolate eaters | 2 × 2 Factorial design | Ex (15 min brisk walking) vs. Con (15 min quiet sitting) (pre-stressor) | Stroop colour-word interference task (low and high demanding) | Ad libitum chocolate consumption | Exercise significantly reduced consumption after both low and high demand stress conditions (p < 0.01) |
| Horsch et al. (2015) [141] | 26 normal weight (NW), 24 overweight (OW) children | 2 × 2 Factorial design | NW Ex (30 min moderate intensity exercise) vs. NW Con (sedentary) vs. OW Ex (30 min moderate exercise) vs. OW Con (pre-stressor) | Trier Social Stress Test for children | Ad libitum food consumption | Exercise significantly reduced low-caloric salty food intake (p < 0.001) and tendency for lower overall carbohydrate intake (p = 0.07) |
| Neumeier et al. (2016) [143] | 38 normal weight university students | Between groups (with each group compared to their baseline rest) | Ex (15 min high intensity interval exercise) vs. Con (rest) (post-stressor) | Graduate entrance level reading comprehension problems and one college entrance math problem | Ad libitum pizza consumption | Con consumed significantly more calories compared to baseline rest (p = 0.02) but EX did not increase intake (p > 0.05) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leow, S.; Jackson, B.; Alderson, J.A.; Guelfi, K.J.; Dimmock, J.A. A Role for Exercise in Attenuating Unhealthy Food Consumption in Response to Stress. Nutrients 2018, 10, 176. https://doi.org/10.3390/nu10020176
Leow S, Jackson B, Alderson JA, Guelfi KJ, Dimmock JA. A Role for Exercise in Attenuating Unhealthy Food Consumption in Response to Stress. Nutrients. 2018; 10(2):176. https://doi.org/10.3390/nu10020176
Chicago/Turabian StyleLeow, Shina, Ben Jackson, Jacqueline A. Alderson, Kym J. Guelfi, and James A. Dimmock. 2018. "A Role for Exercise in Attenuating Unhealthy Food Consumption in Response to Stress" Nutrients 10, no. 2: 176. https://doi.org/10.3390/nu10020176





