3.1. Field Measurements
During the study period in the summers of 2012 and 2013, a total of more than 80 data sets were gathered. The phytoplankton absorption spectra varied both in spectral shape and in magnitude (
Figure 2).
For example,
aph (443) varied from 0.036 to 0.954 m
−1 (
Table 2). The highest variability, spanning over two orders of magnitude, was noted in the case of chl-
a and
aCDOM (443) (
Table 2). The highest values of
aph (λ), as well as chl-
a, were observed in June 2013. These parameters showed also the strongest correlation (
Table 3). However, the correlation between chl-
a and
aph (665) was slightly stronger (R = 0.90) than with
aph (443) (R = 0.88). A strong correlation was also observed between
aCDOM (443) and chl-
a,
aph (443), and
aph (665) (R = 0.80, 0.98, and 0.62, respectively). The weakest, statistically not significant, dependence was observed between
adet (443) and
aph (443) (R = 0.19) and
adet (443) and
aCDOM (443) (R = −0.08) (
Table 3).
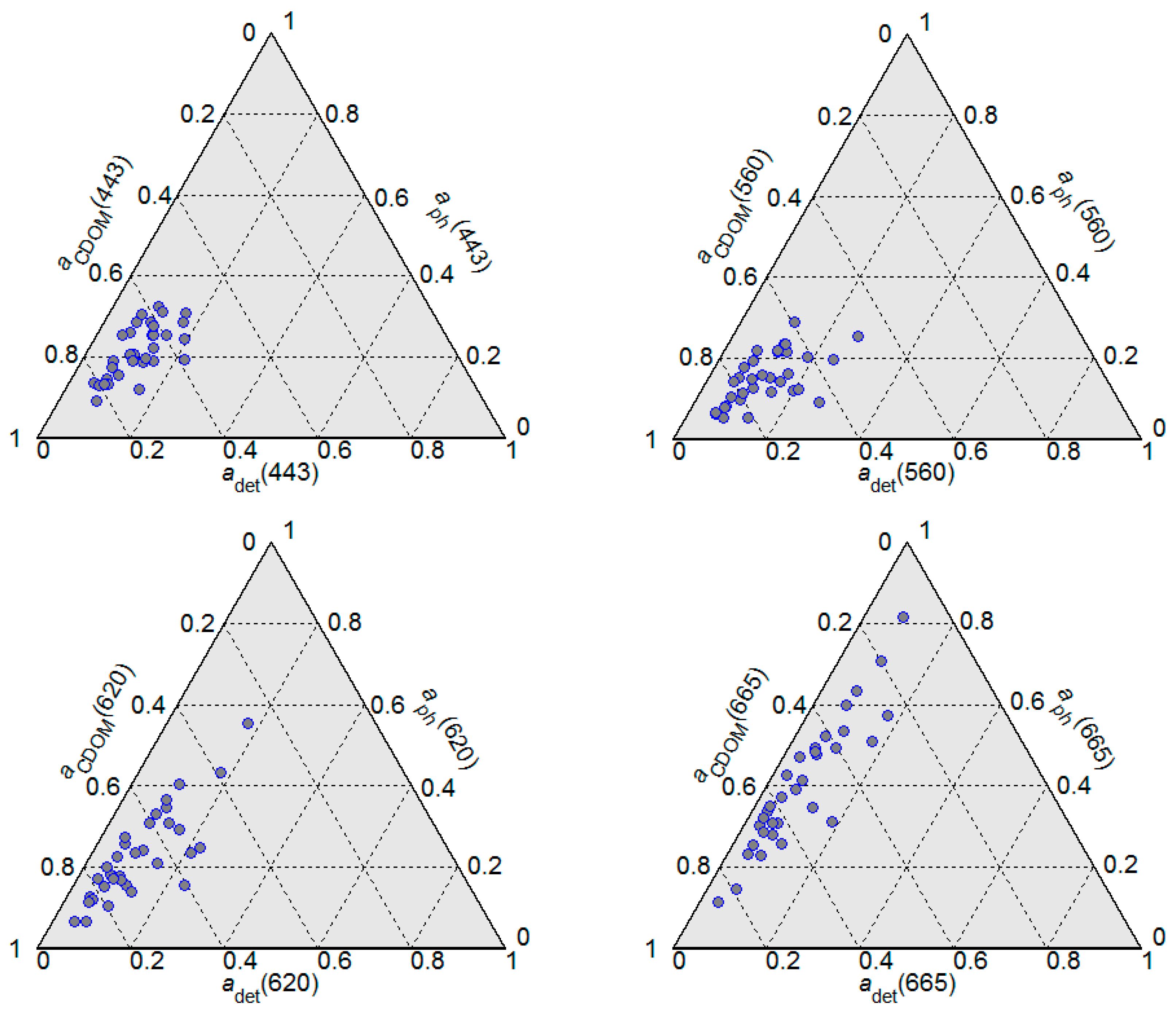
Figure 3 shows the absorption budget for the non-water optically significant seawater constituents, i.e., CDOM, detritus, and phytoplankton pigments at different wavelengths. In all analysed samples, the light absorption at shorter wavelengths (443 nm and 560 nm) was clearly dominated by CDOM which was responsible for roughly 70% of the total absorption. At longer wavelengths, the absorption of light by phytoplankton pigments became more significant. At all analysed wavelengths, the absorption by detritus had the lowest contribution to total absorption.
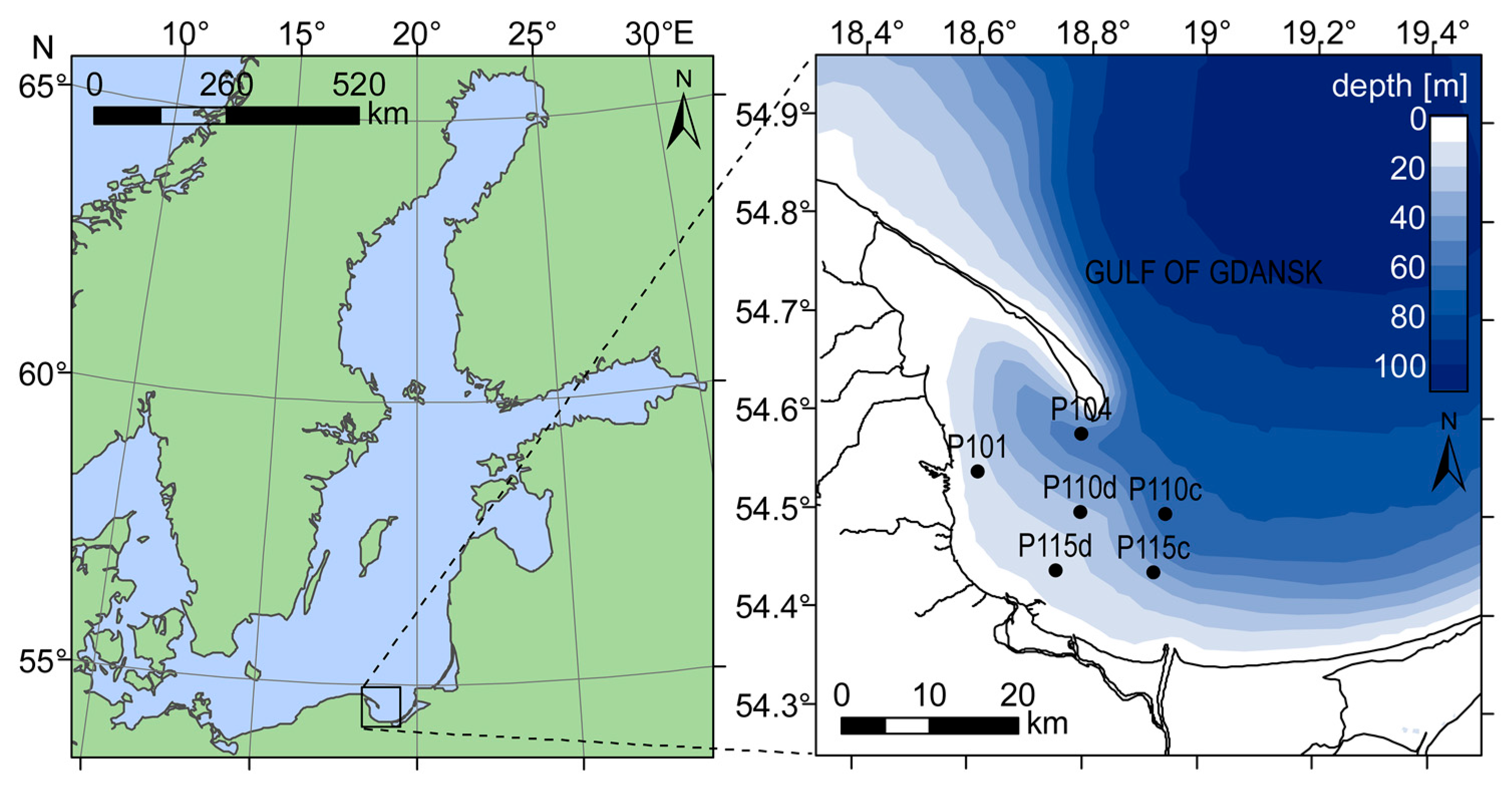
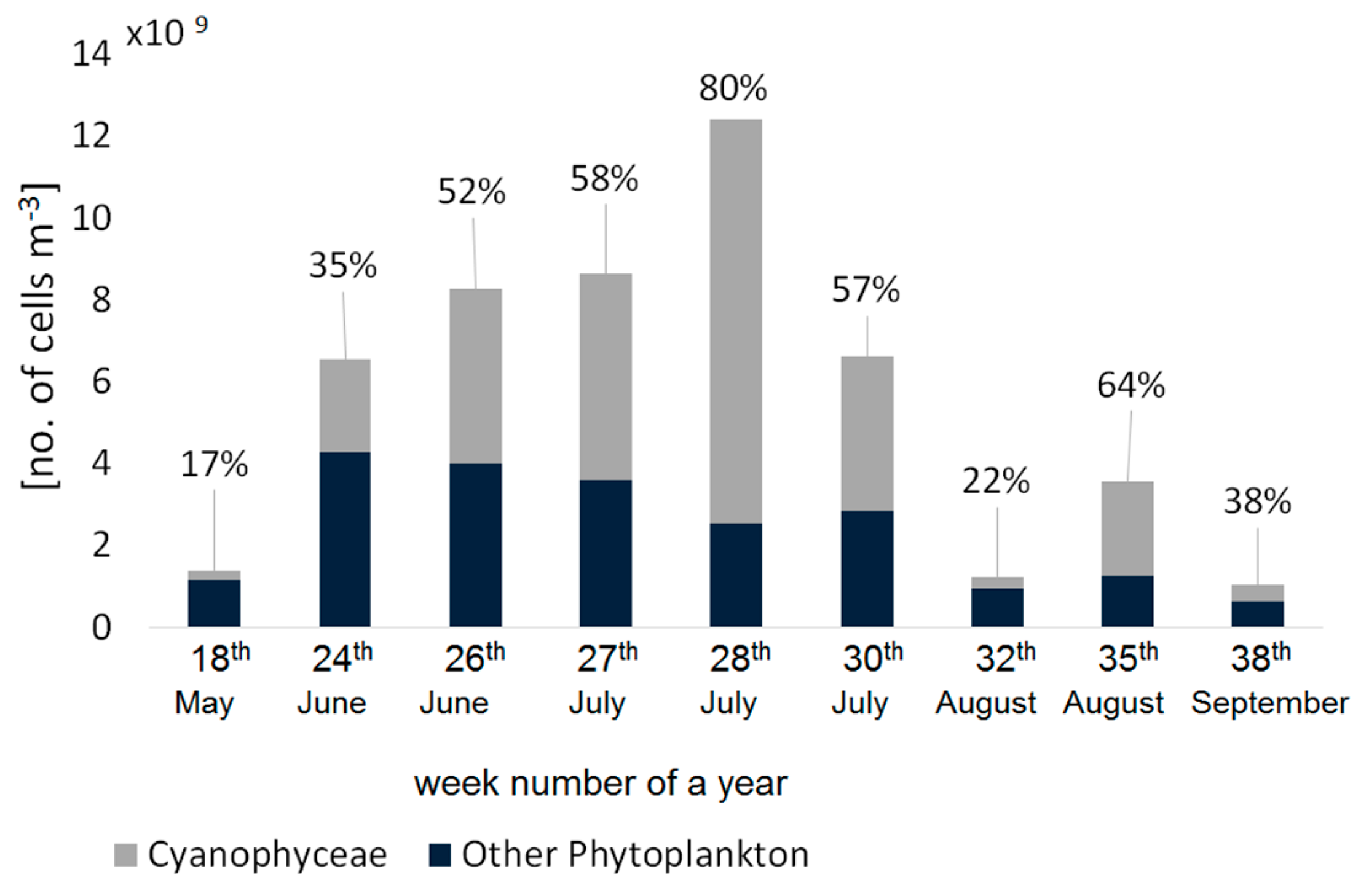
Despite the fact that field measurements were collected only in the Gulf of Gdansk, our dataset covered a wide range of variability of water parameters (
Table 2). The cell counts confirmed that during summer, the phytoplankton composition in the Gulf of Gdansk is dominated by cyanobacteria (
Figure 4), especially in July, when their contribution to total phytoplankton abundance was >50% in terms of the number of cells per cubic meter.
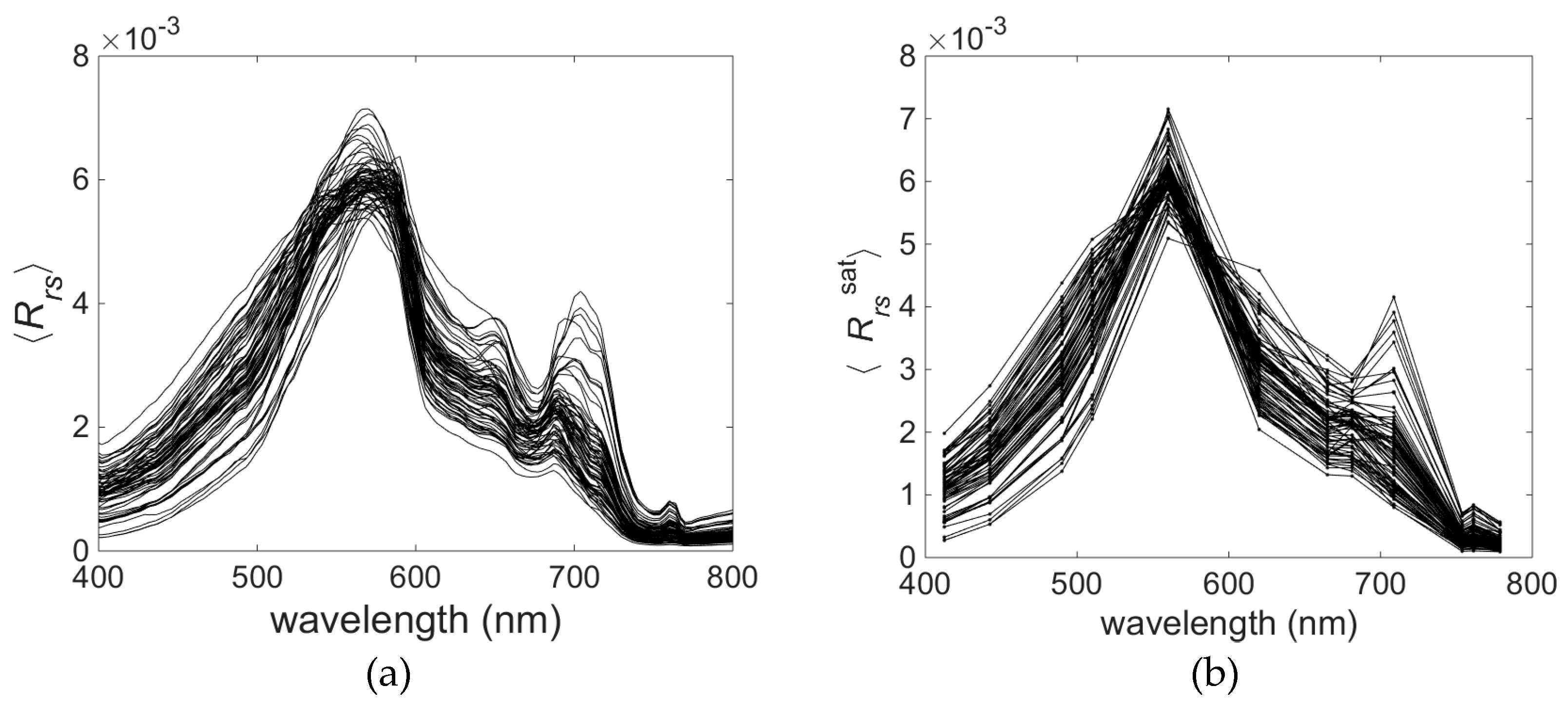
Variability of the optically significant water components influences the shape of the remote sensing reflectance (
Rrs) spectra collected in the field campaigns (
Figure 5a). The collected
Rrs spectra represent quite typical spectral features consistent within optically complex waters [
36]. The shape of normalised
Rrs spectra shows some interesting features related to the phytoplankton pigment absorption and fluorescence. As expected, the highest reflectance is around 570 nm in the ‘green window’ of minimal chlorophyll absorption and where phycoerythrin absorption is strongest. The trough around 620–630 nm indicates the effect of phycocyanin absorption [
59], whereas the trough at about 664 nm indicates the effect of chlorophyll
a absorption. Another small peak around 650 nm can be caused by absorption by these two pigments (chlorophyll
a and phycocyanin) on either side of the peak. Additionally, phycocyanin fluorescence, which has a maximum at 650 nm, may contribute to this spectral feature [
9]. It is difficult to discern the spectral characteristics of phycoerythrin in the
Rrs spectral shape. Maximum absorption and fluorescence of phycoerythrin occur around 565 nm and 576 nm, respectively [
34], where the other optically significant components (e.g., carotenoids) strongly influence the spectra of
Rrs. These features, which are clearly visible in the hyperspectral data (
Figure 5a), become less distinct in the
Rrs data converted to the multispectral cases (
Figure 5b).
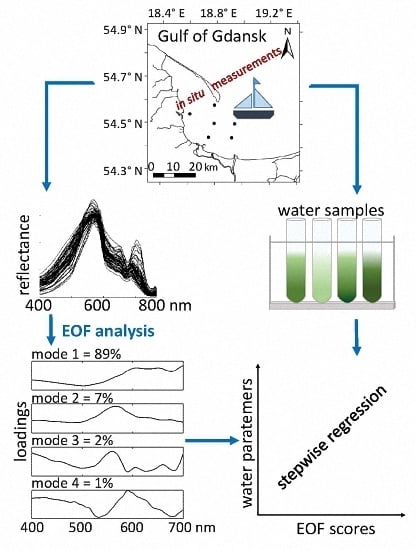
3.2. EOF Models for Phytoplankton Pigments
The Chl-
a model (4) was based on the three EOFs or modes (
Table 4) that were chosen by the stepwise regression. The spectral shapes of these modes, visualised by plotting its loading versus wavelength (
Figure 6), may be interpreted as signatures of changes in the optical properties of the water over samples due to changes in the in-water components. The first mode, which captures 89% of the variability in spectral shape, was chosen as the first model component by stepwise fitting. It exhibits a negative correlation between the short and long wavelength regions of the spectrum, implying shifts between the blue-green and red regions of the spectra. That is, the spectral shapes vary such that, whenever the peak around 500 nm is less than average, the band between 600 nm and 800 nm is larger than average, and vice versa. These spectral shifts may be due in part to changes in the concentrations of detritus and CDOM, which are dominating components of the absorption coefficient in the study area (
Figure 3). However, variations in the longer wavelengths can only be due to changes in the components of the water column that affect these wavelengths (e.g., phytoplankton pigments).
The second mode chosen by stepwise regression was the 3rd EOF. This mode captures variations in spectral shape centred around 400 nm and 560 nm and in the band from about 714 nm to 800 nm that are positive correlated.
It is worth noting that mode 12, which contains only 0.04% of the variance (
Figure 6), was selected by both the SNR procedure and stepwise fit. The numerous spectral inflections, which appear to be coherent signals, are likely related to various phytoplankton absorption and emission (i.e., fluorescence) processes. This strongly implies that mode 12 is not simply noise, as might be expected from such a minor mode of variance, and can bring useful predictive power to the model.
Our model retrieved chl-
a from spectral reflectance with an R
2 value of 0.81 (bias = −0.07 × 10
−15), a tight distribution around the 1:1 line (Slope = 0.88, SE = 0.05, Ratio = 1.02) and a relative error (RMSE) of 0.16, even in very optically complex waters (
Table 5,
Figure 7a). By contrast, the standard OC4 algorithm applied to our dataset resulted in consistent overestimation of chl-
a (R
2 = 0.73, bias = 0.38, Ratio = 2.43, MPD = 143%, Slope = 0.78, SE = 0.04) and a rather high relative error (RMSE = 0.43). It should be noted here that models such as OC4 are global, whereas our chl-
a model was developed using local data. It is therefore not surprising that our model outperformed OC4. However, even with a regionally-tuned band ratio model (Baltic chlor
a 2, Table 4 in [
22]), chl-
a estimates would still be seriously compromised (R
2 = 0.71, RMSE = 0.23) due to the confounding effect of CDOM absorption at the blue end of the spectrum. The significance of this result is that EOF provides a method to derive accurate chl-
a estimates in a scenario where standard approaches provide low quality results.
In summer, the Baltic Sea phytoplankton assemblage is dominated by filamentous cyanobacteria species such as
A. flos-aquae,
N. spumigena, and
Dolichospermum sp., and picocyanobacteria species such as
Synechococcus sp. [
60,
61]. The filamentous species,
A. flos-aquae,
N. spumigena, and
Dolichospermum sp., are rich in phycocyanin, while the non-filamentous
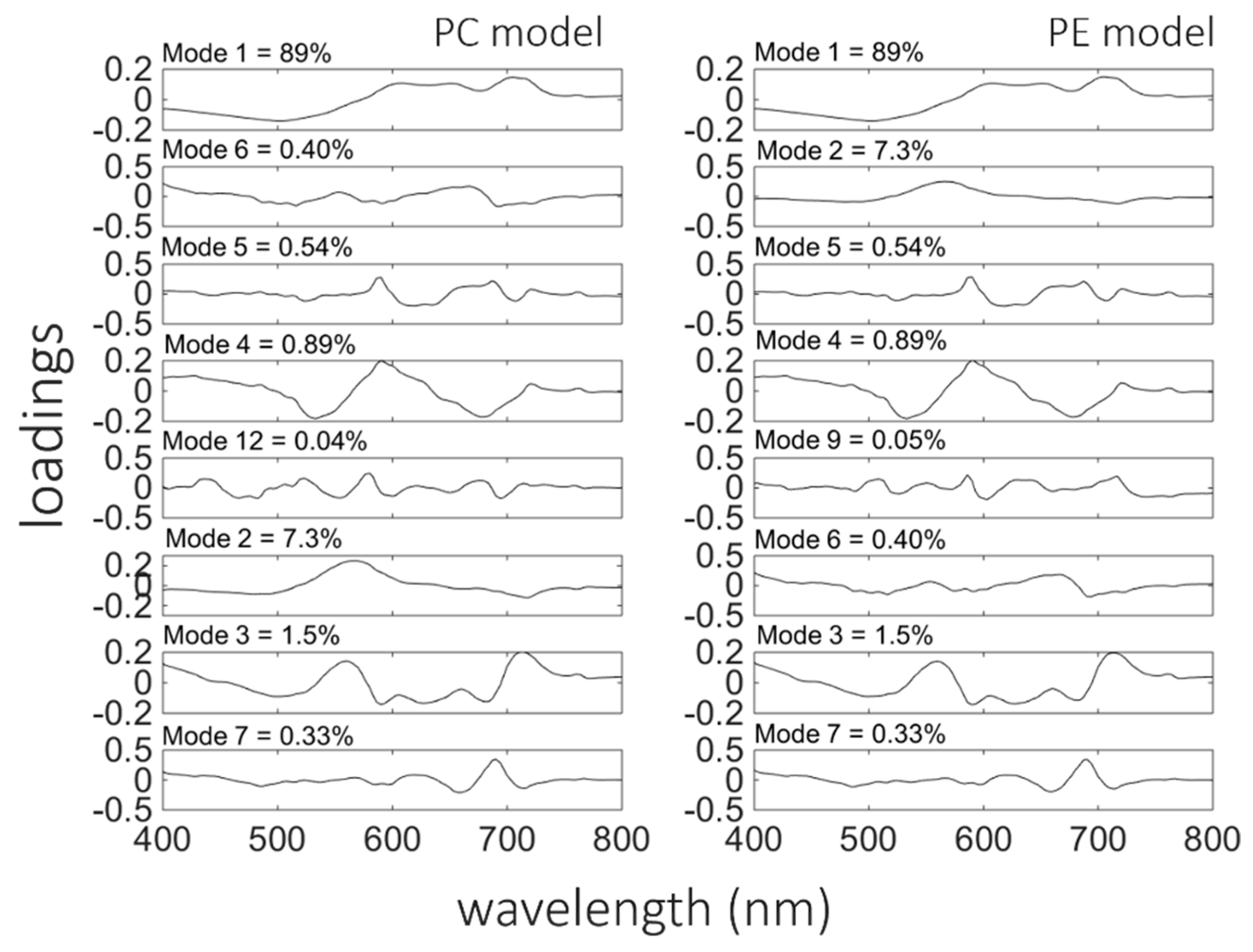
Synechococcus sp., which significantly contributes to total phytoplankton biomass, is rich in phycoerythrin. Both models, for PC and PE contained eight EOFs modes each (
Table 6). In both models, the first mode chosen was the first EOF, which captured 89% of the variance in spectral shape. However, the second component was different in both models, as shown in
Figure 8 (for PC-left panels and PE-right panels), compared to the chl-
a model. In the model for PC estimation, the second component was the 6th mode, which captured only 0.4% of the variance in
Rrs spectral shape, while for PE estimation the 2nd mode (7.3% of total variance) was chosen next. For the PC model, the 6th mode has a characteristic peak around 650 nm, where the local maximum in the
Rrs spectra due to PC fluorescence is located. In the PE model, the 2nd mode had a peak around 560–570 nm, corresponding to the maximum absorption and fluorescence of PE. These associations help to explain why stepwise regression identified these modes as statistically significant predictors even though they captured only a small proportion of the total variance in
Rrs spectral shape.
Our EOF models give approximations of PC with R
2 = 0.73, RMSE = 0.25, MPD = 30% (
Figure 9a), and of PE with R
2 = 0.72, RMSE = 0.15, MPD = 19% (
Figure 9b). The EOF model presented here for estimating PC also shows superior results compared to the band ratio model presented by Wozniak et al. [
62] for the same study area with MPD = 39%.
Overall, our results (
Table 5) compare favourably to a study performed by Bracher et al. [
46] who also used the EOF method to retrieve pigment concentrations in Atlantic waters but over a much wider geographical range (roughly 50°N–40°S). They developed models for chl-
a, carotenoid pigments, and PE, but not for PC. Their model for chl-
a showed a larger relative error with RMSE = 0.49, compared with the RMSE value of 0.16 found in our studies, and a MPD of 43%, compared to our MPD of 19% (
Table 5). In the case of PE estimation, our model showed superior results with RMSE = 0.15 and MPD = 19.5%, compared to Bracher et al. [
46] who observed RMSE = 1.16 and MPD = 139%. The superior performance of the EOF approach in our study can most likely be explained, in large part, by the fact that the Bracher et al. [
46] models were derived over a larger dynamic range, encompassing several biogeographical provinces. In contrast, our study took place exclusively in the Gulf of Gdansk, a body of water that spans over less than 1° of latitude (
Figure 1). In several implementations of the EOF approach, authors have noted that the models perform best when trained in a region-specific manner [
41,
42,
47]. However, more recent studies suggest that global implementations may be possible if a dataset with a dynamic range wide enough in parameter space is used to train the models [
63].
3.3. EOF Models for Spectral Absorption of Phytoplankton and Coloured Detrital Matter
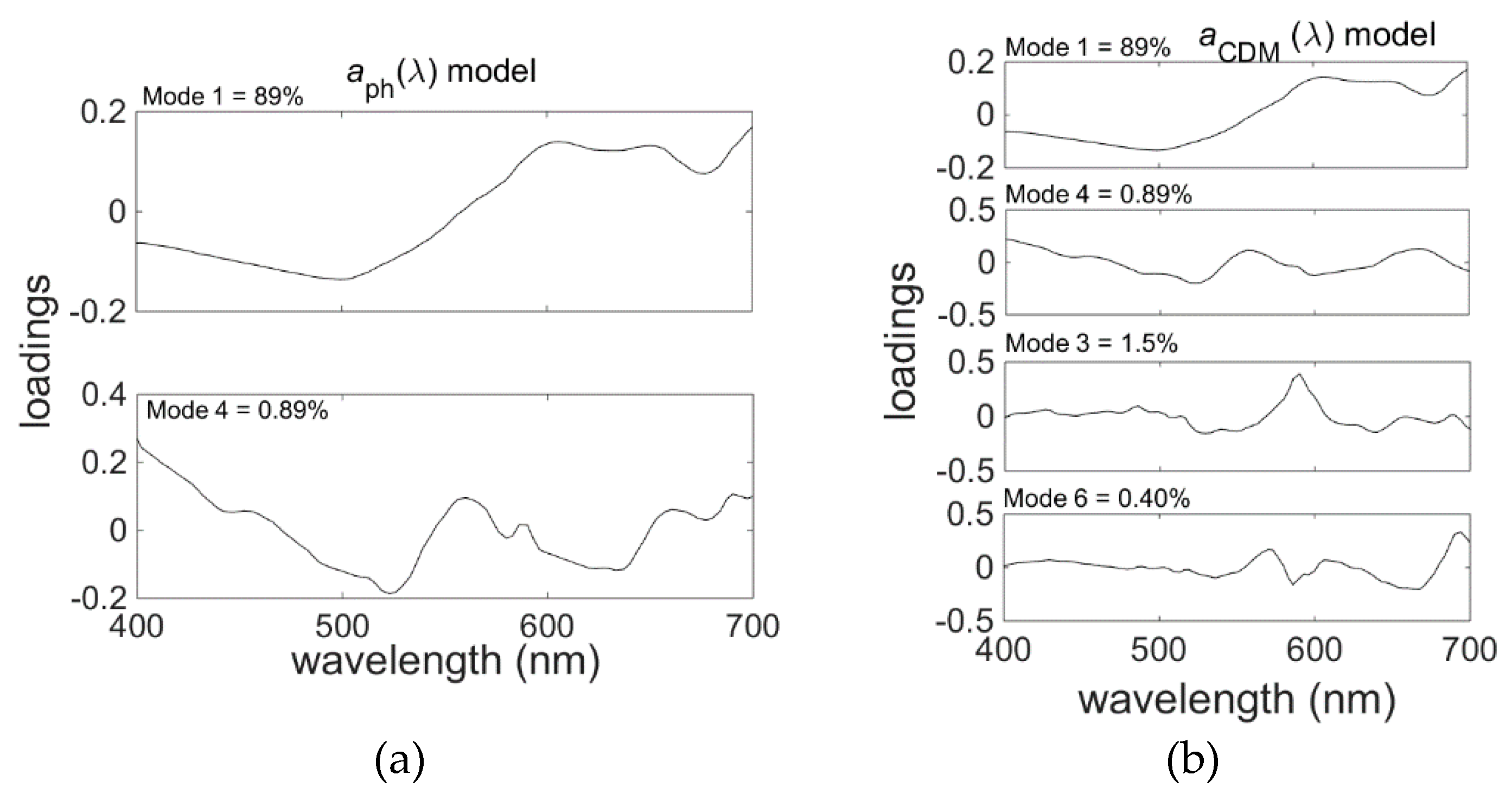
EOF models for phytoplankton and coloured detrital matter (CDM) absorption spectrum were developed separately for each wavelength from the range of 400 nm to 700 nm with a step of 3 nm. Using stepwise regression for each wavelength separately produced spectral discontinuities (
Figure 10). This is in part due to differences in the modes chosen for each wavelength. To remove these discontinuities, it was necessary to select a common set of modes for all wavelengths (
Table 7 and
Table 8,
Figure 11). The resulting models give accurate predictions for the spectral model products (
Table 9). Nevertheless, the discontinuities are the subject of ongoing investigation and future work will seek to develop more objective methods to eliminate this problem.
Knowledge of the shape of the phytoplankton absorption spectra is needed in models that estimate phytoplankton chlorophyll concentrations [
64,
65], or as an input into bio-optical models that predict carbon fixation rates for the global ocean [
66,
67,
68]. The shape and the magnitude of the phytoplankton absorption spectra is controlled primarily by the concentration of various photosynthetic and photoprotective pigments and by the level of the pigment package effect within the cells. The influence of these two processes varies with depth, phytoplankton species composition, and cell size. Quantification of CDM plays an important role in understanding the oceanic carbon cycle. Moreover, CDM absorbs strongly in the UV and blue range of the spectrum, thus determining phytoplankton and bacterial productivity [
69].
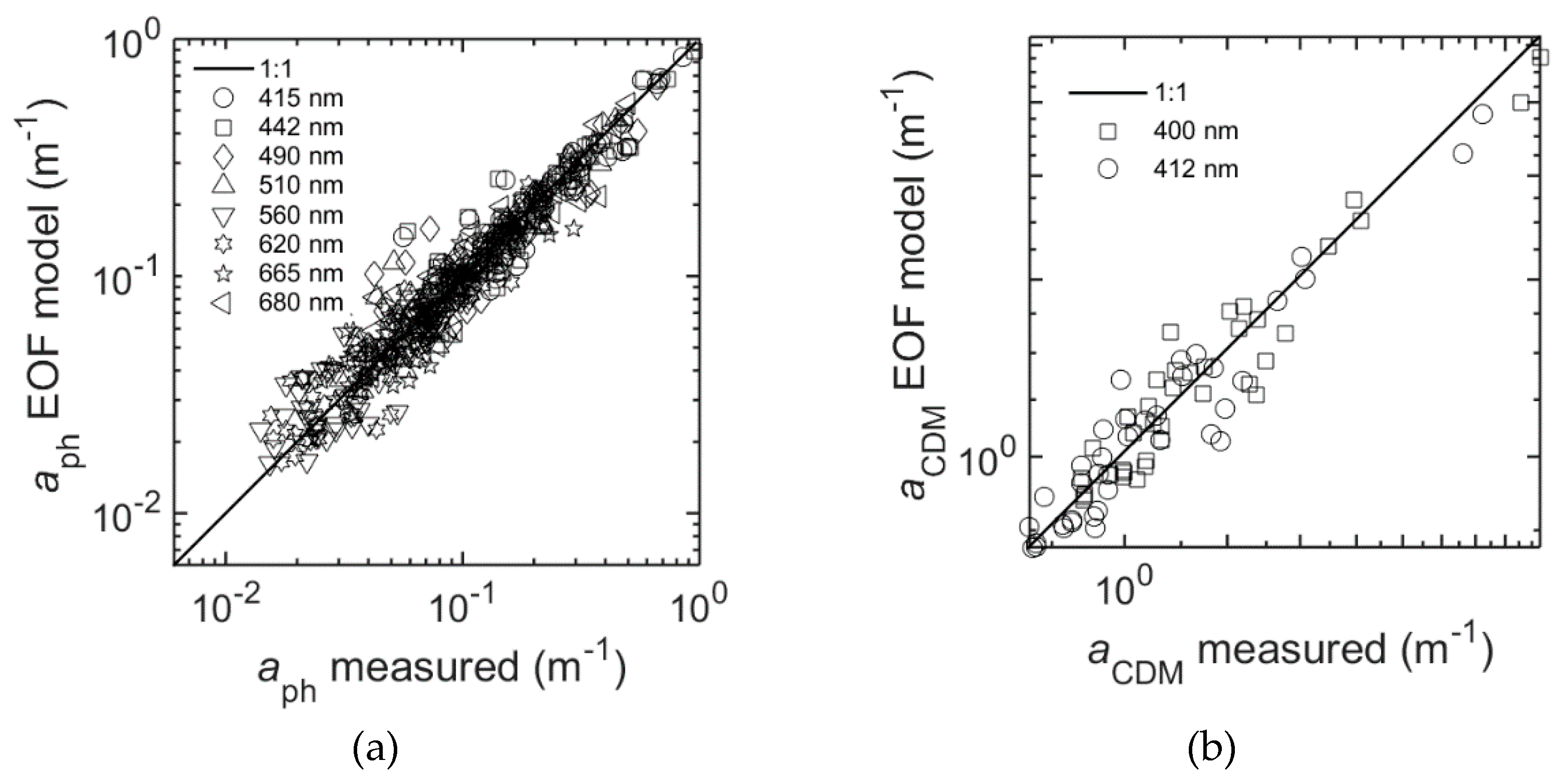
Figure 12a and
Table 9 present the results of the EOF model for
aph at selected wavelengths: R
2 (λ) ranging from 0.80–0.89, and RMSE (λ) = 0.10–0.12.
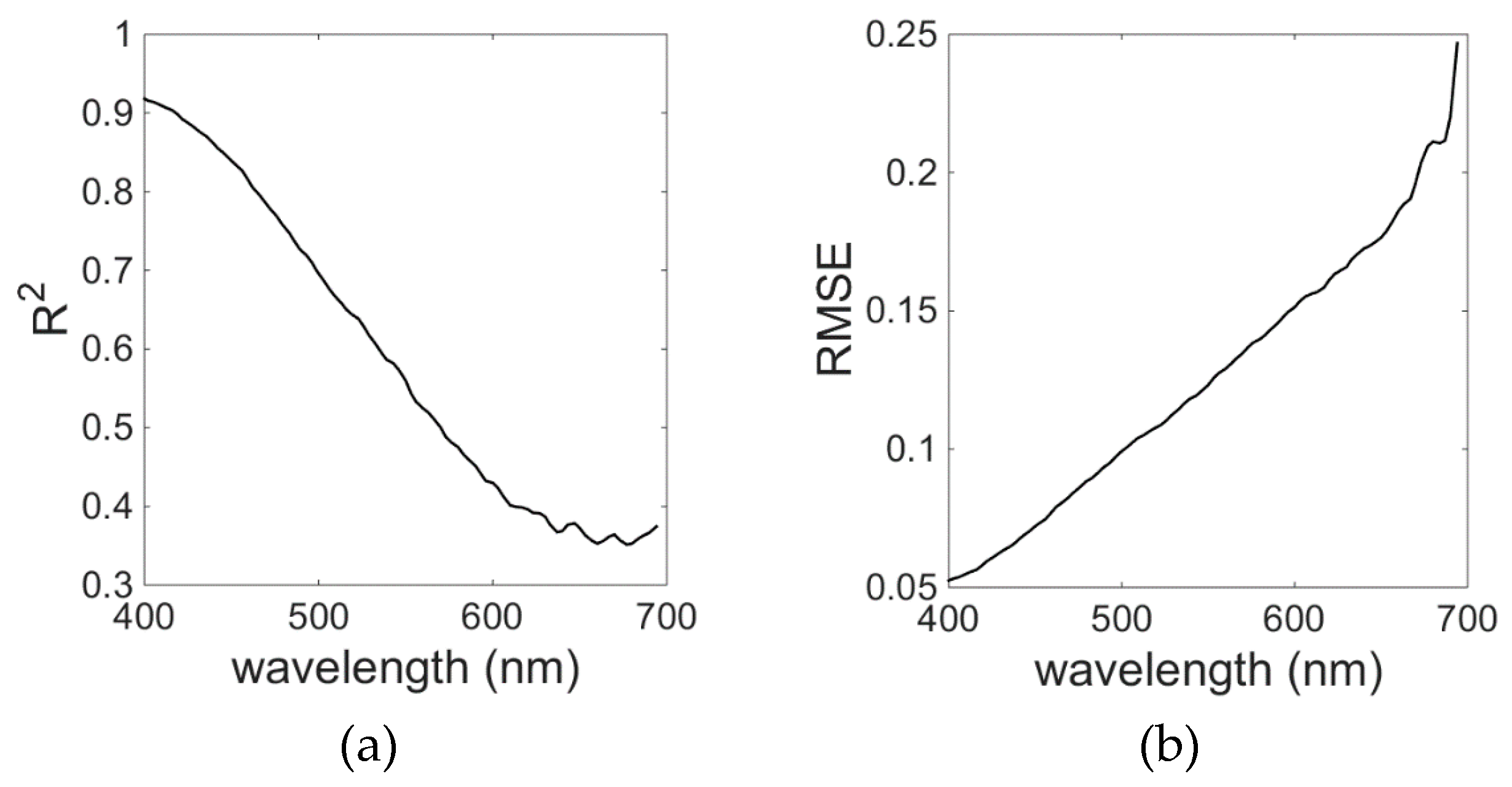
The EOF model for
aCDM gave the best results for the wavelengths in the blue spectral range between 400 and 450 nm; R
2 was higher than 0.6 and RMSE was around 0.06. The model skill decreased with increasing wavelengths (
Figure 13), reaching R
2 below 0.40 and RMSE of about 0.25 for λ = 700 nm. The contribution of CDM to the total absorption decreases approximately exponentially with increasing wavelength, which would explain the model’s decreasing skill towards the red region of the spectrum. However,
aCDM absorption spectra can be derived from the following formula [
1]:
where the slope value S can be retrieved when
aCDM is given for at least two wavelengths, for example 400 and 412 nm (
Figure 12b) which can be estimated with good agreements (
Table 9) by the EOF model.
An inverse semi-analytical model for
aph and
aCDM was presented by Wei and Lee [
1] and Werdell et al. [
70], but in both cases our model showed better results. The model of
aCDM presented by Wei and Lee (2015) also showed similar errors as our model, where model performance was proven to become worse with increasing wavelengths (
Figure 13).
3.4. EOF Models for Synthetic Satellite Data
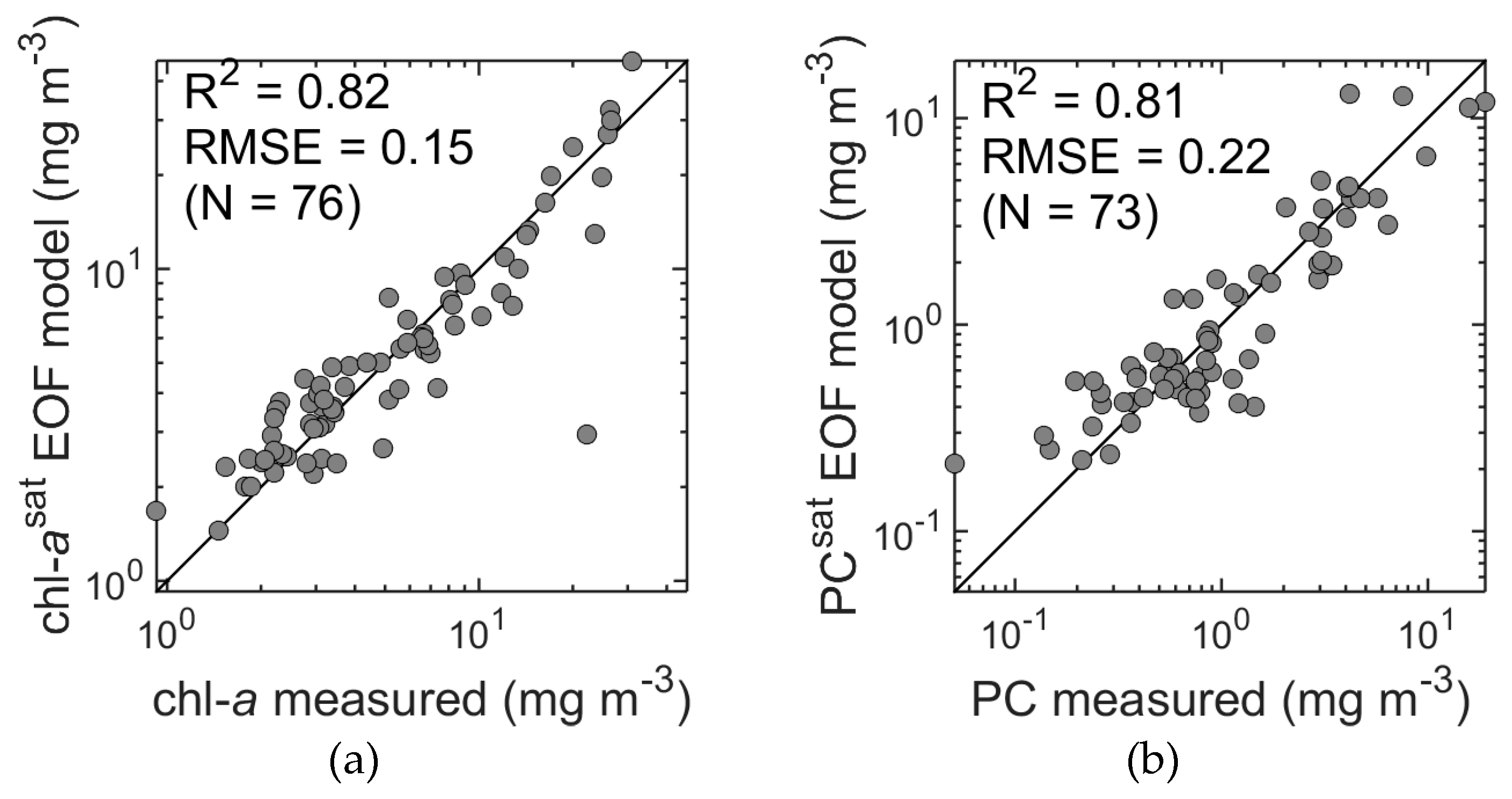
The EOF method applied to the synthetic satellite data set (
Figure 5b) showed accurate results despite the reduced spectral resolution. The accuracy of chl-
a estimates was found to be slightly reduced compared to estimates from hyperspectral data (R
2 = 0.82, RMSE = 0.15 and MPD = 18%, R
2 = 0.84, RMSE = 0.14, and MPD = 17%;
Figure 7a and
Figure 14a, respectively). Unexpectedly, PC estimates (
Figure 14b) were actually more accurate compared to the values obtained from the hyperspectral data in terms of R
2, RMSE, and bias (R
2 = 0.81, RMSE = 0.22, and MPD = 33%, R
2 = 0.77, RMSE = 0.23 and MPD = 23%;
Figure 9a and
Figure 14b, respectively). A possible reason for this may lie in the, as yet, imperfect approach to thresholding noise in the hyperspectral data, i.e., unfiltered hyperspectral noise may degrade the PC estimates. Cross validation was again performed and confirmed the robustness of the models. This demonstrates that a reduction of spectral information from hyper- to the multispectral potentially has little impact on predictive accuracy, and in the case of the PC estimates, actually improved the results. Similar results were found by Craig et al. [
41] for MERIS data, who suggested that as long as spectral information pertinent to the parameter of interest was included in the multispectral waveband set, the EOF models would perform similarly to their hyperspectral versions. In this case, wavebands that included the spectral characteristics of the absorption of chlorophyll
a were included in the synthetic data, hence retaining the information required for their accurate prediction.
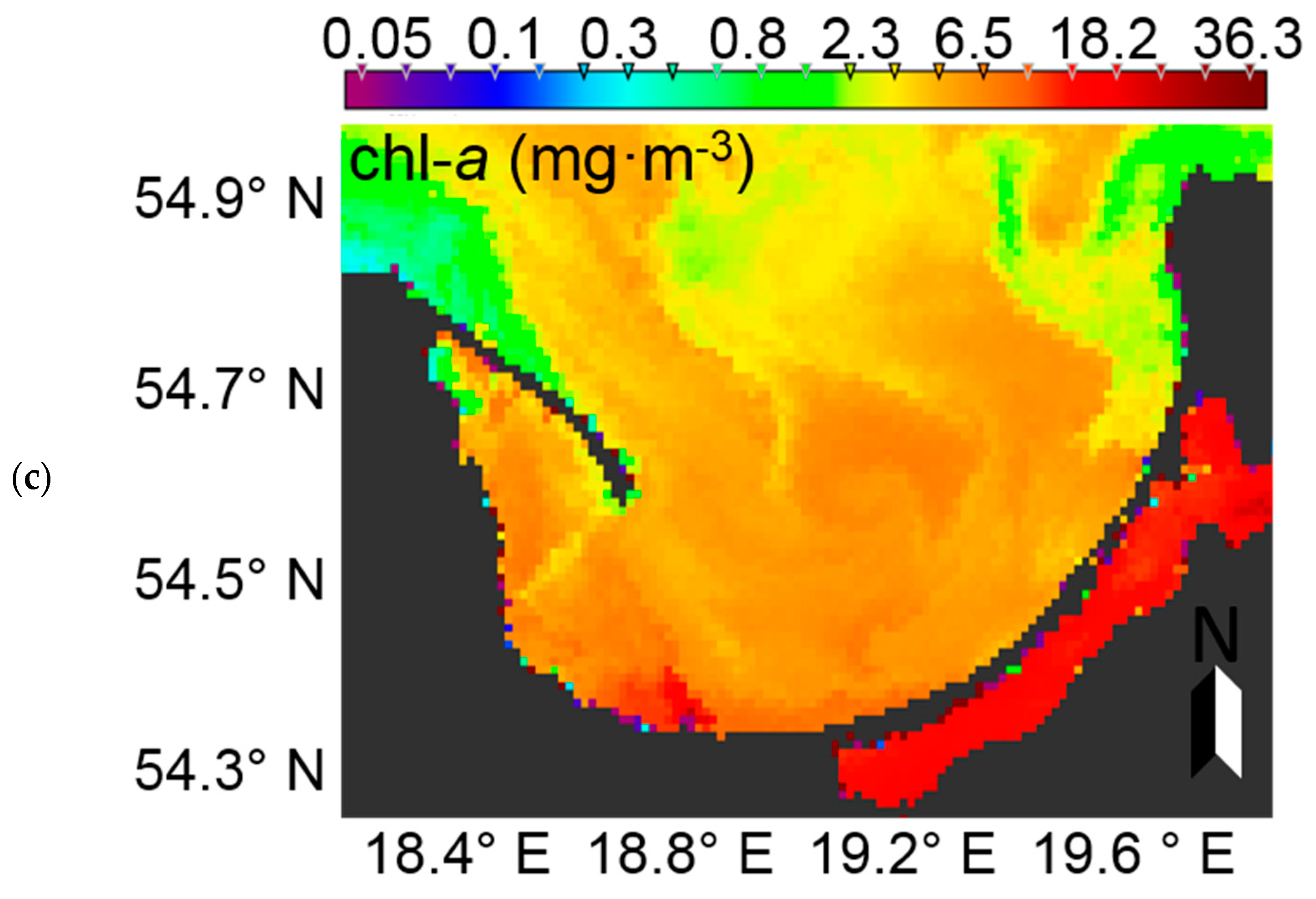
At the time of preparation of the manuscript, summer data from the European Space Agency’s Sentinel-3 instrument, OLCI (Ocean and Land Colour Instrument) radiometer were not yet available. Therefore, to demonstrate the potential usage of EOF models to characterise phytoplankton blooms in the Baltic Sea, MERIS (Envisat) data were used. The Case-2 Regional (C2R) processor [
71] was used to derive remote sensing reflectance from MERIS level 1b data acquired on 5th July 2010 in the Gulf of Gdansk. The EOF models were then run to calculate PC (marker pigment of cyanobacteria in the Baltic Sea) and chl-
a (proxy of phytoplankton biomass). In the surface water of the RGB image, an algal bloom is clearly visible in the northern part of the image (
Figure 15a). Considering the time of data acquisition, it likely represents a cyanobacteria bloom. Evaluating the PC dynamics (
Figure 15b), a similar pattern is evident, further supporting the likelihood that the RGB feature is, indeed, a phytoplankton bloom and most likely dominated by cyanobacteria. In the southern area of the Gulf of Gdansk, where the Vistula River strongly influences the water properties, chl-
a is much higher when compared to the PC concentration. This may be explained by the fact that the input of freshwater increases nutrient concentrations, and thus allows other phytoplankton groups which do not contain PC to outcompete cyanobacteria. These are preliminary results, which may need further investigation and validation, but they demonstrate the potential usefulness of the EOF models for satellite data.