The Prebiotic Inulin Aggravates Accelerated Atherosclerosis in Hypercholesterolemic APOE*3-Leiden Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice and Diet
2.2. Cuff-Induced Atherosclerotic Lesion Formation
2.3. (Immuno)histochemical Staining
2.4. Atherosclerotic Lesion Analysis
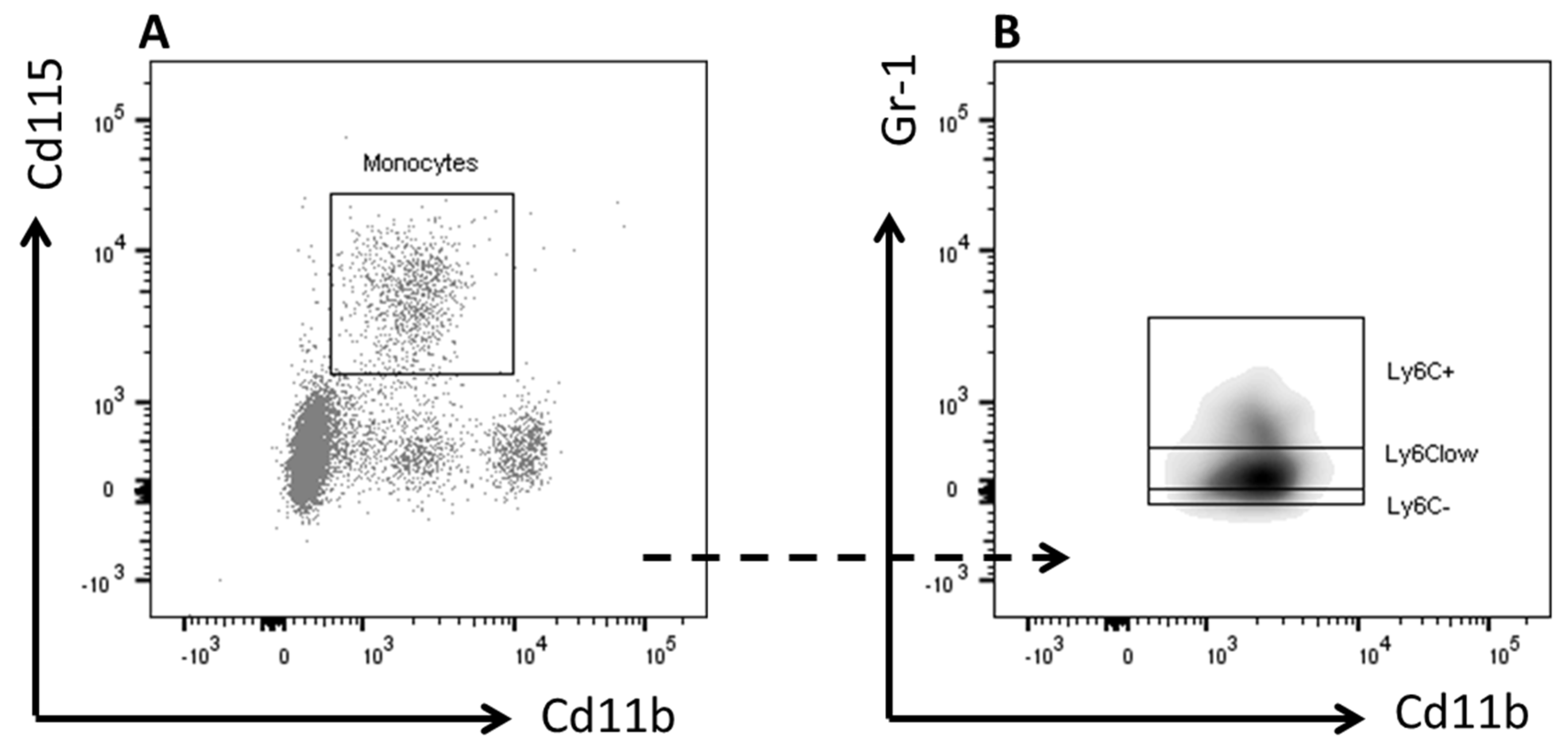
2.5. Flow Cytometry
2.6. Plasma Total Cholesterol
2.7. Statistical Analysis
3. Results
3.1. Inulin Increased Atherosclerotic Lesion Formation and Outward Vascular Remodeling
3.2. Inulin Induced Changes in Lesion Composition
3.3. Inulin did not Affect Blood Monocyte Composition but Increased Total Cholesterol Exposure
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organization (WHO). WHO|The Top 10 Causes of Death; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Hansson, G.K.; Libby, P. The immune response in atherosclerosis: A double-edged sword. Nat. Rev. Immunol. 2006, 6, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- Orford, J.L.; Selwyn, A.P.; Ganz, P.; Popma, J.J.; Rogers, C. The comparative pathobiology of atherosclerosis and restenosis. Am. J. Cardiol. 2000, 86, 6H–11H. [Google Scholar] [CrossRef]
- Tousoulis, D.; Psarros, C.; Demosthenous, M.; Patel, R.; Antoniades, C.; Stefanadis, C. Innate and adaptive inflammation as a therapeutic target in vascular disease: The emerging role of statins. J. Am. Coll. Cardiol. 2014, 63, 2491–2502. [Google Scholar] [CrossRef] [PubMed]
- Jukema, J.W.; Cannon, C.P.; de Craen, A.J.M.; Westendorp, R.G.J.; Trompet, S. The Controversies of Statin Therapy: Weighing the Evidence. J. Am. Coll. Cardiol. 2012, 60, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Dwyer, K.M.; Fan, Z.; Shircore, A.; Fan, J.; Dwyer, J.H. Dietary fiber and progression of atherosclerosis: The Los Angeles Atherosclerosis Study. Am. J. Clin. Nutr. 2003, 78, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Stampfer, M.J.; Hu, F.B.; Giovannucci, E.; Rimm, E.; Manson, J.E.; Hennekens, C.H.; Willett, W.C. Whole-grain consumption and risk of coronary heart disease: Results from the Nurses’ Health Study. Am. J. Clin. Nutr. 1999, 70, 412–419. [Google Scholar] [PubMed]
- Bazzano, L.A.; He, J.; Ogden, L.G.; Loria, C.M.; Whelton, P.K. Dietary Fiber Intake and Reduced Risk of Coronary Heart Disease in US Men and Women: The National Health and Nutrition Examination Survey I Epidemiologic Follow-up Study. Arch. Intern. Med. 2003, 163, 1897. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.S.; Clifton, P.M.; Keogh, J.B. The association between carotid intima media thickness and individual dietary components and patterns. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [PubMed]
- Videla, S.; Vilaseca, J.; Antolin, M.; Garcia-Lafuente, A.; Guarner, F.; Crespo, E.; Casalots, J.; Salas, A.; Malagelada, J.R. Dietary inulin improves distal colitis induced by dextran sodium sulfate in the rat. Am. J. Gastroenterol. 2001, 96, 1486–1493. [Google Scholar] [CrossRef] [PubMed]
- Casellas, F.; Borruel, N.; Torrejón, A.; Varela, E.; Antolin, M.; Guarner, F.; Malagelada, J.-R. Oral oligofructose-enriched inulin supplementation in acute ulcerative colitis is well tolerated and associated with lowered faecal calprotectin. Aliment. Pharmacol. Ther. 2007, 25, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Abhari, K.; Shekarforoush, S.S.; Hosseinzadeh, S.; Nazifi, S.; Sajedianfard, J.; Eskandari, M.H. The effects of orally administered Bacillus coagulans and inulin on prevention and progression of rheumatoid arthritis in rats. Food Nutr. Res. 2016, 60, 30876. [Google Scholar] [CrossRef] [PubMed]
- Vogt, L.; Meyer, D.; Pullens, G.; Faas, M.; Smelt, M.; Venema, K.; Ramasamy, U.; Schols, H.A.; De Vos, P. Immunological properties of inulin-type fructans. Crit. Rev. Food Sci. Nutr. 2015, 55, 414–436. [Google Scholar] [CrossRef] [PubMed]
- Dehghan, P.; Pourghassem Gargari, B.; Asghari Jafar-Abadi, M.; Aliasgharzadeh, A. Inulin controls inflammation and metabolic endotoxemia in women with type 2 diabetes mellitus: A randomized-controlled clinical trial. Int. J. Food Sci. Nutr. 2014, 65, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Rault-Nania, M.-H.; Gueux, E.; Demougeot, C.; Demigné, C.; Rock, E.; Mazur, A. Inulin attenuates atherosclerosis in apolipoprotein E-deficient mice. Br. J. Nutr. 2006, 96, 840–844. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, A.; Poulsen, M.; Frandsen, H. Effect of a long-chained fructan Raftiline HP on blood lipids and spontaneous atherosclerosis in low density receptor knockout mice. Nutr. Res. 2002, 22, 473–480. [Google Scholar] [CrossRef]
- Pedersen, A.; Sandström, B.; Van Amelsvoort, J.M. The effect of ingestion of inulin on blood lipids and gastrointestinal symptoms in healthy females. Br. J. Nutr. 1997, 78, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Van Dokkum, W.; Wezendonk, B.; Srikumar, T.S.; van den Heuvel, E.G. Effect of nondigestible oligosaccharides on large-bowel functions, blood lipid concentrations and glucose absorption in young healthy male subjects. Eur. J. Clin. Nutr. 1999, 53, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Letexier, D.; Diraison, F.; Beylot, M. Addition of inulin to a moderately high-carbohydrate diet reduces hepatic lipogenesis and plasma triacylglycerol concentrations in humans. Am. J. Clin. Nutr. 2003, 77, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Brighenti, F.; Casiraghi, M.C.; Canzi, E.; Ferrari, A. Effect of consumption of a ready-to-eat breakfast cereal containing inulin on the intestinal milieu and blood lipids in healthy male volunteers. Eur. J. Clin. Nutr. 1999, 53, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Davidson, M.H.; Maki, K.C.; Synecki, C.; Torri, S.A.; Drennan, K.B. Effects of dietary inulin on serum lipids in men and women with hypercholesterolemia. Nutr. Res. 1998, 18, 503–517. [Google Scholar] [CrossRef]
- Aliasgharzadeh, A.; Khalili, M.; Mirtaheri, E.; Gargari, B.P.; Tavakoli, F.; Farhangi, M.A.; Babaei, H.; Dehghan, P. A combination of prebiotic inulin and oligofructose improve some of cardiovascular disease risk factors in women with type 2 diabetes: A randomized controlled clinical trial. Adv. Pharm. Bull. 2015, 5, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Van den Maagdenberg, A.; Hofker, M.; Krimpenfort, P.; de Bruijn, I.; van Vlijmen, B.; van der Boom, H.; Havekes, L.; Frants, R. Transgenic mice carrying the apolipoprotein E3-Leiden gene exhibit hyperlipoproteinemia. J. Biol. Chem. 1993, 268, 10540–10545. [Google Scholar] [PubMed]
- Quax, P.H.; Lamfers, M.L.; Lardenoye, J.H.; Grimbergen, J.M.; de Vries, M.R.; Slomp, J.; de Ruiter, M.C.; Kockx, M.M.; Verheijen, J.H.; van Hinsbergh, V.W. Adenoviral expression of a urokinase receptor-targeted protease inhibitor inhibits neointima formation in murine and human blood vessels. Circulation 2001, 103, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Lardenoye, J.H.; Delsing, D.J.; de Vries, M.R.; Deckers, M.M.; Princen, H.M.; Havekes, L.M.; van Hinsbergh, V.W.; van Bockel, J.H.; Quax, P.H. Accelerated atherosclerosis by placement of a perivascular cuff and a cholesterol-rich diet in ApoE*3Leiden transgenic mice. Circ. Res. 2000, 87, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Pires, N.M.M.; Pols, T.W.H.; De Vries, M.R.; Van Tiel, C.M.; Bonta, P.I.; Vos, M.; Arkenbout, E.K.; Pannekoek, H.; Jukema, J.W.; Quax, P.H.A.; et al. Activation of nuclear receptor Nur77 by 6-mercaptopurine protects against neointima formation. Circulation 2007, 115, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Grainger, D.J.; Reckless, J.; McKilligin, E. Apolipoprotein E modulates clearance of apoptotic bodies in vitro and in vivo, resulting in a systemic proinflammatory state in apolipoprotein E-deficient mice. J. Immunol. 2004, 173, 6366–6375. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.H.; Reddick, R.L.; Piedrahita, J.A.; Maeda, N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science 1992, 258, 468–471. [Google Scholar] [CrossRef] [PubMed]
- Zadelaar, S.; Kleemann, R.; Verschuren, L.; de Vries-Van der Weij, J.; van der Hoorn, J.; Princen, H.M.; Kooistra, T. Mouse models for atherosclerosis and pharmaceutical modifiers. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1706–1721. [Google Scholar] [CrossRef] [PubMed]
- Miles, J.P.; Zou, J.; Kumar, M.-V.; Pellizzon, M.; Ulman, E.; Ricci, M.; Gewirtz, A.T.; Chassaing, B. Supplementation of Low- and High-fat Diets with Fermentable Fiber Exacerbates Severity of DSS-induced Acute Colitis. Inflamm. Bowel Dis. 2017, 23, 1133–1143. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, J.L.; Hedin, C.R.H.; Koutsoumpas, A.; Ng, S.C.; McCarthy, N.E.; Hart, A.L.; Kamm, M.A.; Sanderson, J.D.; Knight, S.C.; Forbes, A.; et al. Randomised, double-blind, placebo-controlled trial of fructo-oligosaccharides in active Crohn’s disease. Gut 2011, 60, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Joossens, M.; De Preter, V.; Ballet, V.; Verbeke, K.; Rutgeerts, P.; Vermeire, S. Effect of oligofructose-enriched inulin (OF-IN) on bacterial composition and disease activity of patients with Crohn’s disease: Results from a double-blinded randomised controlled trial. Gut 2012, 61, 958. [Google Scholar] [CrossRef] [PubMed]
- Progatzky, F.; Sangha, N.J.; Yoshida, N.; McBrien, M.; Cheung, J.; Shia, A.; Scott, J.; Marchesi, J.R.; Lamb, J.R.; Bugeon, L.; et al. Dietary cholesterol directly induces acute inflammasome-dependent intestinal inflammation. Nat. Commun. 2014, 5, 5864. [Google Scholar] [CrossRef] [PubMed]
- Goto, H.; Takemura, N.; Ogasawara, T.; Sasajima, N.; Watanabe, J.; Ito, H.; Morita, T.; Sonoyama, K. Effects of fructo-oligosaccharide on DSS-induced colitis differ in mice fed nonpurified and purified diets. J. Nutr. 2010, 140, 2121–2127. [Google Scholar] [CrossRef] [PubMed]
- Vogt, L.; Ramasamy, U.; Meyer, D.; Pullens, G.; Venema, K.; Faas, M.M.; Schols, H.A.; de Vos, P. Immune modulation by different types of β2→1-fructans is toll-like receptor dependent. PLoS ONE 2013, 8, e68367. [Google Scholar] [CrossRef] [PubMed]
- Parnell, J.A.; Reimer, R.A. Effect of prebiotic fibre supplementation on hepatic gene expression and serum lipids: A dose–response study in JCR:LA-cp rats. Br. J. Nutr. 2010, 103, 1577–1584. [Google Scholar] [CrossRef] [PubMed]
- Glagov, S.; Weisenberg, E.; Zarins, C.K.; Stankunavicius, R.; Kolettis, G.J. Compensatory Enlargement of Human Atherosclerotic Coronary Arteries. N. Engl. J. Med. 1987, 316, 1371–1375. [Google Scholar] [CrossRef] [PubMed]
- Pasterkamp, G.; Smits, P.C. Imaging of atherosclerosis. Remodelling of coronary arteries. J. Cardiovasc. Risk 2002, 9, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Galis, Z.S.; Sukhova, G.K.; Lark, M.W.; Libby, P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J. Clin. Investig. 1994, 94, 2493–2503. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Inflammation in atherosclerosis. Nature 2002, 420, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Tarini, J.; Wolever, T.M.S. The fermentable fibre inulin increases postprandial serum short-chain fatty acids and reduces free-fatty acids and ghrelin in healthy subjects. Appl. Physiol. Nutr. Metab. 2010, 35, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Beatty, E.R.; Wang, X.; Cummings, J.H. Selective stimulation of bifidobacteria in the human colon by oligofructose and inulin. Gastroenterology 1995, 108, 975–982. [Google Scholar] [CrossRef]
- Den Besten, G.; Lange, K.; Havinga, R.; van Dijk, T.H.; Gerding, A.; van Eunen, K.; Muller, M.; Groen, A.K.; Hooiveld, G.J.; Bakker, B.M.; et al. Gut-derived short-chain fatty acids are vividly assimilated into host carbohydrates and lipids. AJP Gastrointest. Liver Physiol. 2013, 305, G900–G910. [Google Scholar] [CrossRef] [PubMed]
- Wolever, T.M.; Spadafora, P.; Eshuis, H. Interaction between colonic acetate and propionate in humans. Am. J. Clin. Nutr. 1991, 53, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.I.A.; Gibson, G.R. Effects of consumption of probiotics and prebiotics on serum lipid levels in humans. Crit. Rev. Biochem. Mol. Biol. 2002, 37, 259–281. [Google Scholar] [CrossRef] [PubMed]
- Weitkunat, K.; Schumann, S.; Petzke, K.J.; Blaut, M.; Loh, G.; Klaus, S. Effects of dietary inulin on bacterial growth, short-chain fatty acid production and hepatic lipid metabolism in gnotobiotic mice. J. Nutr. Biochem. 2015. [Google Scholar] [CrossRef] [PubMed]



| Antibody | Fluorochrome | Dilution | Clone, Supplier |
|---|---|---|---|
| CD45.2 | FITC | 1:100 | 104, BioLegend |
| CD11b | Pacific Blue | 1:150 | M1/70, BioLegend |
| CD115-Biotin Streptavidin | n.a. PeCy5 | 1:100 1:100 | AFS98, eBioScience SAV, eBioScience |
| Gr-1 | PeCy7 | 1:1500 | RB6-8C5 |
| Vascular Pathology | Control (n = 11) | Inulin (n = 13) | p-Value |
| (Mean ± SEM) | (Mean ± SEM) | ||
| Intimal thickness (µm2) | 4043 ± 689.5 | 8685 ± 1462 | 0.013 * |
| Intima/media | 0.35 ± 0.05 | 0.65 ± 0.09 | 0.008 * |
| External area (µm2) | 20303 ± 1942 | 28515 ± 2225 | 0.012 * |
| Internal area (µm2) | 9383 ± 1288 | 16203 ± 1715 | 0.005 * |
| Luminal stenosis (%) | 44.21 ± 4.56 | 50.72 ± 5.21 | 0.367 |
| Lumen area (µm2) | 5340 ± 961.1 | 7518 ± 1376 | 0.224 |
| Medial area (µm2) | 10920 ± 723.1 | 12312 ± 755 | 0.201 |
| Medial collagen area (%) | 54.88 ± 3.67 | 56.69 ± 2.76 | 0.692 |
| Intimal collagen area (%) | 33.79 ± 2.62 | 45.6 ± 2.74 | 0.011 * |
| Medial SMC area (%) | 29.74 ± 4.29 | 30.1 ± 4.88 | 0.958 |
| Intimal SMC area (%) | 25.31 ± 2.81 | 41.93 ± 2.57 | 0.001 * |
| Medial macrophages (%) | 4.299 ± 1.92 | 14.85 ± 2.43 | 0.001 * |
| Intimal macrophages (%) | 1.73 ± 0.78 | 6.06 ± 1.33 | 0.002 * |
| Plasma monocytes | Control (n = 11) | Inulin (n = 11) | p-value |
| (mean ± SEM) | (mean ± SEM) | ||
| Granulocytes (%) | 10.55 ± 1.19 | 10.64 ± 1.14 | 0.956 |
| Monocytes (%) | 4.91 ± 0.39 | 6 ± 0.71 | 0.319 |
| Ly6C+ (%) | 0.18 ± 0.12 | 0.36 ± 0.15 | 0.635 |
| LyC6− (%) | 1 ± 0.14 | 1.36 ± 0.24 | 0.23 |
| Ly6Clow (%) | 3.18 ± 0.26 | 3.63 ± 0.51 | 0.737 |
| Plasma cholesterol | Control (n = 11) | Inulin (n = 13) | p-value |
| (mean ± SEM) | (mean ± SEM) | ||
| Plasma TC t = 0 (mM) | 3.83 ± 0.29 | 3.79 ± 0.26 | 0.924 |
| Plasma TC t = 3 (mM) | 13.28 ± 1 | 16.33 ± 0.85 | 0.024 * |
| Plasma TC t = 5 (mM) | 13.12 ± 0.5 | 14.65 ± 1.14 | 0.738 |
| TC exposure (mM*Weeks) | 63.54 ± 3.20 | 72.55 ± 2.38 | 0.03 * |
| Body weight and Food intake | Control (n = 11) | Inulin (n = 13) | p-value |
| (mean ± SEM) | (mean ± SEM) | ||
| Body weight t = 0 (g) | 28.42 ± 0.46 | 27.53 ± 0.54 | 0.832 |
| Body weight t = 1 (g) | 28.35 ± 0.49 | 27.61 ± 0.56 | 0.921 |
| Body weight t = 2 (g) | 28.12 ± 0.55 | 27.49 ± 0.55 | 0.962 |
| Body weight t = 3 (g) | 28.28 ± 0.54 | 27.47 ± 0.56 | 0.884 |
| Body weight t = 4 (g) | 28.01 ± 0.49 | 27.02 ± 0.55 | 0.754 |
| Body weight t = 5 (g) | 29.35 ± 0.54 | 28.59 ± 0.61 | 0.907 |
| Cumulative food intake t = 1 (g) | 24.81 ± 0.78 | 24.4 ± 1.7 | >0.999 |
| Cumulative food intake t = 2 (g) | 47.56 ± 1.51 | 46.3 ± 2.4 | >0.999 |
| Cumulative food intake t = 3 (g) | 81.33 ± 3.22 | 68.16 ± 2.71 | 0.035 * |
| Cumulative food intake t = 4 (g) | 109.94 ± 6.11 | 87.39 ± 2.76 | <0.0001 * |
| Cumulative food intake t = 5 (g) | 131.43 ± 6.52 | 105.81 ± 2.78 | <0.0001 * |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoving, L.R.; De Vries, M.R.; De Jong, R.C.M.; Katiraei, S.; Pronk, A.; Quax, P.H.A.; Van Harmelen, V.; Willems van Dijk, K. The Prebiotic Inulin Aggravates Accelerated Atherosclerosis in Hypercholesterolemic APOE*3-Leiden Mice. Nutrients 2018, 10, 172. https://doi.org/10.3390/nu10020172
Hoving LR, De Vries MR, De Jong RCM, Katiraei S, Pronk A, Quax PHA, Van Harmelen V, Willems van Dijk K. The Prebiotic Inulin Aggravates Accelerated Atherosclerosis in Hypercholesterolemic APOE*3-Leiden Mice. Nutrients. 2018; 10(2):172. https://doi.org/10.3390/nu10020172
Chicago/Turabian StyleHoving, Lisa R., Margreet R. De Vries, Rob C. M. De Jong, Saeed Katiraei, Amanda Pronk, Paul H. A. Quax, Vanessa Van Harmelen, and Ko Willems van Dijk. 2018. "The Prebiotic Inulin Aggravates Accelerated Atherosclerosis in Hypercholesterolemic APOE*3-Leiden Mice" Nutrients 10, no. 2: 172. https://doi.org/10.3390/nu10020172






