Effects of Consuming Preloads with Different Energy Density and Taste Quality on Energy Intake and Postprandial Blood Glucose
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Test Food
2.3. Procedure
2.4. Hedonic, Sensory and Appetite Ratings
2.5. Data Analysis
3. Results
3.1. Participants’ Characteristics
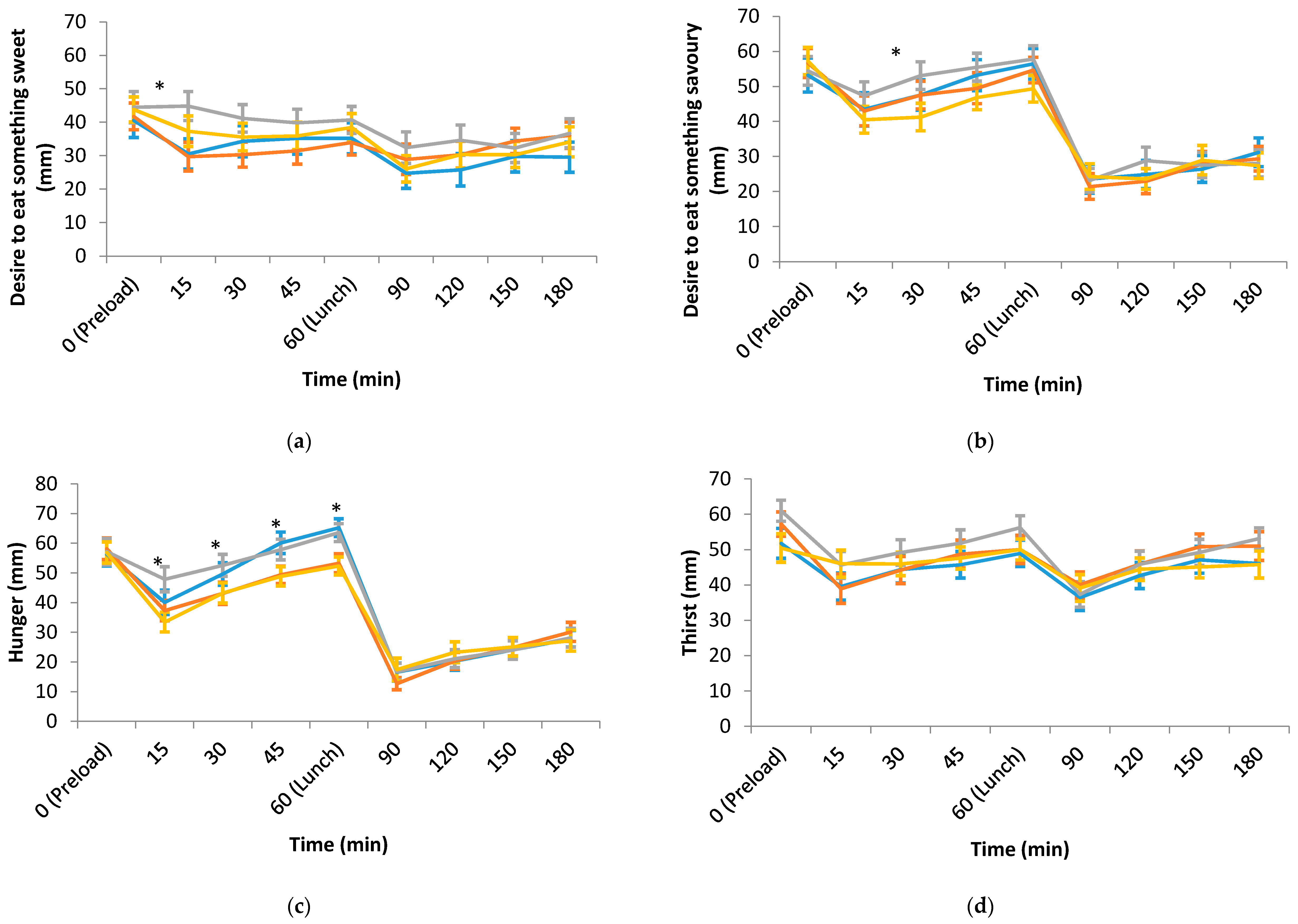
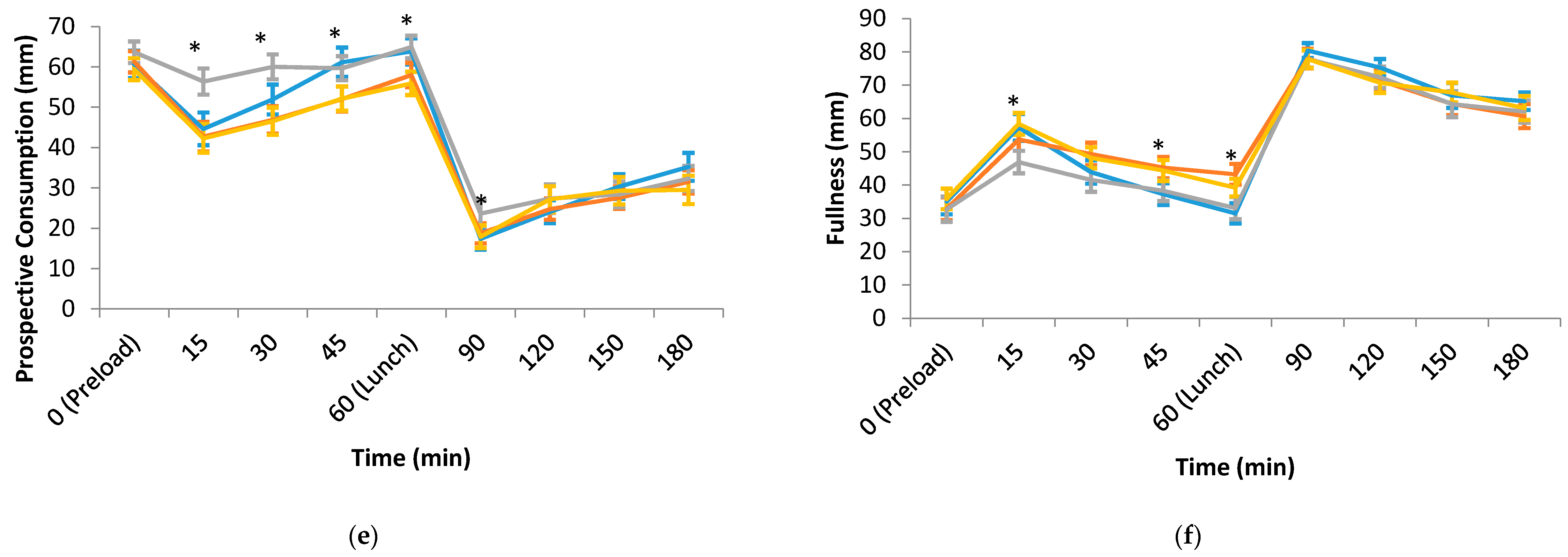
3.2. Hedonic, Sensory and Appetite Ratings of the Test Preloads and Lunch
3.3. Energy Intake
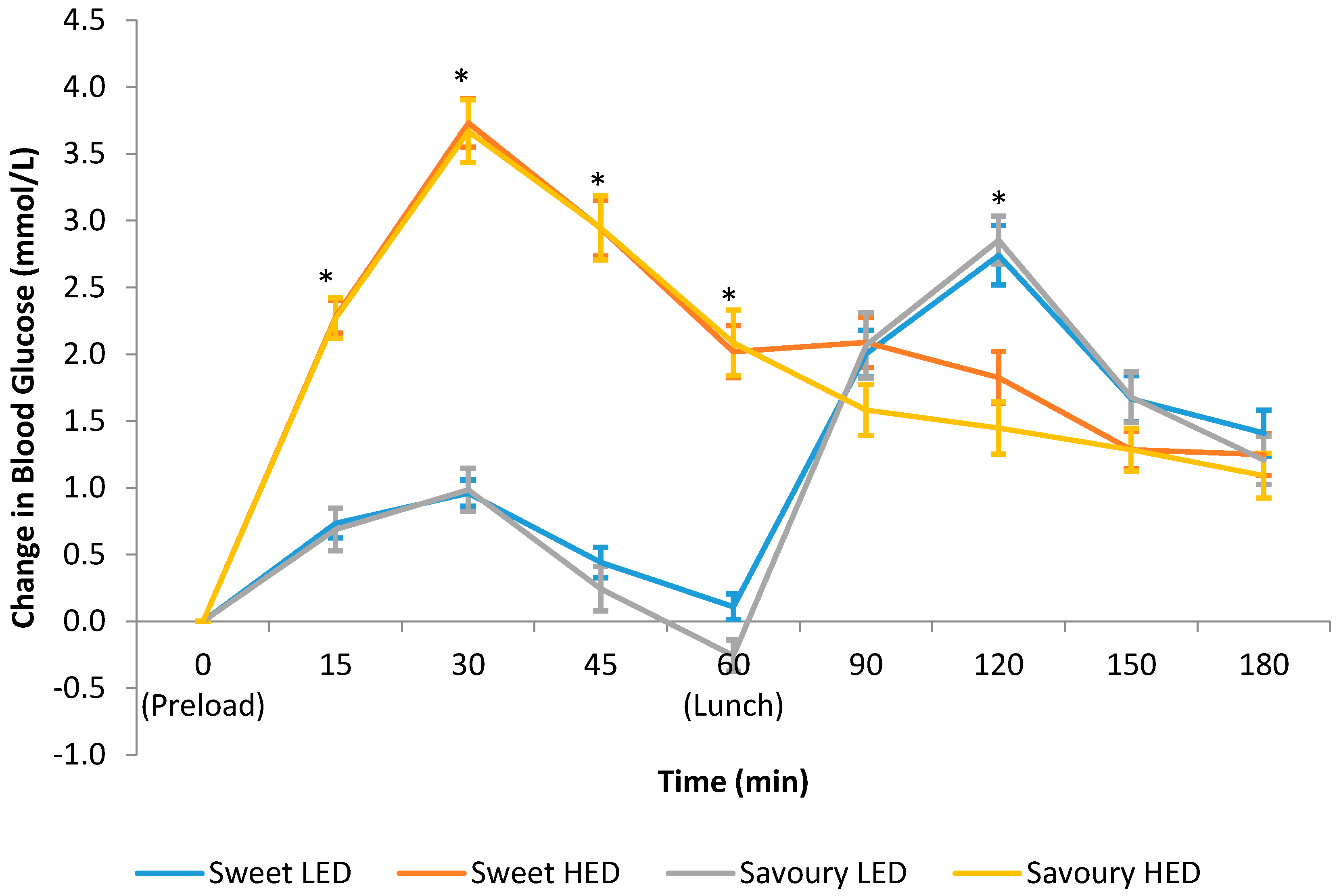
3.4. Postprandial Blood Glucose Response
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Campbell, P.T. Obesity: A certain and avoidable cause of cancer. Lancet 2014, 384, 727–728. [Google Scholar] [CrossRef]
- McPherson, K. Reducing the global prevalence of overweight and obesity. Lancet 2014, 384, 728–730. [Google Scholar] [CrossRef]
- Mozaffarian, D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: A comprehensive review. Circulation 2016, 133, 187–225. [Google Scholar] [CrossRef] [PubMed]
- Withrow, D.; Alter, D.A. The economic burden of obesity worldwide: A systematic review of the direct costs of obesity. Obes. Rev. 2011, 12, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Almiron-Roig, E.; Palla, L.; Guest, K.; Ricchiuti, C.; Vint, N.; Jebb, S.A.; Drewnowski, A. Factors that determine energy compensation: A systematic review of preload studies. Nutr. Rev. 2013, 71, 458–473. [Google Scholar] [CrossRef] [PubMed]
- De Graaf, C. Texture and satiation: The role of oro-sensory exposure time. Physiol. Behav. 2012, 107, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, J.; Running, C.A.; Tucker, R.M.; Mattes, R.D. Effects of food form on appetite and energy balance. Food Qual. Preference 2016, 48, 368–375. [Google Scholar] [CrossRef]
- McCrickerd, K.; Lim, C.M.; Leong, C.; Chia, E.M.; Forde, C.G. Texture-based differences in eating rate reduce the impact of increased energy density and large portions on meal size in adults. J. Nutr. 2017, 147, 1208–1217. [Google Scholar] [CrossRef] [PubMed]
- McCrickerd, K.; Forde, C. Consistency of eating rate, oral processing behaviours and energy intake across meals. Nutrients 2017, 9, 891. [Google Scholar] [CrossRef] [PubMed]
- McCrickerd, K.; Forde, C.G. Sensory influences on food intake control: Moving beyond palatability. Obes. Rev. 2016, 17, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, L.B.; Moller, P.; Flint, A.; Martens, M.; Raben, A. Effect of sensory perception of foods on appetite and food intake: A review of studies on humans. Int. J. Obes. 2003, 27, 1152–1166. [Google Scholar] [CrossRef] [PubMed]
- Yeomans, M.R.; Blundell, J.E.; Leshem, M. Palatability: Response to nutritional need or need-free stimulation of appetite? Br. J. Nutr. 2007, 92, S3–S14. [Google Scholar] [CrossRef]
- Hogenkamp, P.S.; Mars, M.; Stafleu, A.; de Graaf, C. Repeated consumption of a large volume of liquid and semi-solid foods increases ad libitum intake, but does not change expected satiety. Appetite 2012, 59, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Rolls, B.J.; Castellanos, V.H.; Halford, J.C.; Kilara, A.; Panyam, D.; Pelkman, C.L.; Smith, G.P.; Thorwart, M.L. Volume of food consumed affects satiety in men. Am. J. Clin. Nutr. 1998, 67, 1170–1177. [Google Scholar] [PubMed]
- Bell, E.A.; Rolls, B.J. Energy density of foods affects energy intake across multiple levels of fat content in lean and obese women. Am. J. Clin. Nutr. 2001, 73, 1010–1018. [Google Scholar] [PubMed]
- Duffey, K.J.; Popkin, B.M. Energy density, portion size, and eating occasions: Contributions to increased energy intake in the United States, 1977–2006. PLoS Med. 2011, 8, e1001050. [Google Scholar] [CrossRef] [PubMed]
- Ello-Martin, J.A.; Ledikwe, J.H.; Rolls, B.J. The influence of food portion size and energy density on energy intake: Implications for weight management. Am. J. Clin. Nutr. 2005, 82, 236S–241S. [Google Scholar] [PubMed]
- Karl, J.P.; Roberts, S.B. Energy density, energy intake, and body weight regulation in adults. Adv. Nutr. 2014, 5, 835–850. [Google Scholar] [CrossRef] [PubMed]
- Rolls, B.J.; Bell, E.A.; Castellanos, V.H.; Chow, M.; Pelkman, C.L.; Thorwart, M.L. Energy density but not fat content of foods affected energy intake in lean and obese women. Am. J. Clin. Nutr. 1999, 69, 863–871. [Google Scholar] [PubMed]
- Rolls, B.J.; Williams, R.A.; Keller, K.L. The role of dietary energy density in weight management. In Managing and Preventing Obesity; Gill, T., Ed.; Woodhead Publishing: Oxford, UK, 2015; pp. 137–148. [Google Scholar]
- Rouhani, M.H.; Haghighatdoost, F.; Surkan, P.J.; Azadbakht, L. Associations between dietary energy density and obesity: A systematic review and meta-analysis of observational studies. Nutrition 2016, 32, 1037–1047. [Google Scholar] [CrossRef] [PubMed]
- Tey, S.L.; Chia, E.M.E.; Forde, C.G. Impact of dose-response calorie reduction or supplementation of a covertly manipulated lunchtime meal on energy compensation. Physiol. Behav. 2016, 165, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Benton, D. Portion size: What we know and what we need to know. Crit. Rev. Food Sci. Nutr. 2015, 55, 988–1004. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, R.W.; Rydell, S.; Dunn, C.L.; Harnack, L.J.; Levine, A.S.; Pentel, P.R.; Baxter, J.E.; Walsh, E.M. Effects of portion size on chronic energy intake. Int. J. Behav. Nutr. Phys. Act. 2007, 4, 1–5. [Google Scholar] [CrossRef]
- Rolls, B.J.; Roe, L.S.; Meengs, J.S. The effect of large portion sizes on energy intake is sustained for 11 days. Obesity 2007, 15, 1535–1543. [Google Scholar] [CrossRef] [PubMed]
- Wansink, B.; Wansink, C.S. The largest Last Supper: Depictions of food portions and plate size increased over the millennium. Int. J. Obes. 2010, 34, 943–944. [Google Scholar] [CrossRef] [PubMed]
- Mattes, R.D. Gustation as a determinant of ingestion: Methodological issues. Am. J. Clin. Nutr. 1985, 41, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Malik, V.S.; Pan, A.; Willett, W.C.; Hu, F.B. Sugar-sweetened beverages and weight gain in children and adults: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2013, 98, 1084–1102. [Google Scholar] [CrossRef] [PubMed]
- Fernstrom, J.D. Non-nutritive sweeteners and obesity. Annu. Rev. Food Sci. Technol. 2015, 6, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Imamura, F.; O’Connor, L.; Ye, Z.; Mursu, J.; Hayashino, Y.; Bhupathiraju, S.N.; Forouhi, N.G. Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: Systematic review, meta-analysis, and estimation of population attributable fraction. BMJ 2015, 351, h3576. [Google Scholar] [CrossRef] [PubMed]
- Te Morenga, L.; Mallard, S.; Mann, J. Dietary sugars and body weight: Systematic review and meta-analyses of randomised controlled trials and cohort studies. BMJ 2013, 346, e7492. [Google Scholar] [CrossRef] [PubMed]
- Fitch, C.; Keim, K.S. Position of the Academy of Nutrition and Dietetics: Use of nutritive and nonnutritive sweeteners. J. Acad. Nutr. Diet. 2012, 112, 739–758. [Google Scholar] [CrossRef] [PubMed]
- Mattes, R.D. Low calorie sweeteners: Science and controversy: Conference proceedings. Physiol. Behav. 2016, 164, 429–431. [Google Scholar] [CrossRef] [PubMed]
- Rogers, P.J. The role of low-calorie sweeteners in the prevention and management of overweight and obesity: Evidence v. conjecture. Proc. Nutr. Soc. 2017, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tappy, L. What nutritional physiology tells us about diet, sugar and obesity. Int. J. Obes. 2016, 40, S28–S29. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Allman-Farinelli, M.; Heitmann, B.L.; Rangan, A. Substitution of sugar-sweetened beverages with other beverage alternatives: A review of long-term health outcomes. J. Acad. Nutr. Diet. 2015, 115, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Hall, K.D. A review of the carbohydrate-insulin model of obesity. Eur. J. Clin. Nutr. 2017, 71, 323. [Google Scholar] [CrossRef] [PubMed]
- De Graaf, C.; Schreurs, A.; Blauw, Y.H. Short-term effects of different amounts of sweet and nonsweet carbohydrates on satiety and energy intake. Physiol. Behav. 1993, 54, 833–843. [Google Scholar] [CrossRef]
- Anderson, G.H.; Fabek, H.; Akilen, R.; Chatterjee, D.; Kubant, R. Acute effects of monosodium glutamate addition to whey protein on appetite, food intake, blood glucose, insulin and gut hormones in healthy young men. Appetite 2018, 120, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Finlayson, G.; Bordes, I.; Griffioen-Roose, S.; de Graaf, C.; Blundell, J.E. Susceptibility to overeating affects the impact of savory or sweet drinks on satiation, reward, and food intake in nonobese women. J. Nutr. 2012, 142, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Carter, B.E.; Monsivais, P.; Perrigue, M.M.; Drewnowski, A. Supplementing chicken broth with monosodium glutamate reduces hunger and desire to snack but does not affect energy intake in women. Br. J. Nutr. 2011, 106, 1441–1448. [Google Scholar] [CrossRef] [PubMed]
- Masic, U.; Yeomans, M.R. Monosodium glutamate delivered in a protein-rich soup improves subsequent energy compensation. J. Nutr. Sci. 2014, 3, e15. [Google Scholar] [CrossRef] [PubMed]
- Masic, U.; Yeomans, M.R. Does acute or habitual protein deprivation influence liking for monosodium glutamate? Physiol. Behav. 2017, 171, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Griffioen-Roose, S.; Mars, M.; Finlayson, G.; Blundell, J.E.; de Graaf, C. Satiation due to equally palatable sweet and savory meals does not differ in normal weight young adults. J. Nutr. 2009, 139, 2093–2098. [Google Scholar] [CrossRef] [PubMed]
- Rogers, P.J.; Blundell, J.E. Umami and appetite: Effects of monosodium glutamate on hunger and food intake in human subjects. Physiol. Behav. 1990, 48, 801–804. [Google Scholar] [CrossRef]
- Luscombe-Marsh, N.D.; Smeets, A.J.P.G.; Westerterp-Plantenga, M.S. The addition of monosodium glutamate and inosine monophosphate-5 to high-protein meals: Effects on satiety, and energy and macronutrient intakes. Br. J. Nutr. 2009, 102, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Malik, V.S.; Popkin, B.M.; Bray, G.A.; Després, J.-P.; Willett, W.C.; Hu, F.B. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes. Diabetes Care 2010, 33, 2477–2483. [Google Scholar] [CrossRef] [PubMed]
- Stanhope, K.L. Sugar consumption, metabolic disease and obesity: The state of the controversy. Crit. Rev. Clin. Lab. Sci. 2016, 53, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Johnston, C.A.; Stevens, B.; Foreyt, J. The role of low-calorie sweeteners in diabetes. Eur. Endocrinol. 2013, 9, 13–15. [Google Scholar] [CrossRef]
- Tucker, R.M.; Tan, S.-Y. Do non-nutritive sweeteners influence acute glucose homeostasis in humans? A systematic review. Physiol. Behav. 2017, 182, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Tey, S.L.; Salleh, N.B.; Henry, J.; Forde, C.G. Effects of aspartame-, monk fruit-, Stevia-, and sucrose-sweetened beverages on postprandial glucose, insulin and energy intake. Int. J. Obes. 2017, 41, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Tey, S.L.; Salleh, N.B.; Henry, C.J.; Forde, C.G. Effects of non-nutritive (artificial vs. natural) sweeteners on 24-h glucose profiles. Eur. J. Clin. Nutr. 2017, 71, 1129–1132. [Google Scholar] [CrossRef] [PubMed]
- Van Strien, T.; Frijters, J.E.R.; Bergers, G.P.A.; Defares, P.B. The Dutch Eating Behavior Questionnaire (DEBQ) for assessment of restrained, emotional, and external eating behavior. Int. J. Eat. Disord. 1986, 5, 295–315. [Google Scholar] [CrossRef]
- McCrickerd, K.; Salleh, N.B.; Forde, C.G. Removing energy from a beverage influences later food intake more than the same energy addition. Appetite 2016, 105, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Shu-Fen, C.L.; Forde, C.G.; Tey, S.L.; Henry, C.J. Taste perception and diet in people of Chinese ancestry. Asia Pac. J. Clin. Nutr. 2017. [Google Scholar] [CrossRef]
- Shu-Fen, C.L.; Forde, C.G.; Tey, S.L.; Henry, C.J. Taste sensitivities and diet of Chinese and Indians in Singapore. Asia Pac. J. Clin. Nutr. 2017. [Google Scholar] [CrossRef]
- Blundell, J.; De Graaf, C.; Hulshof, T.; Jebb, S.; Livingstone, B.; Lluch, A.; Mela, D.; Salah, S.; Schuring, E.; Van Der Knaap, H.; et al. Appetite control: Methodological aspects of the evaluation of foods. Obes. Rev. 2010, 11, 251–270. [Google Scholar] [CrossRef] [PubMed]
- Gardner, C. Non-nutritive sweeteners: Evidence for benefit vs. risk. Curr. Opin. Lipidol. 2014, 25, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.E.; Perez, V. Low-calorie sweeteners and body weight and composition: A meta-analysis of randomized controlled trials and prospective cohort studies. Am. J. Clin. Nutr. 2014, 100, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.C.; Beck, J. Low Calorie Sweetener (LCS) use and energy balance. Physiol. Behav. 2016, 164, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Rogers, P.J.; Hogenkamp, P.S.; de Graaf, C.; Higgs, S.; Lluch, A.; Ness, A.R.; Penfold, C.; Perry, R.; Putz, P.; Yeomans, M.R.; et al. Does low-energy sweetener consumption affect energy intake and body weight? A systematic review, including meta-analyses, of the evidence from human and animal studies. Int. J. Obes. 2016, 40, 381–394. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 4. Lifestyle management: Standards of medical care in diabetes—2018. Diabetes Care 2018, 41, S38–S50. [Google Scholar]
- Mattes, R.D.; Popkin, B.M. Nonnutritive sweetener consumption in humans: Effects on appetite and food intake and their putative mechanisms. Am. J. Clin. Nutr. 2009, 89, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hetherington, M.; Havermans, R.C. 14—Sensory-specific satiation and satiety. In Satiation, Satiety and the Control of Food Intake; Blundell, J.E., Bellisle, F., Eds.; Woodhead Publishing: Sawston, UK, 2013; pp. 253–269. [Google Scholar]
- Hetherington, M.; Rolls, B.J.; Burley, V.J. The time course of sensory-specific satiety. Appetite 1989, 12, 57–68. [Google Scholar] [CrossRef]
- Rolls, B.J.; Rolls, E.T.; Rowe, E.A.; Sweeney, K. Sensory specific satiety in man. Physiol. Behav. 1981, 27, 137–142. [Google Scholar] [CrossRef]
- Tey, S.L.; Brown, R.C.; Gray, A.R.; Chisholm, A.W.; Delahunty, C.M. Long-term consumption of high energy-dense snack foods on sensory-specific satiety and intake. Am. J. Clin. Nutr. 2012, 95, 1038–1047. [Google Scholar] [CrossRef] [PubMed]
- Soong, Y.Y.; Lim, W.X.; Leow, M.K.S.; Siow, P.C.; Teh, A.L.; Henry, C.J. Combination of soya protein and polydextrose reduces energy intake and glycaemic response via modulation of gastric emptying rate, ghrelin and glucagon-like peptide-1 in Chinese. Br. J. Nutr. 2016, 115, 2130–2137. [Google Scholar] [CrossRef] [PubMed]
- Livesey, G. Glycaemic responses and toleration. In Sweeteners and Sugar Alternatives in Food Technology; Wiley-Blackwell: Hoboken, NJ, USA, 2012; pp. 1–26. [Google Scholar]
- Smeets, P.A.; de Graaf, C.; Stafleu, A.; van Osch, M.J.; van der Grond, J. Functional magnetic resonance imaging of human hypothalamic responses to sweet taste and calories. Am. J. Clin. Nutr. 2005, 82, 1011–1016. [Google Scholar] [PubMed]
- Ford, H.E.; Peters, V.; Martin, N.M.; Sleeth, M.L.; Ghatei, M.A.; Frost, G.S.; Bloom, S.R. Effects of oral ingestion of sucralose on gut hormone response and appetite in healthy normal-weight subjects. Eur. J. Clin. Nutr. 2011, 65, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Takahashi, C. Interactions of monosodium glutamate and sodium chloride on saltiness and palatability of a clear soup. J. Food Sci. 1984, 49, 82–85. [Google Scholar] [CrossRef]
- Huxley, R.; James, W.P.T.; Barzi, F.; Patel, J.V.; Lear, S.A.; Suriyawongpaisal, P.; Janus, E.; Caterson, I.; Zimmet, P.; Prabhakaran, D.; et al. Ethnic comparisons of the cross-sectional relationships between measures of body size with diabetes and hypertension. Obes. Rev. 2008, 9, 53–61. [Google Scholar] [CrossRef] [PubMed]
- James, W.P.T. The challenge of diabetes in Asia. Eur. J. Clin. Nutr. 2017, 71, 803–804. [Google Scholar] [CrossRef] [PubMed]
- Nanditha, A.; Ma, R.C.W.; Ramachandran, A.; Snehalatha, C.; Chan, J.C.N.; Chia, K.S.; Shaw, J.E.; Zimmet, P.Z. Diabetes in Asia and the Pacific: Implications for the global epidemic. Diabetes Care 2016, 39, 472–485. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, A.; Snehalatha, C.; Shetty, A.S.; Nanditha, A. Trends in prevalence of diabetes in Asian countries. World J. Diabetes 2012, 3, 110–117. [Google Scholar] [CrossRef] [PubMed]

| Participant Characteristics | Means (SD) |
|---|---|
| Age (years) | 28.9 (7.5) |
| Height (cm) | 172.3 (6.5) |
| Weight (kg) | 65.6 (7.3) |
| Body mass index (kg/m2) | 22.1 (1.8) |
| Waist circumference (cm) | 73.9 (5.3) |
| Hip circumference (cm) | 89.4 (3.6) |
| Bodpod basal metabolic rate (kcal) | 1431 (159) |
| Bodpod fat (%) | 17.5 (6.3) |
| Bodpod fat free mass (%) | 82.5 (6.3) |
| Fasting blood glucose (mmol/L) | 4.5 (0.4) |
| Systolic blood pressure (mmHg) | 119.6 (9.6) |
| Diastolic blood pressure (mmHg) | 70.7 (7.3) |
| All values are means (SD) |
| Sweet LED | Sweet HED | Savoury LED | Savoury HED | p Value | |
|---|---|---|---|---|---|
| Ratings after a spoonful of the preload | |||||
| Pleasantness (mm) | 46.3 (3.5) a,b | 53.5 (3.1) b | 37.5 (3.8) a | 43.2 (4.0) a | 0.008 |
| Thickness (mm) | 24.8 (3.5) | 27.7 (4.1) | 31.3 (4.1) | 30.1 (3.8) | 0.266 |
| Desire to eat something sweet (mm) | 40.7 (4.8) | 46.9 (4.6) | 46.9 (4.8) | 41.7 (4.5) | 0.487 |
| Desire to eat something savoury (mm) | 51.4 (4.3) | 56.8 (3.6) | 50.8 (4.4) | 51.2 (4.3) | 0.475 |
| Bitterness (mm) | 22.6 (3.9) | 12.0 (2.4) | 20.3 (4.3) | 18.2 (4.3) | 0.076 |
| Sweetness (mm) | 53.2 (4.4) a | 63.6 (3.5) b | 19.5 (3.9) c | 19.2 (3.5) c | <0.001 |
| Expected Fullness (mm) | 48.1 (4.0) | 47.2 (3.4) | 48.4 (4.0) | 51.8 (3.6) | 0.627 |
| Salty (mm) | 21.0 (3.4) a | 27.0 (3.8) a | 62.8 (3.7) b | 60.7 (4.0) b | <0.001 |
| Savoury (mm) | 38.5 (4.8) | 37.2 (4.3) | 49.2 (3.6) | 47.6 (4.3) | 0.060 |
| Flavour intensity (mm) | 35.7 (3.4) a | 49.0 (3.3) b | 48.1 (3.7) b | 53.8 (3.7) b | <0.001 |
| Ratings after a spoonful of the lunch | |||||
| Pleasantness (mm) | 58.0 (2.6) | 59.8 (2.4) | 61.0 (3.4) | 56.6 (2.9) | 0.493 |
| Sweetness (mm) | 26.7 (3.6) | 27.0 (3.7) | 26.8 (3.8) | 24.2 (3.8) | 0.734 |
| Saltiness (mm) | 43.2 (3.1) | 44.2 (3.0) | 41.5 (3.6) | 43.6 (2.8) | 0.838 |
| Familiarity (mm) | 73.9 (3.0) | 74.6 (3.0) | 80.1 (3.1) | 74.6 (3.3) | 0.134 |
| Hunger (mm) | 64.6 (3.3) a | 58.3 (2.9) b | 65.1 (3.0) a | 54.7 (3.2) b | 0.001 |
| Desire to eat something sweet (mm) | 35.5 (4.6) | 35.6 (4.0) | 35.7 (4.6) | 35.8 (4.2) | 1.000 |
| Desire to eat something savoury (mm) | 58.8 (4.0) | 59.5 (3.5) | 61.2 (4.0) | 54.8 (3.8) | 0.236 |
| Prospective consumption (mm) | 62.7 (3.4) a | 61.9 (3.0) a | 65.5 (3.0) a | 53.5 (2.9) b | <0.001 |
| Thirst (mm) | 45.7 (3.7) a | 53.1 (3.5) b | 54.7 (3.3) b | 49.3 (3.3) a,b | 0.029 |
| Fullness (mm) | 31.3 (3.1) a | 38.1 (2.8) a,b | 32.7 (3.2) a | 41.4 (3.1) b | 0.026 |
| Savoury (mm) | 55.6 (3.2) | 53.4 (2.7) | 52.8 (3.7) | 53.8 (3.0) | 0.854 |
| Flavour intensity (mm) | 51.1 (3.0) | 56.3 (2.3) | 54.3 (3.5) | 52.3 (2.4) | 0.407 |
| Energy intake | |||||
| Ad libitum lunch (kcal) | 762.5 (44.4) a | 721.7 (48.4) a,b | 761.5 (43.5) a | 692.8 (41.5) b | 0.012 |
| Subsequent meals (kcal) | 714.1 (67.3) | 698.6 (65.8) | 822.7 (65.6) | 714.8 (48.6) | 0.284 |
| Total daily intake (kcal) | 2061 (85.8) | 2203 (80.7) | 2178 (94.3) | 2199 (61.8) | 0.214 |
| Blood glucose response | |||||
| Glucose iAUC 0 to 180 min | 249.1 (18.6) a | 354.8 (22.3) b | 252.5 (18.5) a | 327.6 (26.4) b | <0.001 |
| Glucose total AUC 0 to 180 min | 1040 (12.9) a | 1131 (18.9) b | 1043 (13.2) a | 1131 (15.2) b | <0.001 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tey, S.L.; Salleh, N.; Henry, C.J.; Forde, C.G. Effects of Consuming Preloads with Different Energy Density and Taste Quality on Energy Intake and Postprandial Blood Glucose. Nutrients 2018, 10, 161. https://doi.org/10.3390/nu10020161
Tey SL, Salleh N, Henry CJ, Forde CG. Effects of Consuming Preloads with Different Energy Density and Taste Quality on Energy Intake and Postprandial Blood Glucose. Nutrients. 2018; 10(2):161. https://doi.org/10.3390/nu10020161
Chicago/Turabian StyleTey, Siew Ling, Nurhazwani Salleh, Christiani Jeyakumar Henry, and Ciaran G. Forde. 2018. "Effects of Consuming Preloads with Different Energy Density and Taste Quality on Energy Intake and Postprandial Blood Glucose" Nutrients 10, no. 2: 161. https://doi.org/10.3390/nu10020161





