Remote Estimation of Vegetation Fraction and Flower Fraction in Oilseed Rape with Unmanned Aerial Vehicle Data
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Unmanned Aerial Vehicle Data
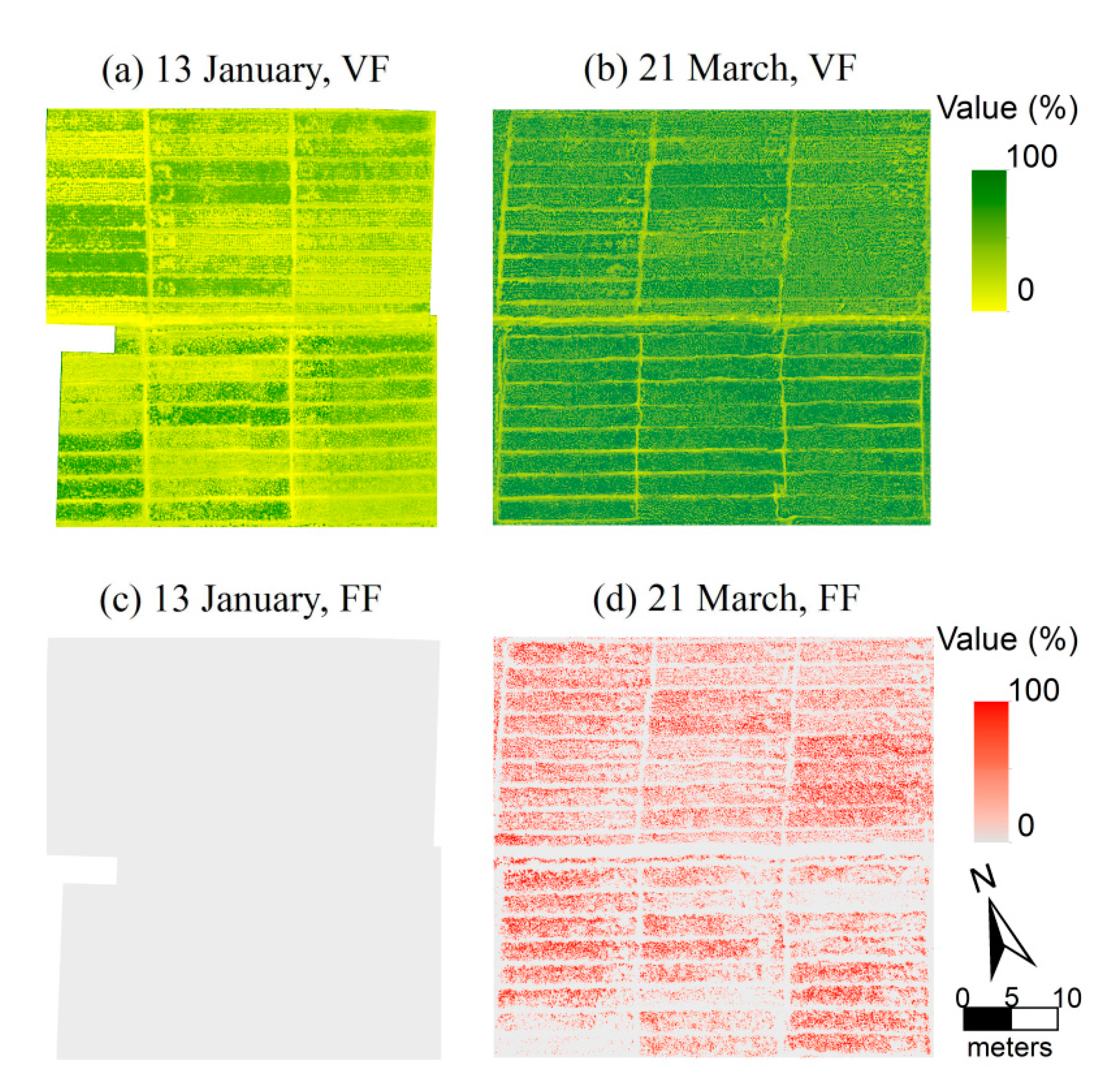
2.3. Vegetation Fraction and Flower Fraction Determined by Image Classification
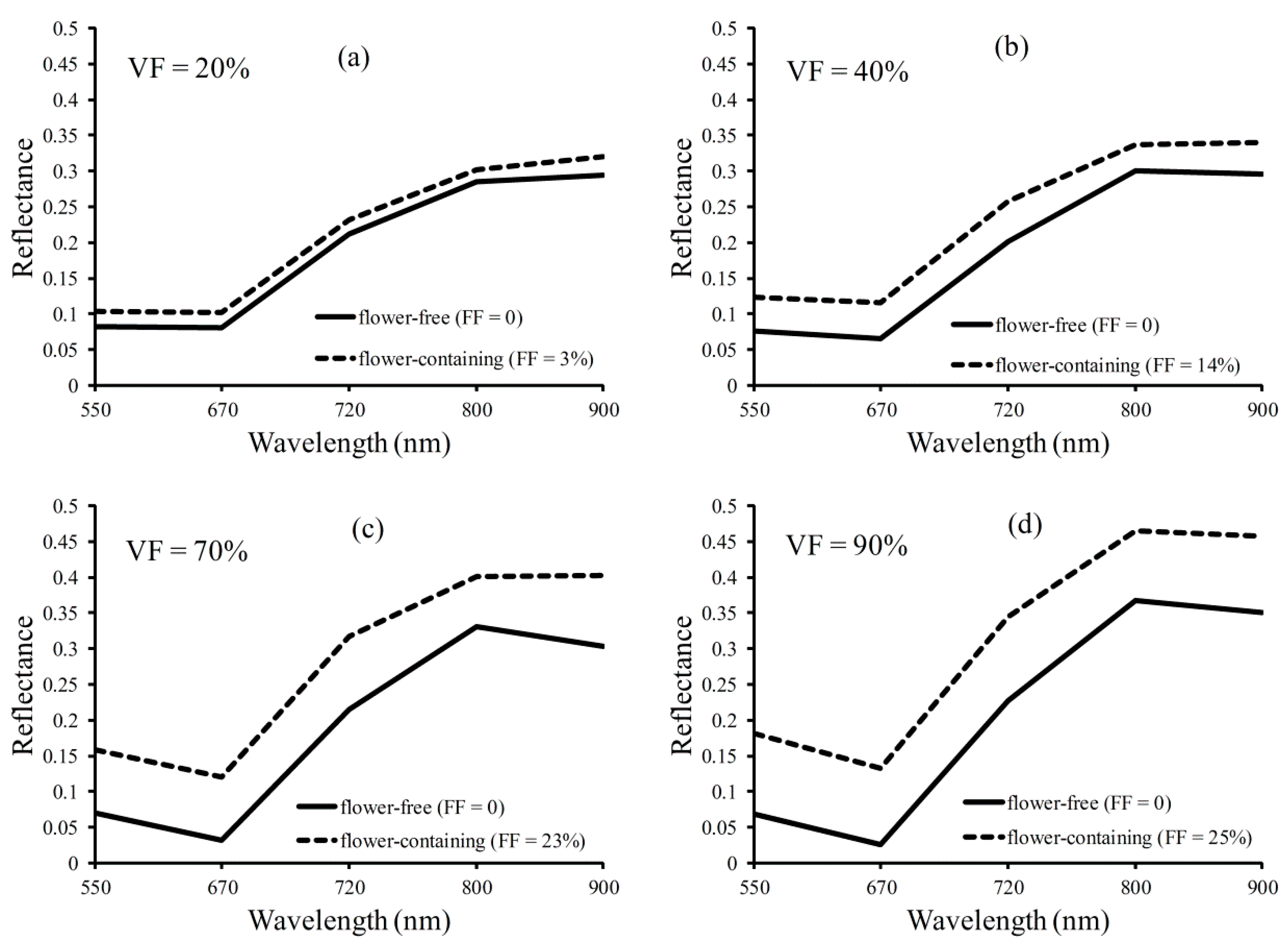
2.4. Surface Reflectance
2.5. Vegetation Indices
3. Results and Discussion
3.1. Relationship of Vegetation Fraction and Vegetation Index in Oilseed Rape
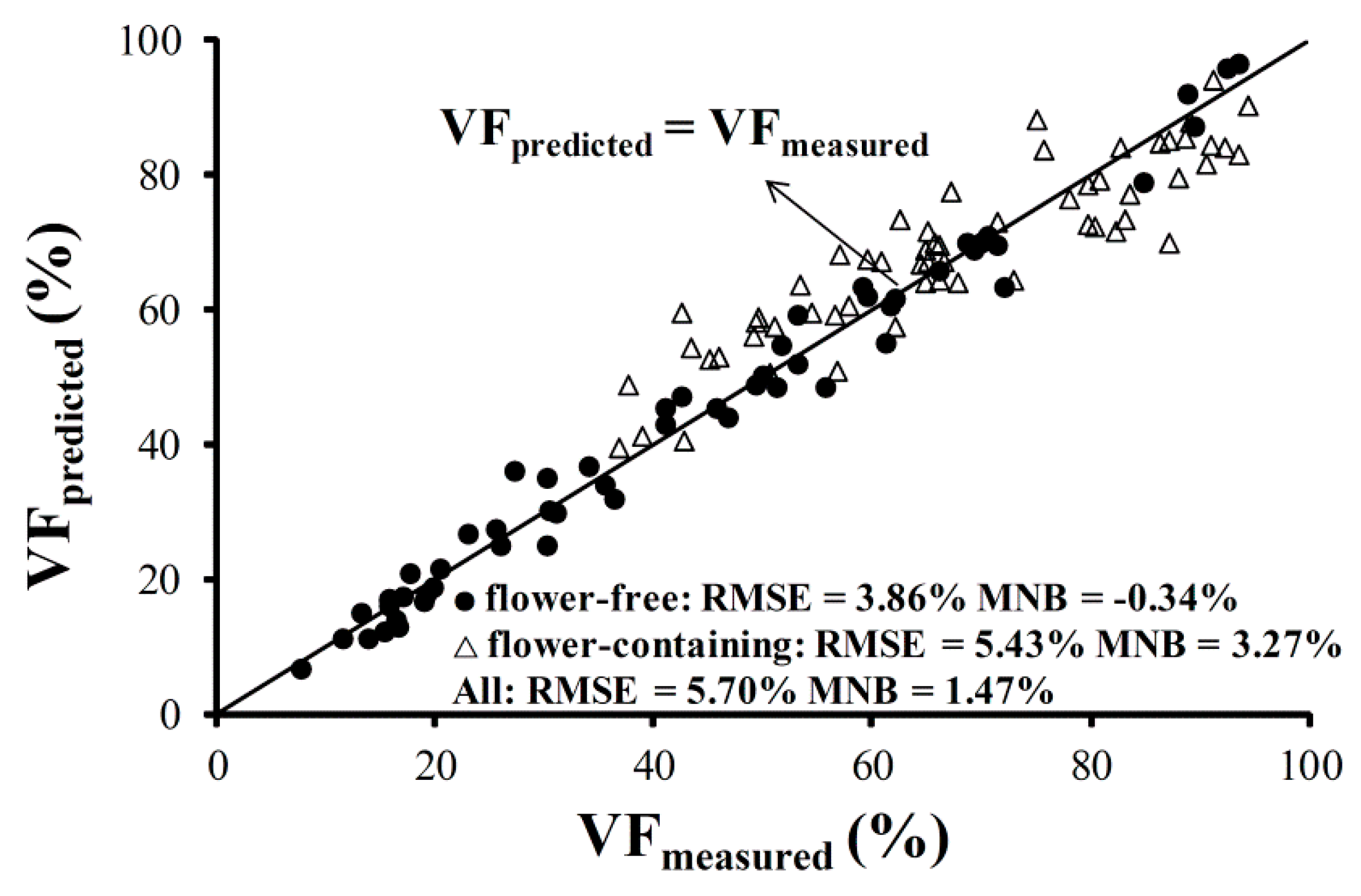
3.2. Remote Estimation of Vegetation Fraction in Oilseed Rape
- Flagging the sample based on NGVI value: if NGVI > 0.6, the sample was flagged as a flower-free sample; if NGVI ≤ 0.6, it was flagged as a flower-containing sample.
- Applying the algorithm to estimate VF for the sample: if flagged as a flower-free sample, VF = 1.31 × VARIgreen + 0.25; if flagged as a flower-containing sample, VF = 2.41 × EVI2 – 0.40.
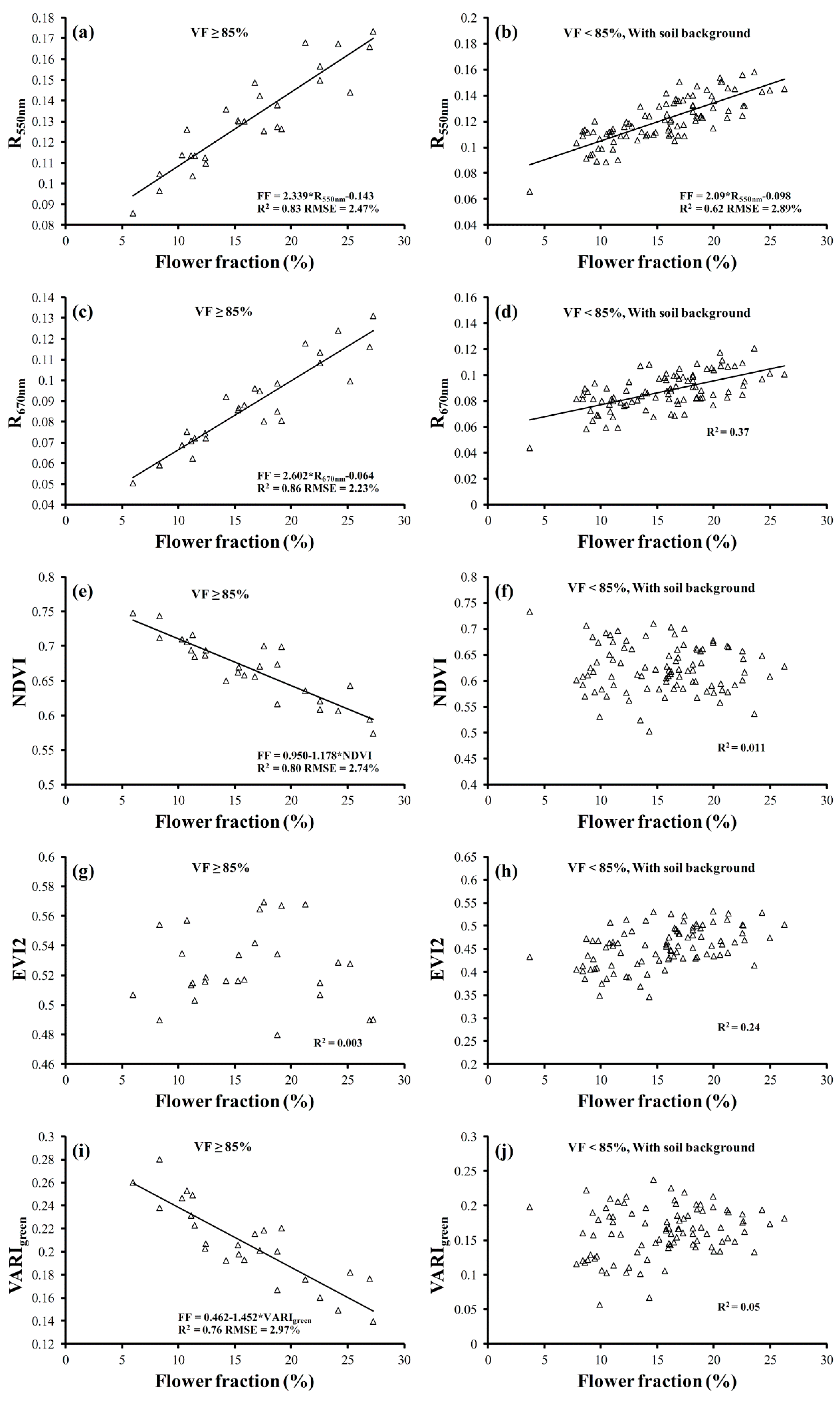
3.3. Remote Estimation of Flower Fraction in Oilseed Rape
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| VF | Vegetation Fraction |
| FF | Flower Fraction |
| NDVI | Normalized Difference Vegetation Index |
| VARIgreen | Visible Atmospherically Resistant Index |
| EVI | Enhanced Vegetation Index |
| MSAVI | Modified Soil-Adjusted Vegetation Index |
| RMSE | Root Mean Square Error |
| MNB | Mean Normalized Bia |
References
- Purevdorj, T.S.; Tateishi, R.; Ishiyama, T.; Honda, Y. Relationships between percent vegetation cover and vegetation indices. Int. J. Remote Sens. 1998, 19, 3519–3535. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Kaufman, Y.J.; Stark, R.; Rundquist, D. Novel algorithms for remote estimation of vegetation fraction. Remote Sens. Environ. 2002, 80, 76–87. [Google Scholar] [CrossRef]
- Carlson, T.N.; Gillies, R.R.; Perry, E.M. A method to make use of thermal infrared temperature and NDVI measurements to infer surface soil water content and fractional vegetation cover. Remote Sens. Rev. 1994, 9, 161–173. [Google Scholar] [CrossRef]
- Owen, T.W.; Carlson, T.N.; Gillies, R.R. An assessment of satellite remotely-sensed land cover parameters in quantitatively describing the climatic effect of urbanization. Int. J. Remote Sens. 1998, 19, 1663–1681. [Google Scholar] [CrossRef]
- Steven, M.D.; Biscoe, P.V.; Jaggard, K.W.; Paruntu, J. Foliage cover and radiation interception. Field Crop. Res. 1986, 13, 75–87. [Google Scholar] [CrossRef]
- Monsi, M.; Saeki, T. Uber den lichtfaktor in den Pflanzengesellschaften undseine Bedeutung fur die stoffproduktion. Jpn. J. Bot. 1952, 14, 22–52. [Google Scholar]
- Li, X.; Zhang, J. Derivation of the green vegetation fraction of the whole China from 2000 to 2010 from MODIS data. Earth. Interact. 2015, 20, 1–16. [Google Scholar] [CrossRef]
- Graetz, R.D.; Pech, R.P.; DAVIS, A.W. The assessment and monitoring of sparsely vegetated rangelands using calibrated Landsat data. Int. J. Remote Sens. 1988, 9, 1201–1222. [Google Scholar] [CrossRef]
- North, P.R.J. Estimation of fAPAR, LAI, and vegetation fractional cover from ATSR-2 imagery. Remote Sens. Environ. 2002, 80, 114–121. [Google Scholar] [CrossRef]
- Nguyrobertson, A.; Gitelson, A.A.; Peng, Y.; Walter-Shea, E.; Leavitt, B.; Arkebauer, T. Continuous monitoring of crop reflectance, vegetation fraction, and identification of developmental stages using a four band radiometer. Agron. J. 2013, 105, 1769–1779. [Google Scholar] [CrossRef]
- Baret, F.; Clevers, J.G.P.W.; Steven, M.D. The robustness of canopy gap fraction estimates from red and near-infrared reflectances: A comparison of approaches. Remote Sens. Environ. 1995, 54, 141–151. [Google Scholar] [CrossRef]
- Peddle, D.R.; Smith, A.M. Spectral mixture analysis of agricultural crops: Endmember validation and biophysical estimation in potato plots. Int. J. Remote Sens. 2005, 26, 4959–4979. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Gitelson, A.A.; Schepers, J.S.; Walthall, C.L. Application of spectral remote sensing for agronomic decisions. Agron. J. 2008, 100, 117–131. [Google Scholar] [CrossRef]
- Maire, G.L.; François, C.; Soudani, K.; Berveiller, D.; Pontailler, J.Y.; Bréda, N.; Genet, H.; Davi, H.; Dufrêne, E. Calibration and validation of hyperspectral indices for the estimation of broadleaved forest leaf chlorophyll content, leaf mass per area, leaf area index and leaf canopy biomass. Remote Sens. Environ. 2008, 112, 3846–3864. [Google Scholar] [CrossRef]
- Verstraete, M.M.; Pinty, B.; Myneni, R.B. Potential and limitations of information extraction on the terrestrial biosphere from satellite remote sensing. Remote Sens. Environ. 1996, 58, 201–214. [Google Scholar] [CrossRef]
- Liu, J.; Miller, J.R.; Haboudane, D.; Pattey, E.; Hochheim, K. Crop fraction estimation from casi hyperspectral data using linear spectral unmixing and vegetation indices. Can. J. Remote Sens. 2008, 34, S124–S138. [Google Scholar] [CrossRef]
- Alexandridis, T.K.; Oikonomakis, N.; Gitas, I.Z.; Silleos, K. The performance of vegetation indices for operational monitoring of CORINE vegetation types. Int. J. Remote Sens. 2014, 35, 3268–3285. [Google Scholar] [CrossRef]
- Everitt, J.H.; Alaniz, M.A.; Escobar, D.E.; Davis, M.R. Using remote sensing to distinguish common (Isocoma coronopifolia) and Drummond Goldenweed (Isocoma drummondii). Weed Sci. 1992, 4, 621–628. [Google Scholar]
- Everitt, J.H.; Davis, M.R. Light reflectance characteristics and remote sensing of big Bend Loco (Astragalus mollissimus var. earlei) and Wooton Loco (Astragalus wootonii). Weed Sci. 1994, 42, 115–122. [Google Scholar]
- Ge, S.; Everitt, J.; Carruthers, R.; Gong, P.; Anderson, G. Hyperspectral characteristics of canopy components and structure for phenological assessment of an invasive weed. Environ. Monit. Assess. 2006, 120, 109–126. [Google Scholar] [CrossRef] [PubMed]
- Vina, A.; Gitelson, A.A.; Rundquist, D.C.; Keydan, G.; Leavitt, B.; Schepers, J. Monitoring maize (Zea mays L.) phenology with remote sensing. Remote Sens. 2009, 2, 2729–2747. [Google Scholar]
- Gitelson, A.A.; Vina, A.; Arkebauer, T.J.; Rundquist, D.C.; Keydan, G.; Leavitt, B. Remote estimation of leaf area index and green leaf biomass in maize canopies. Geophys. Res. Lett. 2003, 30. [Google Scholar] [CrossRef]
- Verma, K.S.; Saxena, R.K.; Hajare, T.N. Spectral response of gram varieties under variable soil condition. Int. J. Remote Sens. 2002, 23, 313–324. [Google Scholar] [CrossRef]
- Behrens, T.; Müller, J.; Diepenbrock, W. Utilization of canopy reflectance to predict properties of oilseed rape (Brassica napus L.) and barley (Hordeum vulgare L.) during ontogenesis. Eur. J. Agron. 2006, 25, 345–355. [Google Scholar] [CrossRef]
- Shen, M.; Chen, J.; Zhu, X.; Tang, Y. Yellow flowers can decrease NDVI and EVI values: Evidence from a field experiment in an alpine meadow. Can. J. Remote Sens. 2009, 35, 99–106. [Google Scholar] [CrossRef]
- Sulik, J.J.; Long, D.S. Spectral indices for yellow canola flower. Int. J. Remote Sens. 2015, 36, 2751–2765. [Google Scholar]
- Angadi, S.V.; Cutforth, H.W.; Miller, P.R.; McConkey, B.G.; Entz, M.H.; Brandt, S.A.; Volkmar, K.M. Response of three Brassica species to high temperature stress during reproductive growth. Can. J. Plant. Sci. 2000, 80, 693–701. [Google Scholar] [CrossRef]
- Qian, W.; Chen, X.; Fu, D.; Zou, J.; Meng, J. Intersubgenomic heterosis in seed yield potential observed in a new type of Brassica napus introgressed with partial Brassica rapa genome. Theor. Appl. Genet. 2005, 110, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Nowosad, K.; Liersch, A.; Popławska, W.; Bocianowski, J. Genotype by environment interaction for seed yield in rapeseed (Brassica napus L.) using additive main effects and multiplicative interaction model. Euphytica 2015, 208, 187–194. [Google Scholar] [CrossRef]
- Wittkop, B.; Snowdon, R.J.; Friedt, W. Status and perspectives of breeding for enhanced yield and quality of oilseed crops for Europe. Euphytica 2009, 170, 131–140. [Google Scholar] [CrossRef]
- Morrison, M.J.; Stewart, D.W. Heat Stress during Flowering in Summer Brassica. Crop. Sci. 2002, 42, 797–803. [Google Scholar] [CrossRef]
- Gan, Y.; Angadi, S.V.; Cutforth, H.; Potts, D.; Angadi, V.V.; McDonald, C.L. Canola and mustard response to short periods of temperature and water stress at different developmental stages. Can. J. Plant. Sci. 2004, 84, 697–704. [Google Scholar] [CrossRef]
- Faraji, A. Flower formation and pod/flower ratio in canola (Brassica napus L.) affected by assimilates supply around flowering. Int. J. Plant Prod. 2010, 4, 271–280. [Google Scholar]
- Klemas, V. Remote sensing of wetlands: Case studies comparing practical techniques. J. Coastal. Res. 2011, 27, 418–427. [Google Scholar] [CrossRef]
- Jensen, J.R. Remote Sensing of the Environment, 2nd ed.; Pearson: Upper Saddle River, NJ, USA, 2007. [Google Scholar]
- Klemas, V.V. Coastal and environmental remote sensing from unmanned aerial vehicles: An overview. J. Coastal. Res. 2015, 315, 1260–1267. [Google Scholar] [CrossRef]
- Hunt, E.R.; Hively, W.D.; Fujikawa, S.J.; Linden, D.S.; Daughtry, C.S.T.; McCarty, G.W. Acquisition of NIR-green-blue digital photographs from unmanned aircraft for crop monitoring. Remote Sens. 2010, 2, 290–305. [Google Scholar] [CrossRef]
- Ma, N.; Yuan, J.; Li, M. Ideotype population exploration: Growth, photosynthesis, and yield components at different planting densities in winter oilseed rape (Brassica napus L.). PLoS ONE 2014, 9, e114232. [Google Scholar]
- Thenkabail, P.S.; Lyon, G.J.; Huete, A. Hyperspectral Remote Sensing of Vegetation, 1st ed.; CRC Press-Taylor and Francis Group: Boca Raton, FL, USA, 2011. [Google Scholar]
- Kira, O.; Linker, R.; Gitelson, A.A. Non-destructive estimation of foliar chlorophyll and carotenoid contents: Focus on informative spectral bands. Int. J. Appl. Earth. Obs. 2015, 38, 251–260. [Google Scholar] [CrossRef]
- Ray, S.S.; Jain, N.; Miglani, A.; Singh, J.P.; Singh, A.K.; Panigrahy, S.; Parihar, J.S. Defining optimum spectral narrow bands and bandwidths for agricultural applications. Curr. Sci. India. 2010, 93, 1365–1369. [Google Scholar]
- TETRACAM. Available online: http://www.tetracam.com/Products-Mini_MCA.htm (accessed on 11 May 2016).
- Turner, D.; Lucieer, A.; Malenovský, Z.; King, D.H.; Robinson, S.A. Patial co-registration of ultra-high resolution visible, multispectral and thermal images acquired with a, Micro-UAV over Antarctic Moss Beds. Remote Sens. 2014, 6, 4003–4024. [Google Scholar] [CrossRef]
- Zhang, W.H.; Li, Y.C.; Li, D.L.; Teng, C.S.; Liu, J. Distortion correction algorithm for UAV remote sensing image based on CUDA. In Proceedings of the 35th International Symposium on Remote Sensing of Environment, Beijing, China, 22–26 April 2013.
- Schwieder, M.; Leitao, P.J.; Suess, S.; Senf, C.; Hostert, P. Estimating fractional shrub cover using simulated EnMAP data: A comparison of three machine learning regression techniques. Remote Sens. 2014, 6, 3427–3445. [Google Scholar] [CrossRef]
- Lehnert, L.W.; Meyer, H.; Wang, Y.; Miehe, G.; Thies, B.; Reudenbach, C.; Bendix, J. Retrieval of grassland plant coverage on the Tibetan Plateau based on a multi-scale, multi-sensor and multi-method approach. Remote Sens. Environ. 2015, 164, 197–207. [Google Scholar] [CrossRef]
- Dwyer, J.L.; Kruse, K.A.; Lefkoff, A.B. Effects of empirical versus model-based reflectance calibration on automated analysis of imaging spectrometer data: A case study from the Drum Mountains, Utah. Photogramm. Eng. Remote Sens. 1995, 61, 1247–1254. [Google Scholar]
- Laliberte, A.S.; Goforth, M.A.; Steele, C.M.; Rango, A. Multispectral Remote Sensing from Unmanned Aircraft: Image processing workflows and applications for rangeland environments. Remote Sens. 2011, 3, 2529–2551. [Google Scholar] [CrossRef]
- Farrand, W.H.; Singer, R.B.; Merényi, E. Retrieval of apparent surface reflectance from AVIRIS data: A comparison of empirical line, radiative transfer, and spectral mixture methods. Remote Sens. Environ. 1994, 47, 311–321. [Google Scholar] [CrossRef]
- Wang, C.; Myint, S.W. A simplified empirical line method of radiometric calibration for small unmanned aircraft systems-based remote sensing. IEEE J-STARS 2015, 8, 1–10. [Google Scholar] [CrossRef]
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W. Monitoring vegetation systems in the Great Plains with ERTS. Nasa Special Publ. 1974, 351, 309–309. [Google Scholar]
- Qi, J.; Chehbouni, A.; Huete, A.R.; Kerr, Y.H.; Sorooshian, S. A modified soil adjusted vegetation index. Remote Sens. Environ. 1994, 48, 119–126. [Google Scholar] [CrossRef]
- Jiang, Z.; Huete, A.R.; Didan, K.; Miura, T. Development of a two-band enhanced vegetation index without a blue band. Remote Sens. Environ. 2015, 112, 3833–3845. [Google Scholar] [CrossRef]
- Gitelson, A.A. Remote estimation of crop fractional vegetation cover: The use of noise equivalent as an indicator of performance of vegetation indices. Int. J. Remote Sens. 2013, 34, 1–13. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Gritz, Y.; Merzlyak, M.N. Relationships between leaf chlorophyll content and spectral reflectance and algorithms for non-destructive chlorophyll assessment in higher plant leaves. J. Plant. Physiol. 2003, 160, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Brisco, B.; Short, N.; Sanden, J.V.D.; Landry, R.; Raymond, D. A semi-automated tool for surface water mapping with RADARSAT-1. Can. J. Remote Sens. 2009, 35, 336–344. [Google Scholar] [CrossRef]
- Li, J.; Wang, S. An automatic method for mapping inland surface waterbodies with Radarsat-2 imagery. Int. J. Remote Sens. 2015, 36, 1367–1384. [Google Scholar] [CrossRef]
- Adar, S.; Shkolnisky, Y.; Ben Dor, E. A new approach for thresholding spectral change detection using multispectral and hyperspectral image data, a case study over Sokolov, Czech Republic. Int. J. Remote Sens. 2014, 35, 1563–1584. [Google Scholar] [CrossRef]
- Penuelas, J.; Filella, I.; Serrano, L.; Save, R. Cell wall elasticity and Water Index (R970 nm/R900 nm) in wheat under different nitrogen availabilities. Int. J. Remote Sens. 1996, 17, 373–382. [Google Scholar] [CrossRef]
- Diepenbrock, W. Yield analysis of winter oilseed rape (Brassica napus L.): A review. Field Crop. Res. 2000, 67, 35–49. [Google Scholar] [CrossRef]








| Vegetation Indices | Formula | References |
|---|---|---|
| Normalized Difference Vegetation Index (NDVI) | (R800 – R670)/(R800 + R670) | Rouse et al. [51] |
| Visible Atmospherically Resistant Index (VARIgreen) | (R550 – R670)/(R550 + R670) | Gitelson et al. [2] |
| Modified Soil-Adjusted Vegetation Index (MSAVI) | Qi et al. [52] | |
| Enhanced Vegetation Index (EVI2) | 2.5 × (R800 – R670)/(1 + R800 + 2.4 × R670) | Jiang et al. [53] |
| Index | Equation | R2 | RMSE (%) |
|---|---|---|---|
| VARIgreen | VF = 1.31x + 0.25 | 0.98 | 3.56 |
| NDVI | VF = 4.38x2 − 4.45x + 1.28 | 0.94 | 6.56 |
| MSAVI | VF = 0.87x2 + 0.44x − 0.02 | 0.92 | 7.69 |
| EVI2 | VF = 0.82x2 + 0.56x − 0.08 | 0.90 | 8.39 |
| Index | Equation | R2 | RMSE (%) |
|---|---|---|---|
| EVI2 | VF = 2.41x − 0.40 | 0.84 | 5.65 |
| MSAVI | VF = 2.43x − 0.37 | 0.83 | 5.74 |
| VARIgreen | VF = 2.57x + 0.29 | 0.60 | 9.0 |
| NDVI | - | 0.33 | - |
| Reflectance/Index | Threshold | Overall Accuracy | Kappa Coefficient |
|---|---|---|---|
| (R900nm − R550nm)/(R900nm + R550nm) | 0.60 | 81.02% | 0.58 |
| R900nm | 0.4 | 78.94% | 0.57 |
| R550nm | 0.12 | 75.69% | 0.54 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, S.; Tang, W.; Peng, Y.; Gong, Y.; Dai, C.; Chai, R.; Liu, K. Remote Estimation of Vegetation Fraction and Flower Fraction in Oilseed Rape with Unmanned Aerial Vehicle Data. Remote Sens. 2016, 8, 416. https://doi.org/10.3390/rs8050416
Fang S, Tang W, Peng Y, Gong Y, Dai C, Chai R, Liu K. Remote Estimation of Vegetation Fraction and Flower Fraction in Oilseed Rape with Unmanned Aerial Vehicle Data. Remote Sensing. 2016; 8(5):416. https://doi.org/10.3390/rs8050416
Chicago/Turabian StyleFang, Shenghui, Wenchao Tang, Yi Peng, Yan Gong, Can Dai, Ruhui Chai, and Kan Liu. 2016. "Remote Estimation of Vegetation Fraction and Flower Fraction in Oilseed Rape with Unmanned Aerial Vehicle Data" Remote Sensing 8, no. 5: 416. https://doi.org/10.3390/rs8050416






