Effects of Metformin Combined with Lactoferrin on Lipid Accumulation and Metabolism in Mice Fed with High-Fat Diet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Diets
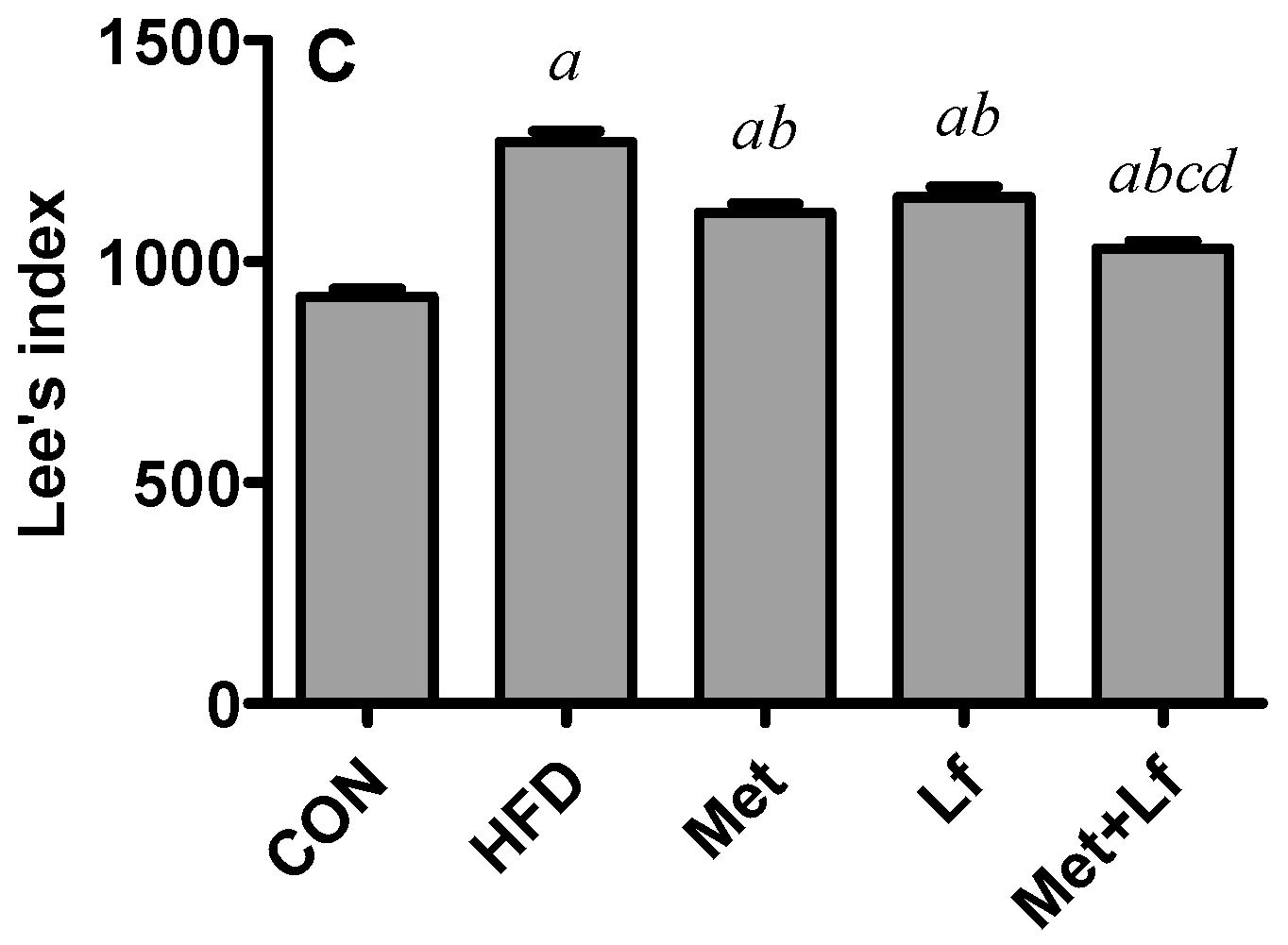
2.2. Body Weight, Waist Circumference, and Lee’s Index
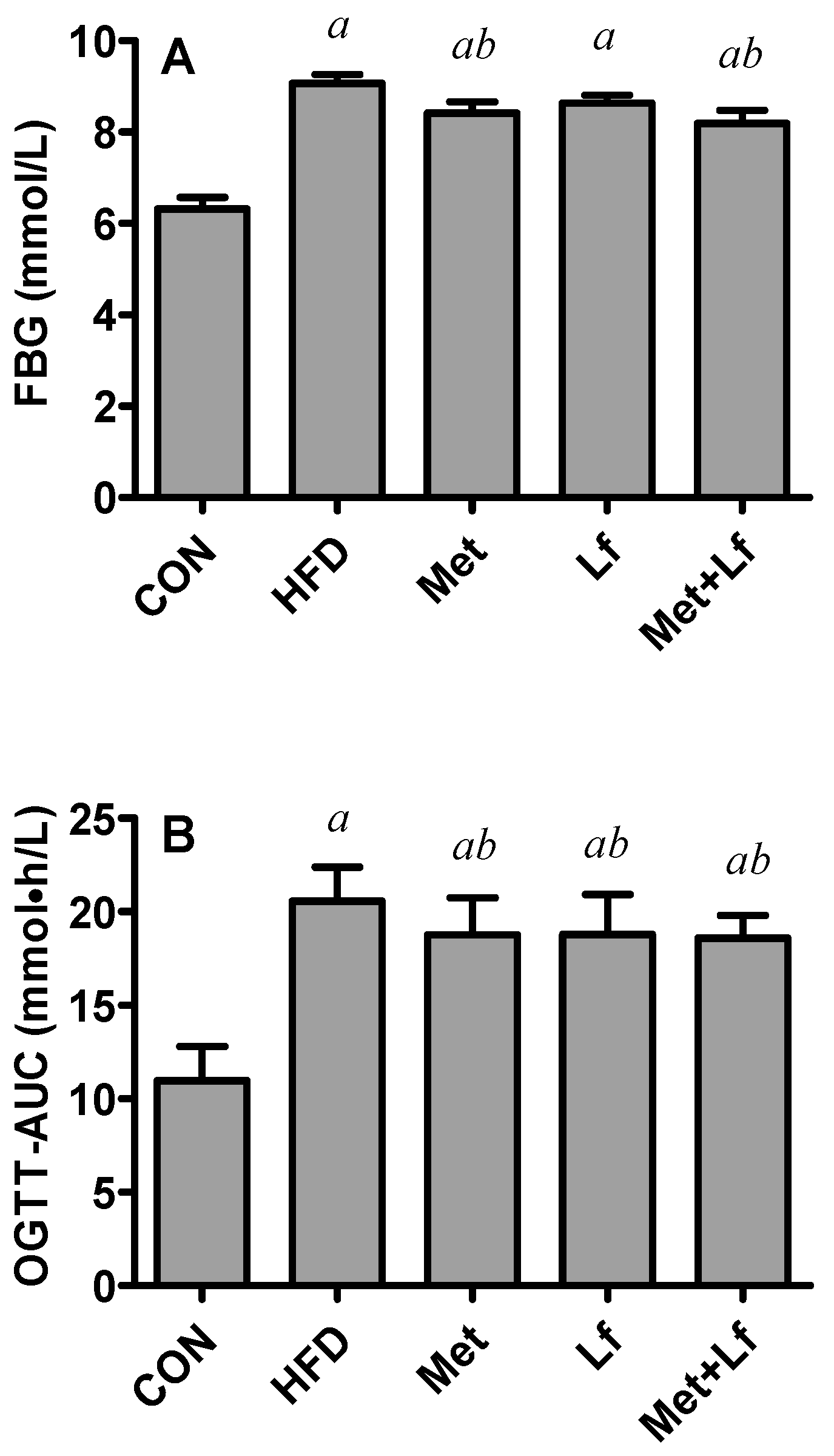
2.3. Oral Glucose Tolerance Test (OGTT)
2.4. Sample Collection at Autopsy
2.5. Serum Biochemical Determination
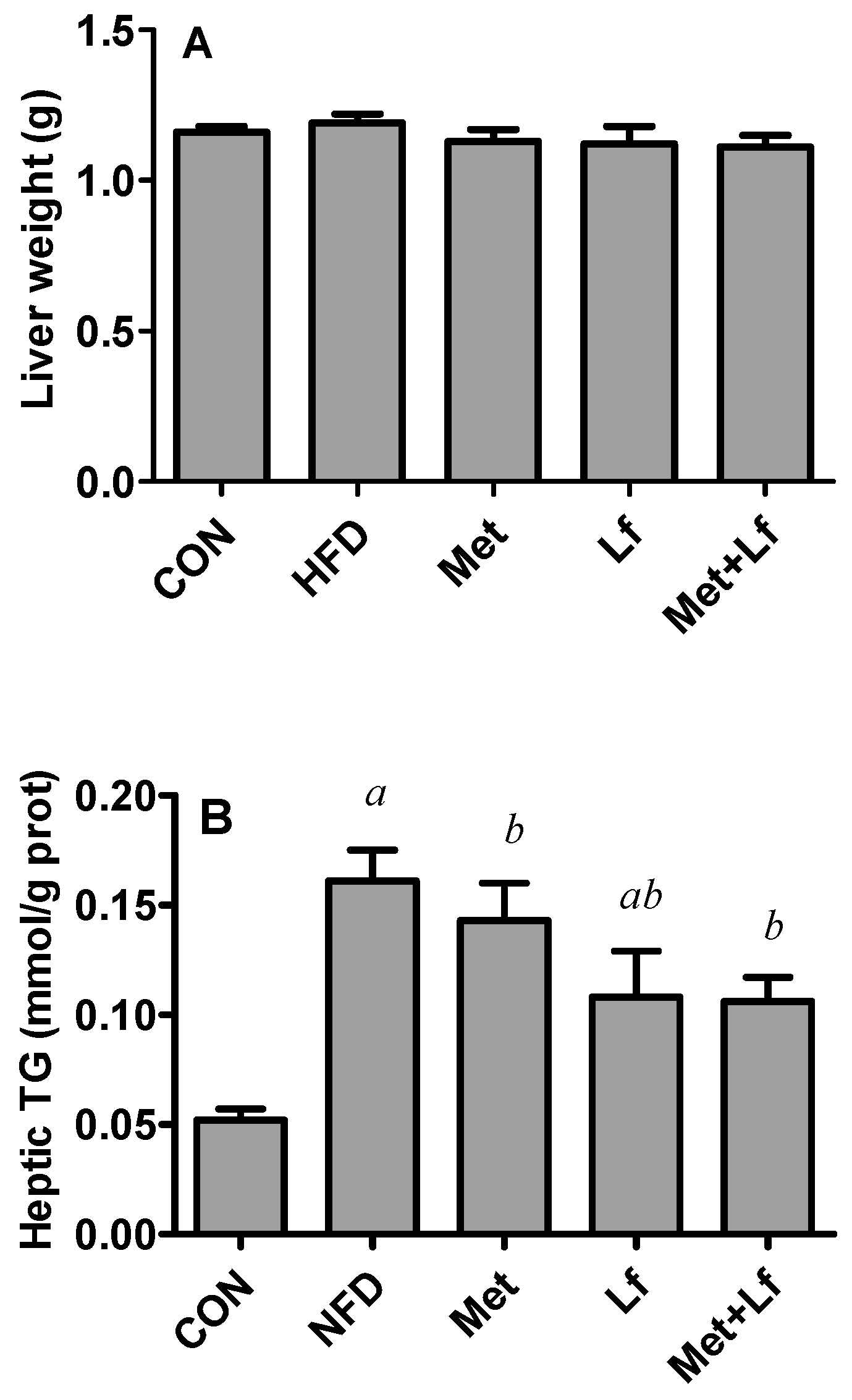
2.6. Hepatic TG and TC Contents
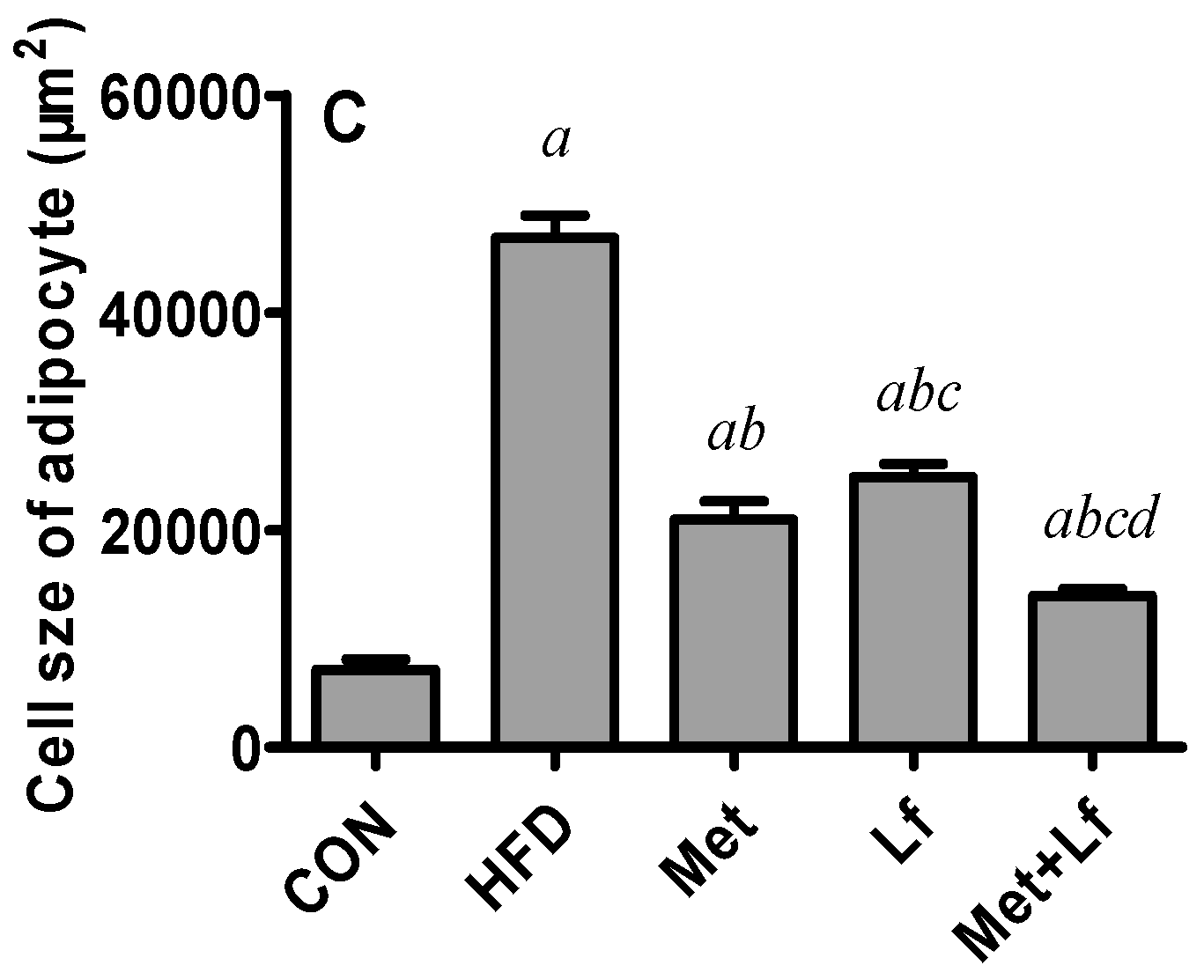
2.7. Histology of Liver and Perirenal Fat
2.8. Western Blot Analysis in Liver
2.9. Statistical Analysis
3. Results
3.1. Body Weight, Waist Circumference, and Lee’s Index
3.2. FBG and OGTT
3.3. Serum Lipid Profiles, Adipocytokines, and Transaminases
3.4. Hepatic Weight and Lipids
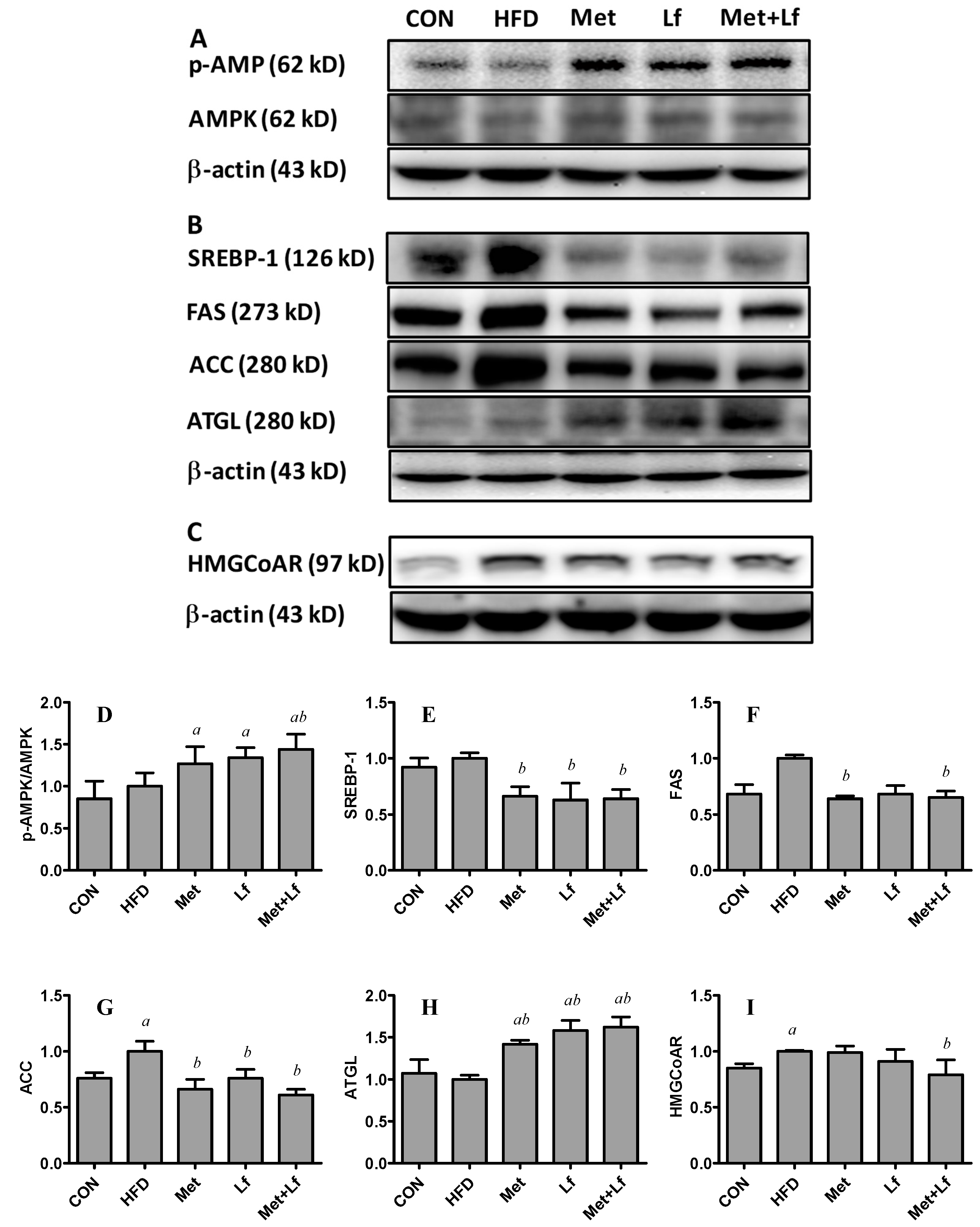
3.5. Hepatic Protein Expression
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Moreno-Navarrete, J.M.; Ortega, F.; Sabater, M.; Ricart, W.; Fernández-Real, J.M. Proadipogenic effects of lactoferrin in human subcutaneous and visceral preadipocytes. J. Nutr. Biochem. 2011, 22, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.S.; Sendra, S.; Lloret, J.; Bosch, I. Systems and WBANs for Controlling Obesity. J. Healthc. Eng. 2018, 2018, 1564748. [Google Scholar] [CrossRef] [PubMed]
- Desilets, A.R.; Dhakal-Karki, S.; Dunican, K.C. Role of metformin for weight management in patients without type 2 diabetes. Ann. Pharmacother. 2008, 42, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Seifarth, C.; Schehler, B.; Schneider, H.J. Effectiveness of metformin on weight loss in non-diabetic individuals with obesity. Exp. Clin. Endocrinol. Diabetes 2013, 121, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, F.J.; Jiang, C. A Western diet-induced mouse model reveals a possible mechanism by which metformin decreases obesity. Eur. J. Clin. Pharmacol. 2017, 73, 1337–1339. [Google Scholar] [CrossRef] [PubMed]
- Marchesini, G.; Brizi, M.; Bianchi, G.; Tomassetti, S.; Zoli, M.; Melchionda, N. Metformin in non-alcoholic steatohepatitis. Lancet 2001, 358, 893–894. [Google Scholar] [CrossRef]
- Li, M.; Sharma, A.; Yin, C.; Tan, X.; Xiao, Y. Metformin ameliorates hepatic steatosis and improves the induction of autophagy in HFD induced obese mice. Mol. Med. Rep. 2017, 16, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.L.; Xu, H.; Li, H.; Zhao, Y.; Hu, X.; Zhao, J.; Guo, X.; Guo, T.; Botchlett, R.; Qi, T.; et al. Metformin ameliorates hepatic steatosis and inflammation without altering adipose phenotype in diet-induced obesity. PLoS ONE 2014, 9, e91111. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Woo, S.L.; Hu, X.; Botchlett, R.; Chen, L.; Huo, Y.; Wu, C. Metformin and metabolic diseases: A focus on hepatic aspects. Front. Med. 2015, 9, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.H.; Hung, Y.J.; Hsieh, P.S. Additional effect of metformin and celecoxib against lipid dysregulation and adipose tissue inflammation in high-fat fed rats with insulin resistance and fatty liver. Eur. J. Pharmacol. 2016, 789, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.R.; Bose, S.; Wang, J.H.; Ansari, A.; Lim, S.K.; Chin, Y.W.; Choi, H.S.; Kim, H. Flos lonicera combined with metformin ameliorates hepatosteatosis and glucose intolerance in association with gut microbiota modulation. Front. Microbiol. 2017, 8, 2271. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Bruckbauer, A.; Li, F.; Cao, Q.; Cui, X.; Wu, R.; Shi, H.; Zemel, M.B.; Xue, B. Interaction between metformin and leucine in reducing hyperlipidemia and hepatic lipid accumulation in diet-induced obese mice. Metabolism 2015, 64, 1426–1434. [Google Scholar] [CrossRef] [PubMed]
- Shojaei Zarghani, S.; Abbaszadeh, S.; Alizadeh, M.; Rameshrad, M.; Garjani, A.; Soraya, H. The effect of metformin combined with calcium-vitamin D3 against diet-induced nonalcoholic fatty liver disease. Adv. Pharm. Bull. 2018, 8, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Expósito, L.; Illescas-Montes, R.; Melguizo-Rodríguez, L.; Ruiz, C.; Ramos-Torrecillas, J.; de Luna-Bertos, E. Multifunctional capacity and therapeutic potential of lactoferrin. Life Sci. 2018, 195, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Morishita, S.; Ono, T.; Fujisaki, C.; Ishihara, Y.; Murakoshi, M.; Kato, H.; Hosokawa, M.; Miyashita, K.; Sugiyama, K.; Nishino, H. Bovine lactoferrin reduces visceral fat and liver triglycerides in ICR mice. J. Oleo Sci. 2013, 62, 97–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeuchi, T.; Shimizu, H.; Ando, K.; Harada, E. Bovine lactoferrin reduces plasma triacylglycerol and NEFA accompanied by decreased hepatic cholesterol and triacylglycerol contents in rodents. Br. J. Nutr. 2004, 91, 533–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, J.; Finckenberg, P.; Martonen, E.; Ahlroos-Lehmus, A.; Pilvi, T.K.; Korpela, R.; Mervaala, E.M. Metabolic effects of lactoferrin during energy restriction and weight regain in diet-induced obese mice. J. Funct. Foods 2012, 4, 66–78. [Google Scholar] [CrossRef]
- Sun, J.; Ren, F.; Xiong, L.; Zhao, L.; Guo, H. Bovine lactoferrin suppresses high-fat diet induced obesity and modulates gut microbiota in C57BL/6J mice. J. Funct. Foods 2016, 22, 189–200. [Google Scholar] [CrossRef]
- Xiong, L.; Ren, F.; Lv, J.; Zhang, H.; Guo, H. Lactoferrin attenuates high-fat diet-induced hepatic steatosis and lipid metabolic dysfunctions by suppressing hepatic lipogenesis and down-regulating inflammation in C57BL/6J mice. Food Funct. 2018, 9, 4328–4339. [Google Scholar] [CrossRef] [PubMed]
- Ono, T.; Murakoshi, M.; Suzuki, M.; Iida, N.; Ohdera, M.; Iigo, M.; Yoshida, T.; Sugiyama, K.; Nishino, H. Potent anti-obesity effect of enteric-coated lactoferrin: Decrease in visceral fat accumulation in Japanese men and women with abdominal obesity after 8-week administration of enteric-coated lactoferrin tablets. Br. J. Nutr. 2010, 104, 1688–1695. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.K.; Marcinko, K.; Desjardins, E.M.; Lally, J.S.; Ford, R.J.; Steinberg, G.R. Treatment of nonalcoholic fatty liver disease: Role of AMPK. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E730–E740. [Google Scholar] [CrossRef] [PubMed]
- Cuccioloni, M.; Mozzicafreddo, M.; Spina, M.; Tran, C.N.; Falconi, M.; Eleuteri, A.M.; Angeletti, M. Epigallocatechin-3-gallate potently inhibits the in vitro activity of hydroxy-3-methyl-glutaryl-CoA reductase. J. Lipid Res. 2011, 52, 897–907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geerling, J.J.; Boon, M.R.; van der Zon, G.C.; van den Berg, S.A.; van den Hoek, A.M.; Lombès, M.; Princen, H.M.; Havekes, L.M.; Rensen, P.C.; Guigas, B. Metformin lowers plasma triglycerides by promoting VLDL-triglyceride clearance by brown adipose tissue in mice. Diabetes 2014, 63, 880–891. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, M.; Kawakita, T.; Inaba, T.; Okada, N.; Ito, M.; Shimmura, S.; Watanabe, M.; Shinmura, K.; Tsubota, K. Dietary lactoferrin alleviates age-related lacrimal gland dysfunction in mice. PLoS ONE 2012, 7, e33148. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Babcook, M.A.; Shukla, S.; Shankar, E.; Wang, Z.; Liu, G.; Erokwu, B.O.; Flask, C.A.; Lu, L.; Daneshgari, F.; et al. Obesity-initiated metabolic syndrome promotes urinary voiding dysfunction in a mouse model. Prostate 2016, 76, 964–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Zhang, R.; Qin, Z.Z.; Chen, J.P.; Xu, J.Y.; Qin, L.Q. Effects of chronic whey protein supplementation on atherosclerosis in ApoE−/− mice. J. Nutr. Sci. Vitaminol. (Tokyo) 2018, 64, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Viscarra, J.; Kim, S.J.; Sul, H.S. Transcriptional regulation of hepatic lipogenesis. Nat. Rev. Mol. Cell Biol. 2015, 16, 678–689. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Yan, H.; Xia, M.; Chang, X.; Xu, X.; Wang, L.; Sun, X.; Lu, Y.; Bian, H.; Li, X.; et al. Metformin attenuates triglyceride accumulation in HepG2 cells through decreasing stearyl-coenzyme A desaturase 1 expression. Lipids Health Dis. 2018, 17, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno-Navarrete, J.M.; Ortega, F.J.; Ricart, W.; Fernandez-Real, J.M. Lactoferrin increases (172Thr) AMPK phosphorylation and insulin-induced (p473Ser) AKT while impairing adipocyte differentiation. Int. J. Obes. (Lond.) 2009, 33, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Zapata, R.C.; Singh, A.; Pezeshki, A.; Nibber, T.; Chelikani, P.K. Whey protein components—Lactalbumin and lactoferrin—Improve energy balance and metabolism. Sci. Rep. 2017, 7, 9917. [Google Scholar] [CrossRef] [PubMed]
- Cao, H. Adipocytokines in obesity and metabolic disease. J. Endocrinol. 2014, 220, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Galic, S.; Oakhill, J.S.; Steinberg, G.R. Adipose tissue as an endocrine organ. Mol. Cell Endocrinol. 2010, 316, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Su, J.R.; Lu, Z.H.; Su, Y.; Zhao, N.; Dong, C.L.; Sun, L.; Zhao, S.F.; Li, Y. Relationship of serum adiponectin levels and metformin therapy in patients with type 2 diabetes. Horm. Metab. Res. 2016, 48, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Ida, S.; Murata, K.; Kaneko, R. Effects of metformin treatment on blood leptin and ghrelin levels in patients with type 2 diabetes mellitus. J. Diabetes 2017, 9, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Lutchman, G.; Kleiner, D.E.; Ricks, M.; Feld, J.J.; Borg, B.B.; Modi, A.; Nagabhyru, P.; Sumner, A.E.; Liang, T.J.; et al. Clinical trial: Pilot study of metformin for the treatment of non-alcoholic steatohepatitis. Aliment. Pharmacol. Ther. 2009, 29, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ba, T.; Chen, L.; Shan, C.; Zheng, M.; Wang, Y.; Ren, H.; Chen, J.; Xu, J.; Han, F.; et al. Effects of metformin and sitagliptin on glycolipid metabolism in type 2 diabetic rats on different diets. Arch. Med. Sci. 2016, 12, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, S.; Mihaylova, M.M.; Zheng, B.; Hou, X.; Jiang, B.; Park, O.; Luo, Z.; Lefai, E.; Shyy, J.Y.; et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011, 13, 376–388. [Google Scholar] [CrossRef] [PubMed]
- Ruderman, N.B.; Xu, X.J.; Nelson, L.; Cacicedo, J.M.; Saha, A.K.; Lan, F.; Ido, Y. AMPK and SIRT1: A long-standing partnership? Am. J. Physiol. Endocrinol. Metab. 2010, 298, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, J.; Bruckbauer, A.; Zemel, M.B. Activation of the AMPK/Sirt1 pathway by a leucine-metformin combination increases insulin sensitivity in skeletal muscle, and stimulates glucose and lipid metabolism and increases life span in Caenorhabditis elegans. Metabolism 2016, 65, 1679–1691. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.K.; Lee, S.H.; Jhun, J.Y.; Byun, J.K.; Jeong, J.H.; Lee, S.Y.; Kim, J.K.; Choi, J.Y.; Cho, M.L. Metformin prevents fatty liver and improves balance of white/brown adipose in an obesity mouse model by inducing FGF21. Mediat. Inflamm. 2016, 2016, 5813030. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Kishida, T.; Ejima, A.; Tateyama, R.; Morishita, S.; Ono, T.; Murakoshi, M.; Sugiyama, K.; Nishino, H.; Mazda, O. Bovine lactoferrin promotes energy expenditure via the cAMP-PKA signaling pathway in human reprogrammed brown adipocytes. Biometals 2018. [Google Scholar] [CrossRef] [PubMed]



| TG (mmol/L) | TC (mmol/L) | HDL (mmol/L) | LDL (mmol/L) | Leptin (ng/mL) | Adiponectin (ng/mL) | AST (U/L) | ALT (U/L) | |
|---|---|---|---|---|---|---|---|---|
| CON | 1.55 ± 0.1 | 3.57 ± 0.41 | 1.51 ± 0.07 | 0.16 ± 0.02 | 2.21 ± 0.16 | 8.97 ± 0.26 | 152.94 ± 9.61 | 37.04 ± 2.39 |
| HFD | 1.78 ± 0.06 a | 8.89 ± 0.72 a | 2.85 ± 0.17 a | 0.29 ± 0.01 a | 7.48 ± 0.96 a | 7.79 ± 0.36 a | 156.83 ± 13.25 | 44.47 ± 2.39 a |
| Met | 1.51 ± 0.06 b | 7.41 ± 0.51 ab | 3.29 ± 0.06 ab | 0.23 ± 0.01 b | 2.13 ± 0.27 b | 9.15 ± 0.41 b | 144.57 ± 5.40 | 33.89 ± 1.35 b |
| Lf | 1.4 ± 0.04 b | 6.81 ± 0.52 ab | 2.9 ± 0.08 ac | 0.24 ± 0.01 a | 2.64 ± 0.37 b | 8.11 ± 0.33 c | 150.46 ± 5.39 | 34.28 ± 1.71 b |
| Met + Lf | 1.41 ± 0.04 b | 5.64 ± 0.19 abcd | 3.27 ± 0.08 abd | 0.24 ± 0.01 ab | 1.95 ± 0.13 b | 9.61 ± 0.48 bd | 138.59 ± 7.78 | 33.26 ± 2.1 b |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Min, Q.-Q.; Qin, L.-Q.; Sun, Z.-Z.; Zuo, W.-T.; Zhao, L.; Xu, J.-Y. Effects of Metformin Combined with Lactoferrin on Lipid Accumulation and Metabolism in Mice Fed with High-Fat Diet. Nutrients 2018, 10, 1628. https://doi.org/10.3390/nu10111628
Min Q-Q, Qin L-Q, Sun Z-Z, Zuo W-T, Zhao L, Xu J-Y. Effects of Metformin Combined with Lactoferrin on Lipid Accumulation and Metabolism in Mice Fed with High-Fat Diet. Nutrients. 2018; 10(11):1628. https://doi.org/10.3390/nu10111628
Chicago/Turabian StyleMin, Qing-Qing, Li-Qiang Qin, Zhen-Zhen Sun, Wen-Ting Zuo, Lin Zhao, and Jia-Ying Xu. 2018. "Effects of Metformin Combined with Lactoferrin on Lipid Accumulation and Metabolism in Mice Fed with High-Fat Diet" Nutrients 10, no. 11: 1628. https://doi.org/10.3390/nu10111628




