Functional Genomics of Allergen Gene Families in Fruits
Abstract
:1. Introduction
| Families | Type member | Properties | Gene symbol |
|---|---|---|---|
| PR-1* | Tobacco PR-1a | Unknown | Ypr1 |
| PR-2* | Tobacco PR-2 | β-1,3-glucanase | Ypr2, [Gns2 (‘Glb’)] |
| PR-3* | Tobacco P, Q | Chitinase type I,II, IV,V,VI,VII | Ypr3, Chia |
| PR-4 | Tobacco ‘R’ | Chitinase type I,II | Ypr4, Chid |
| PR-5* | Tobacco S | Thaumatin-like | Ypr5 |
| PR-6 | Tomato Inhibitor I | Proteinase-inhibitor | Ypr6, Pis (´Pin´) |
| PR-7 | Tomato P69 | Endoproteinase | Ypr7 |
| PR-8* | Cucumber chitinase | Chitinase type III | Ypr8, Chib |
| PR-9 | Tobacco “lignin-forming peroxidise” | Peroxidase | Ypr9, Prx |
| PR-10* | Parsley “PR1” | Ribonuclease-like | Y pr10 |
| PR-11 | Tobacco “class V” chitinase | Chitinase, type I | Ypr11, Chic |
| PR-12 | Radish Rs-AFP3 | Defensin | Ypr12 |
| PR-13 | Arabidopsis THI2.1 | Thionin | Ypr13, Thi |
| PR-14* | Barley LTP4 | Lipid-transfer protein | Ypr14, Ltp |
| PR-15 | Barley OxOa (germin) | Oxalate oxidase | Ypr15 |
| PR-16 | Barley OxOLP | Oxalate oxidase-like | Yrp16 |
| PR-17 | Tobacco PRp27 | Unknown | Yrp17 |
2. Genomic Approach

3. Development of Patient Independent Detection Tools for Characterization of Fruit Allergen


4. Detection Tools for Characterization of Fruit Allergens Involving Patient Sera
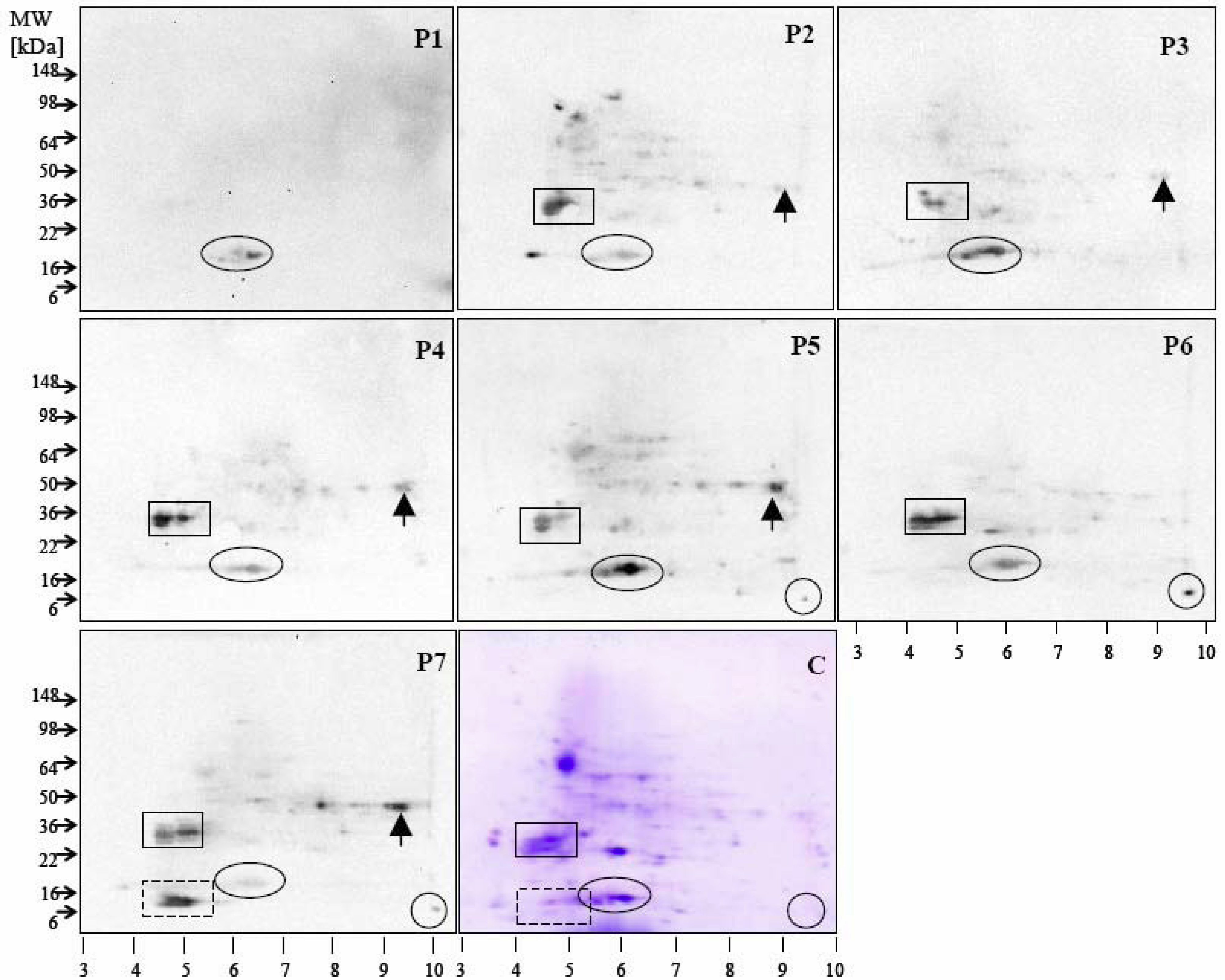
5. Strategies towards Low-Allergenic Fruits
Acknowledgements
Appendix
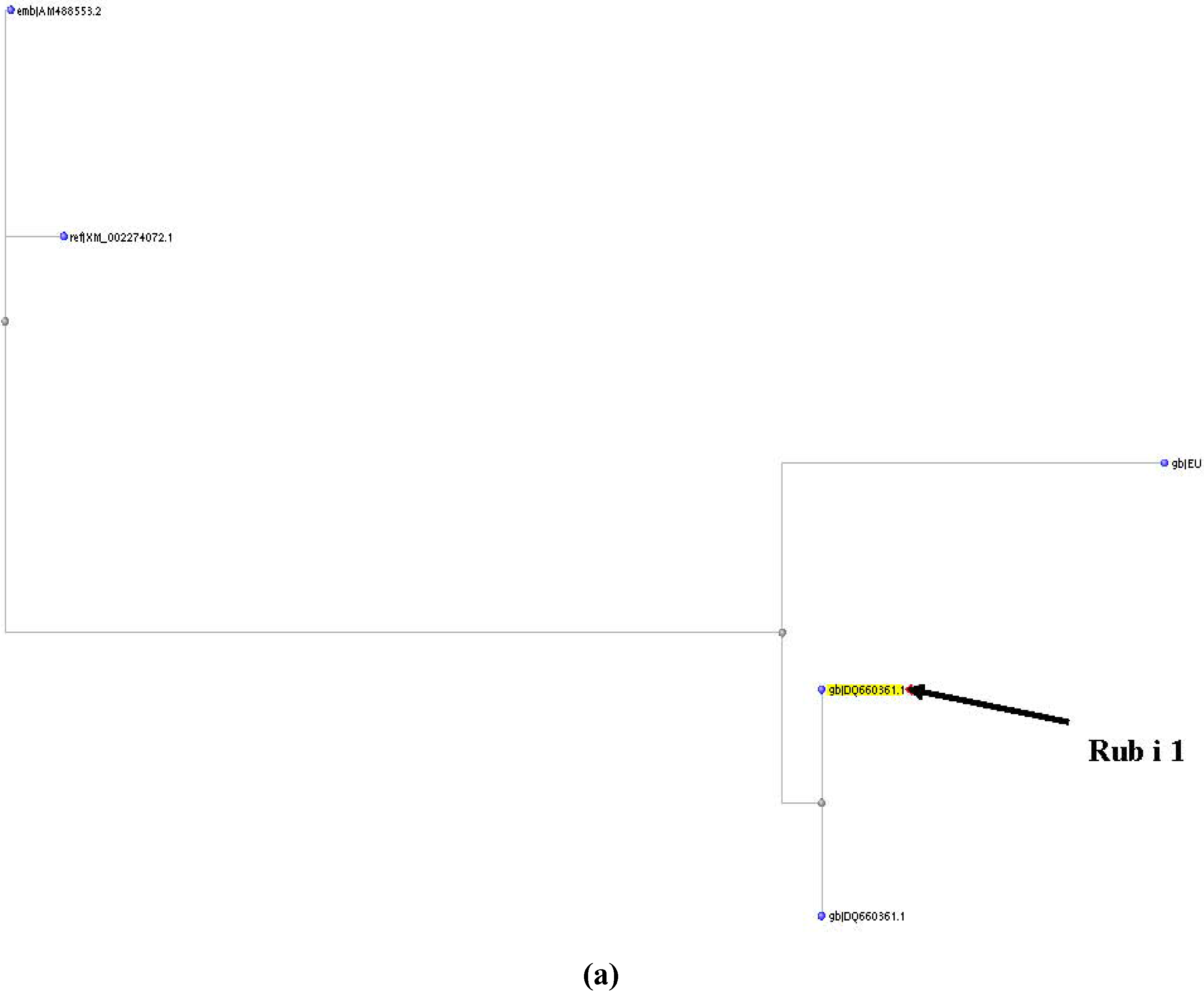
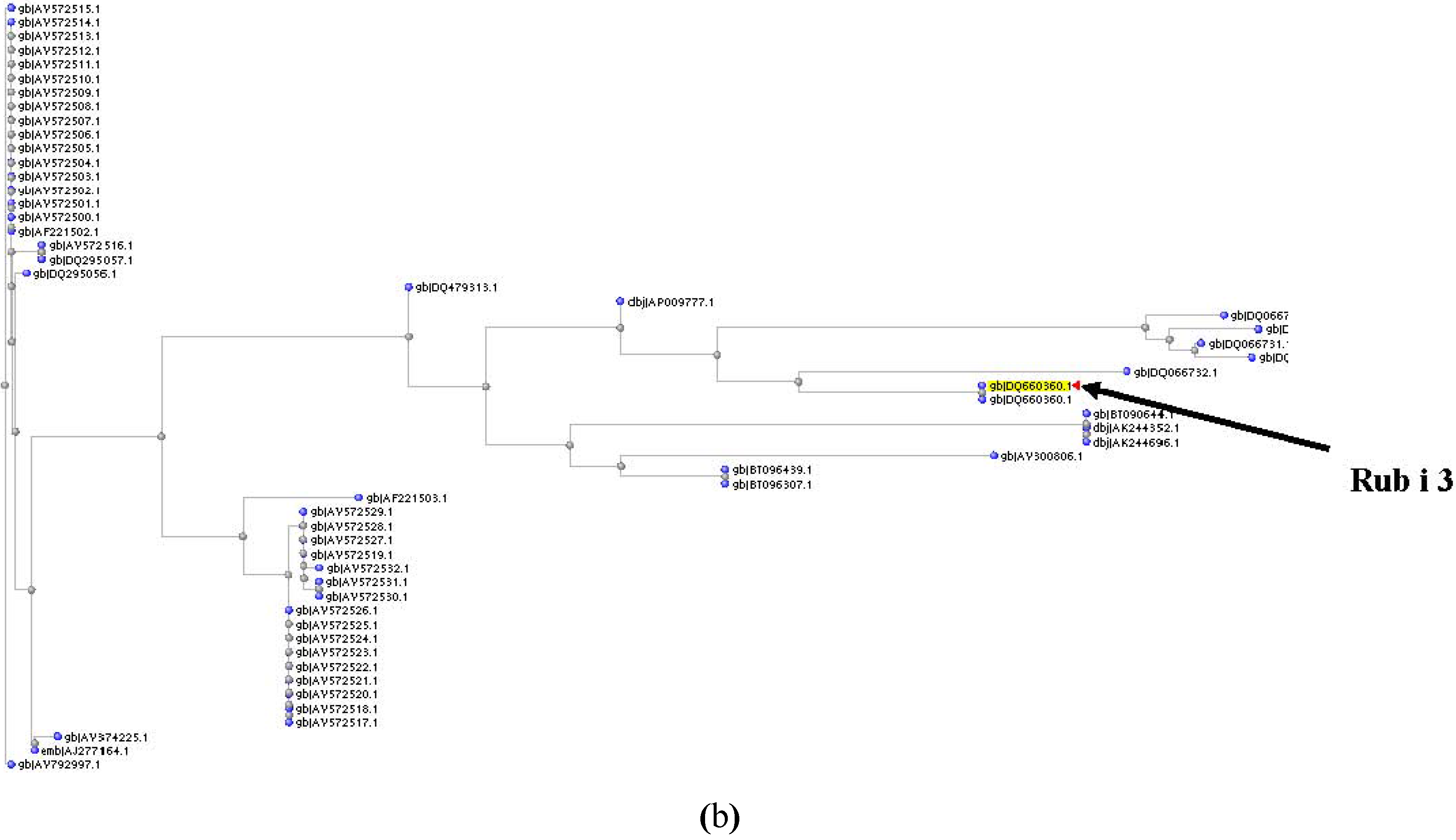
References
- Hoffmann-Sommergruber, K. SAFE Consortium. The SAFE project: plant food allergies: field to table strategies for reducing their incidence in Europe an EC-funded study. Allergy 2005, 60, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Rona, R.J.; Keil, T.; Summers, C.; Gislason, D.; Zuidmeer, L.; Sodergren, E.; Sigurdardottir, S.T.; Lindner, T.; Goldhahn, K.; Dahlstrom, J.; McBride, D.; Madsen, C. The prevalence of food allergy: A meta-analysis. J. Allergy Clin. Immunol. 2007, 120, 638–646. [Google Scholar]
- Young, E.; Stoneham, M.D.; Petruckevitch, A.; Barton, J.; Rona, R. A population study of food intolerance. Lancet 1994, 343, 1127–1130. [Google Scholar]
- Jansen, J.J.N.; Kardinaal, A.F.M.; Huijbers, G.; Vlieg-Boerstra, B.J.; Martens, B.P.M.; Ockhuizen, T. Prevalence of food allergy and intolerance in the adults dutch population. J. Allergy Clin. Immunol. 1994, 93, 446–456. [Google Scholar]
- Zuberbier, T.; Edenharter, G.; Worm, M.; Ehlers, I.; Reimann, S.; Hantke, T.; Roehr, C.C.; Bergmann, K.E.; Niggermann, B. Prevalence of adverse reactions to food in Germany a population study. Allergy 2004, 59, 338–345. [Google Scholar]
- Kanny, G.; Moneret-Vautrin, D.A.; Flabbee, J.; Beaudouin, E.; Morisset, M.; Thevenin, F. Population study of food allergy in France. J. Allergy Clin. Immunol. 2001, 108, 133–140. [Google Scholar]
- Munoz-Furlong, A. Food allergy in schools: concerns for allergists, pediatricians, parents, and school staff. Ann. Allergy Asthma Immunol. 2004, 93, 47–50. [Google Scholar] [CrossRef]
- Sampson, H.A. Update on food allergy. J. Allergy Clin. Immunol. 2004, 113, 805–819. [Google Scholar] [CrossRef] [PubMed]
- Thong, B.Y.; Hourihane, J.O. Monitoring of IgE-mediated food allergy in childhood. Acta Paediatr. 2004, 93, 759–764. [Google Scholar]
- Fernandez-Rivas, M.; Gonzalez-Mancebo, E.; Rodriguez-Perez, R.; Benito, C.; Sanchez-Monge, R.; Salcedo, G.; Alonso, M.D.; Rosado, A.; Tejedor, M.A.; Vila, C.; Casas, M.L. Clinically relevant peach allergy is related to peach lipid transfer protein, Pru p 3, in the Spanish population. J. Allergy Clin. Immunol. 2003, 112, 789–795. [Google Scholar] [PubMed]
- Fernández-Rivas, M.; Bolhaar, S.; González-Mancebo, E.; Asero, R.; van Leeuwen, A.; Bohle, B.; Ma, Y.; Ebner, C.; Rigby, N.; Sancho, A.I.; Miles, S.; Zuidmeer, L.; Knulst, A.; Breiteneder, H.; Mills, C.; Hoffmann-Sommergruber, K.; van Ree, R. Apple allergy across Europe: how allergen sensitization profiles determine the clinical expression of allergies to plant foods. J. Allergy Clin. Immunol. 2006, 118, 481–488. [Google Scholar] [PubMed]
- Astwood, J.D.; Leach, J.N.; Fuchs, R.L. Stability of food allergens to digestion in vitro. Nat. Biotechnol. 1996, 14, 1269–1273. [Google Scholar] [CrossRef] [PubMed]
- Aalberse, R.C. Structural biology of allergens. J. Allergy Clin. Immunol. 2000, 106, 228–238. [Google Scholar]
- Yagami, T. Allergies to cross-reactive plant proteins. Latex-fruit syndrome is comparable with pollen-food allergy syndrome. Int. Arch. Allergy Immunol. 2002, 128, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, F.; Hirtenlehner, K.; Jilek, A.; Godnik-Cvar, J.; Breiteneder, H.; Grimm, R.; Hoffmann-Sommergruber, K.; Scheiner, O.; Kraft, D.; Breitenbach, M.; Rheinberger, H.; Ebner, C. Dissection of immunoglobulin E and T lymphocyte reactivity of isoforms of the major birch pollen allergen Bet v 1: potential use of hypoallergenic isoforms for immunotherapy. J. Exp. Med. 1996, 183, 599–609. [Google Scholar]
- Breiteneder, H.; Ebner, C. Molecular and biochemical classification of plant-derived food allergens. J. Allergy Clin. Immunol. 2000, 106, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Aalberse, R.C.; Akkerdaas, J.; van Ree, R. Cross-reactivity of IgE antibodies to allergens. Allergy 2001, 56, 478–490. [Google Scholar]
- Ferreira, F.; Hawranek, T.; Gruber, P.; Wopfner, N.; Mari, A. Allergic cross-reactivity: from gene to the clinic. Allergy 2004, 59, 243–267. [Google Scholar]
- Marzban, G.; Herndl, A.; Kolarich, D.; Maghuly, F; Mansfeld, A.; Hemmer, W.; Katinger, H.; Laimer, M. Identification of four IgE-reactive proteins in raspberry (Rubus ideaeus L.). Mol. Nutr. Food Res. 2008, 52, 1497–1506. [Google Scholar] [CrossRef] [PubMed]
- van Loon, L.C.; Rep, M.; Pieterse, C.M. Significance of inducible defense-related proteins in infected plants. Annu. Rev. Phytopathol. 2006, 44, 135–162. [Google Scholar] [CrossRef] [PubMed]
- Ebner, C.; Hoffmann-Sommergruber, K.; Breiteneder, H. Plant food allergens homologous to pathogenesis-related proteins. Allergy 2001, 56, 43–44. [Google Scholar]
- Odjakova, M.; Hadjiivanova, C. The complexity of pathogen defense in plants. Bulg. J. Plant Physiol. 2001, 27, 101–109. [Google Scholar]
- Edreva, A. Pathogenesis-related proteins: research progress in the last 15 years. Gen. Appl. Plant Physiology 2005, 31, 105–124. [Google Scholar]
- Wei, Y.; Zhang, Z.; Andersen, C.H.; Schmelzer, E.; Gregersen, P.L.; Collinge, D.B.; Smedegaard-Petersen, V.; Thordal-Christensen, H. An epidermis/papilla-specific oxalate oxidase-like protein in the defence response of barley attacked by the powdery mildew fungus. Plant Mol. Biol. 1998, 36, 101–112. [Google Scholar]
- Brandazza, A.; Angeli, S.; Tegoni, M.; Cambillau, C.; Pelosi, P. Plant stress proteins of the thaumatin-like family discovered in animals. FEBS Lett. 2004, 572, 3–7. [Google Scholar]
- Pühringer, H.; Moll, D.; Hoffmann-Sommergruber, K.; Watillon, B.; Katinger, H.; Laimer da Câmara Machado, M. The promoter of an apple Ypr10 gene, encoding the major allergen Mal d 1, is stress- and pathogen-inducible. Plant Sci. 2000, 152, 35–50. [Google Scholar] [CrossRef]
- Kader, J.C. Lipid-Transfer Proteins in Plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996, 47, 627–654. [Google Scholar]
- Jensen-Jarolim, E.; Untersmayr, E. Food safety: in vitro digestion tests are non-predictive for allergenic potential of food in stomach insufficiency. Immunol. Lett. 2006, 102, 118–119. [Google Scholar]
- Díaz-Perales, A.; Tabar, A.; Sánchez-Monge, R.; García, B.; Gómez, B.; Barber, D.; Salcedo, G. Characterization of asparagus allergens: a relevant role of lipid transfer proteins. J. Allergy Clin. Immunol. 2002, 110, 790–796. [Google Scholar]
- Pastorello, E.A.; Ortolani, C.; Farioli, L.; Pravettoni, V.; Ispano, M.; Borga, A.; Bengtsson, A.; Incorvaia, C.; Berti, C.; Zanussi, C. Allergenic cross-reactivity among peach, apricot, plum, and cherry in patients with oral allergy syndrome: an in vivo and in vitro study. J. Allergy Clin. Immunol. 1994, 94, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Marzban, G.; Mansfeld, A.; Hemmer, W.; Stoyanova, E.; Katinger, H.; da Câmara Machado, M. Fruit cross-reactive allergens: a theme of uprising interest for consumers' health. Biofactors 2005, 23, 235–241. [Google Scholar]
- Marzban, G.; Herndl, A.; Pietrozotto, S.; Banerjee, S.; Obinger, C.; Maghuly, F.; Hahn, R.; Boscia, D.; Katinger, H.; Laimer, M. Conformational changes of Mal d 2, a thaumatin-like apple allergen, induced by food processing. Food Chem. 2009, 112, 803–811. [Google Scholar] [CrossRef]
- Zuidmeer, L.; Salentijn, E.; Rivas, M.F.; Mancebo, E.G.; Asero, R.; Matos, C.I.; Pelgrom, K.T.; Gilissen, L.J.; van Ree, R. The role of profilin and lipid transfer protein in strawberry allergy in the Mediterranean area. Clin. Exp. Allergy 2006, 36, 666–675. [Google Scholar]
- Marzban, G.; Pühringer, H.; Dey, R.; Brynda, S.; Ma, Y.; Martinelli, A.; Zaccarini, M.; Van der Weg, E.; Housley, Z.; Kolarich, D.; Altmann, F.; Laimer, M. Localisation and distribution of the major allergens in apple fruits. Plant Sci. 2005, 169, 387–394. [Google Scholar]
- Herndl, A.; Marzban, G.; Kolarich, D.; Hahn, R.; Boscia, D.; Hemmer, W.; Maghuly, F.; Stoyanova, E.; Katinger, H.; Laimer, M. Mapping of Malus domestica allergens by two-dimensional electrophoresis and IgE- reactivity. Electrophoresis 2007, 28, 437–448. [Google Scholar]
- Sander, I.; Flagge, A.; Merget, R.; Halder, T.M.; Meyer, H.E.; Baur, X. Identification of wheat flour allergens by means of 2-dimensional immunoblotting. J. Allergy Clin. Immunol. 2001, 107, 907–913. [Google Scholar]
- Osterballe, M.; Hansen, T.K.; Mortz, C.G.; Bindslev-Jensen, C. The clinical relevance of sensitization to pollen-related fruits and vegetables in unselected pollen-sensitized adults. Allergy 2005, 60, 218–225. [Google Scholar]
- Vieths, S.; Scheurer, S.; Ballmer-Weber, B. Current understanding of cross-reactivity of food allergens and pollen. Ann. N. Y. Acad. Sci. 2002, 964, 47–68. [Google Scholar]
- Hemmer, W.; Focke, M.; Marzban, G.; Laimer, M.; Jarisch, R. Identification of fig and other Moraceae fruits as new birch pollen-associated foods. Clin. Exp. Allergy 2009. (submitted). [Google Scholar]
- Marzban, G.; Mansfeld, A.; Herndl, A.; Jäger, S.; Stoyanova, M.E.; Hemmer, W.; Katinger, H.; Laimer, M. Direct evidence for the presence of allergens in Rosaceae fruit-tree pollen. Aerobiologia 2006, 22, 237–245. [Google Scholar]
- Arus, P. Integrating genomics into Rosaceae fruit breeding. Acta. Horticulturae 2007, 738, 29–35. [Google Scholar]
- Maghuly, F.; Borroto-fernandez, E.G.; Khan, M.A.; Herndl, A.; Marzban, G.; Laimer, M. Expression of calmodulin and lipid transfer protein genes in Prunus incisa x serrula under different stress conditions. Tree Physiol. 2009, 29, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Marzban, G.; Maghuly, F.; Herndl, A.; Katinger, H.; Laimer, M. Screening and identification of putative allergens in berry fruits of the Rosaceae family: technical challenges. Biofactors 2008, 33, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, S.; Illa, E.; Song, L.; Wu, S.; Howad, W.; Arús, P.; van de Weg, E.; Chen, K.; Gao, Z. Genomic characterization of putative allergen genes in peach/almond and their synteny with apple. BMC Genomics 2008, 9, 543. [Google Scholar]
- Gao, Z.S.; van de Weg, W.E.; Schaart, J.G.; Schouten, H.J.; Tran, D.H.; Kodde, L.P.; van der Meer, I.M.; van der Geest, A.H.; Kodde, J.; Breiteneder, H.; Hoffmann-Sommergruber, K.; Bosch, D.; Gilissen, L.J. Genomic cloning and linkage mapping of the Mal d 1 (PR-10) gene family in apple (Malus domestica). Theor. Appl. Genet. 2005, 111, 171–183. [Google Scholar]
- Gao, Z.S.; Weg, W.E.; Schaart, J.G.; Arkel, G.; Breiteneder, H.; Hoffmann-Sommergruber, K.; Gilissen, L.J. Genomic characterization and linkage mapping of the apple allergen genes Mal d 2 (thaumatin-like protein) and Mal d 4 (profilin). Theor. Appl. Genet. 2005, 111, 1087–1097. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.S.; van de Weg, W.E.; Schaart, J.G.; van der Meer, I.M.; Kodde, L.; Laimer, M.; Breiteneder, H.; Hoffmann-Sommergruber, K.; Gilissen, L.J. Linkage map positions and allelic diversity of two Mal d 3 (non-specific lipid transfer protein) genes in the cultivated apple (Malus domestica). Theor. Appl. Genet. 2005, 110, 479–491. [Google Scholar]
- Pühringer, H.; Zinoecker, I.; Marzban, G.; Katinger, H.; Laimer, M. MdAP, a novel protein in apple, is associated with the major allergen Mal d 1. Gene 2003, 321, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Bjorksten, F.; Halmepuro, L.; Hannuksela, M.; Lahti, A. Extraction and properties of apple allergens. Allergy 1980, 35, 671–677. [Google Scholar]
- Marzban, G.; Herndl, A.; Maghuly, F.; Katinger, H.; Laimer, M. Mapping of fruit allergens by two-dimensional electrophoresis and immuno-detection. Exp. Rev. Proteomics 2008, 5, 61–75. [Google Scholar]
- Rodrigues, S.P.; Ventura, J.A.; Zingal, R.B.; Fernandes, P.M. Evaluation of sample preparation methods for the analysis of papaya leaf proteins through two-dimensional gel electrophoresis. Phytochem. Anal. 2009. [Google Scholar]
- Docena, G.H; Fossati, C.A. Serological prevalence of anti-latex IgE antibodies in unselected blood donors in Argentina. J. Allergol. Clin. Immunol. 2005, 15, 46–49. [Google Scholar]
- Tang, G.L.; Galili, G. Using RNAi to improve plant nutritional value: from mechanism to application. Trends Biotechnol. 2004, 22, 463–469. [Google Scholar]
- Waterhouse, P.M.; Helliwell, C.A. Exploring plant genomes by RNA-induced gene silencing. Nature Rev.: Genet. 2003, 4, 29–38. [Google Scholar] [CrossRef]
- Gallo, M.; Sayre, R. Removing allergens and reducing toxins from food crops. Curr. Opinion Biotechnol. 2009, 20, 191–196. [Google Scholar]
- Gilissen, L.J.; Bolhaar, S.T.; Matos, C.I.; Rouwendal, G.J.; Boone, M.J.; Krens, F.A.; Zuidmeer, L.; van Leewen, A.; Akkerdaas, J.; Hoffmann-Sommergruber, K.; Knulst, A.C.; Bosch, D.; van der Weg, W.; van Ree, R. Silencing the major apple allergen Mal d 1 by using the RNA interference approach. J. Allergy Clin. Immunol. 2005, 115, 364–369. [Google Scholar]
- Le, L.Q.; Lorenz, Y.; Scheurer, S.; Fotisch, K.; Enrique, E.; Bartra, J.; Biemelt, S.; Vieths, S.; Sonnewald, U. Design of tomato fruits with reduced allergenicity by dsRNAi-mediated inhibition of ns-LTP (Lyc e 3) expression. Plant Biotechnol. J. 2006, 4, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Le, L.Q.; Mahler, V.; Lorenz, Y.; Scheurer, S.; Biemelt, S.; Vieths, S.; Sonnewald, U. Reduced allergenicity of tomato fruits harvested from Lyc e 1-silenced transgenic tomato plants. J. Allergy Clin. Immunol. 2006, 118, 1176–1183. [Google Scholar] [CrossRef] [PubMed]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Maghuly, F.; Marzban, G.; Laimer, M. Functional Genomics of Allergen Gene Families in Fruits. Nutrients 2009, 1, 119-132. https://doi.org/10.3390/nu1020119
Maghuly F, Marzban G, Laimer M. Functional Genomics of Allergen Gene Families in Fruits. Nutrients. 2009; 1(2):119-132. https://doi.org/10.3390/nu1020119
Chicago/Turabian StyleMaghuly, Fatemeh, Gorji Marzban, and Margit Laimer. 2009. "Functional Genomics of Allergen Gene Families in Fruits" Nutrients 1, no. 2: 119-132. https://doi.org/10.3390/nu1020119




