Examining the Influence of Seasonality, Condition, and Species Composition on Mangrove Leaf Pigment Contents and Laboratory Based Spectroscopy Data
Abstract
:1. Introduction
2. Methods
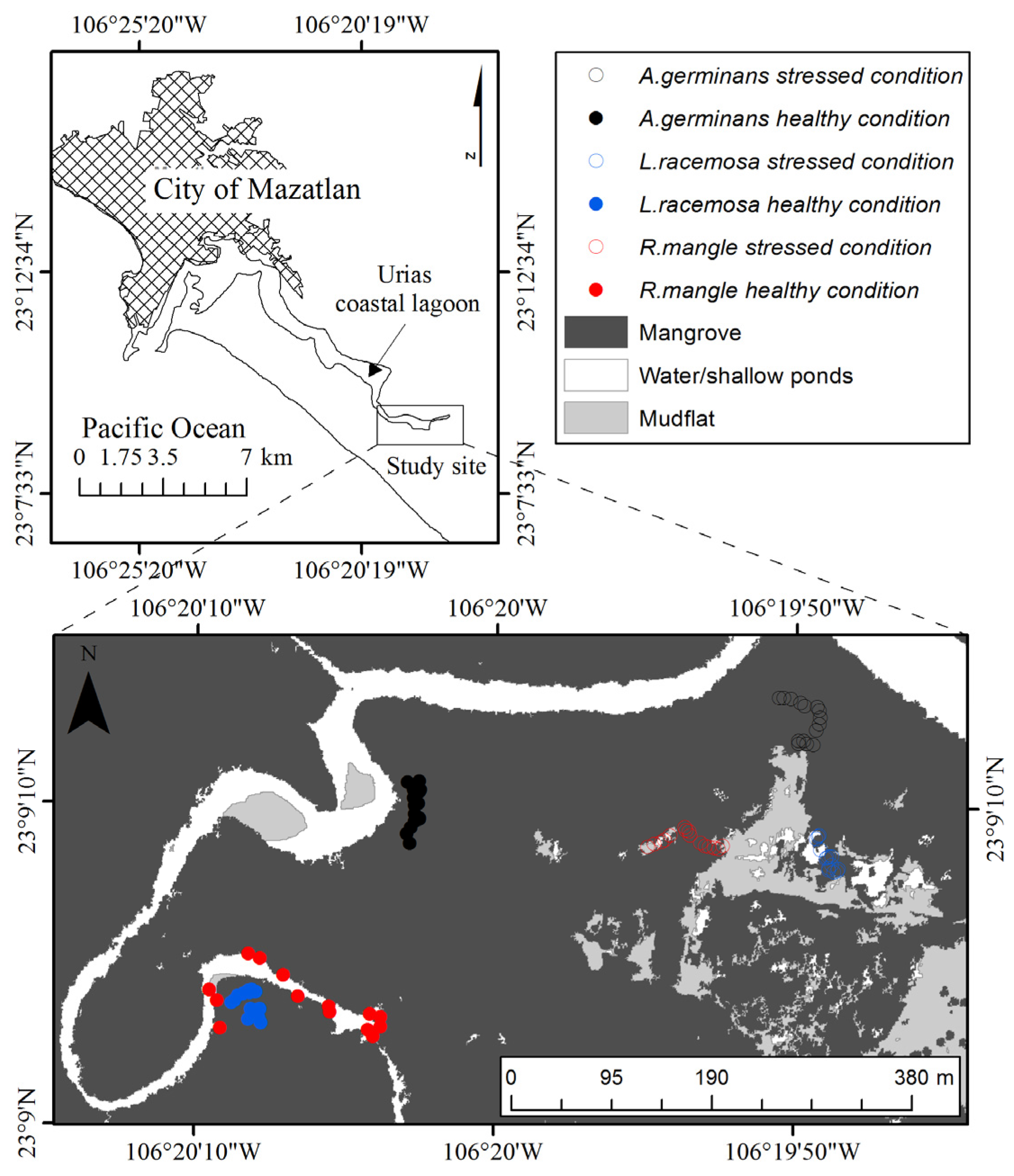
2.1. Study Site and Data Collection
2.2. Leaf Spectroscopy Data
2.3. Leaf Pigment Contents
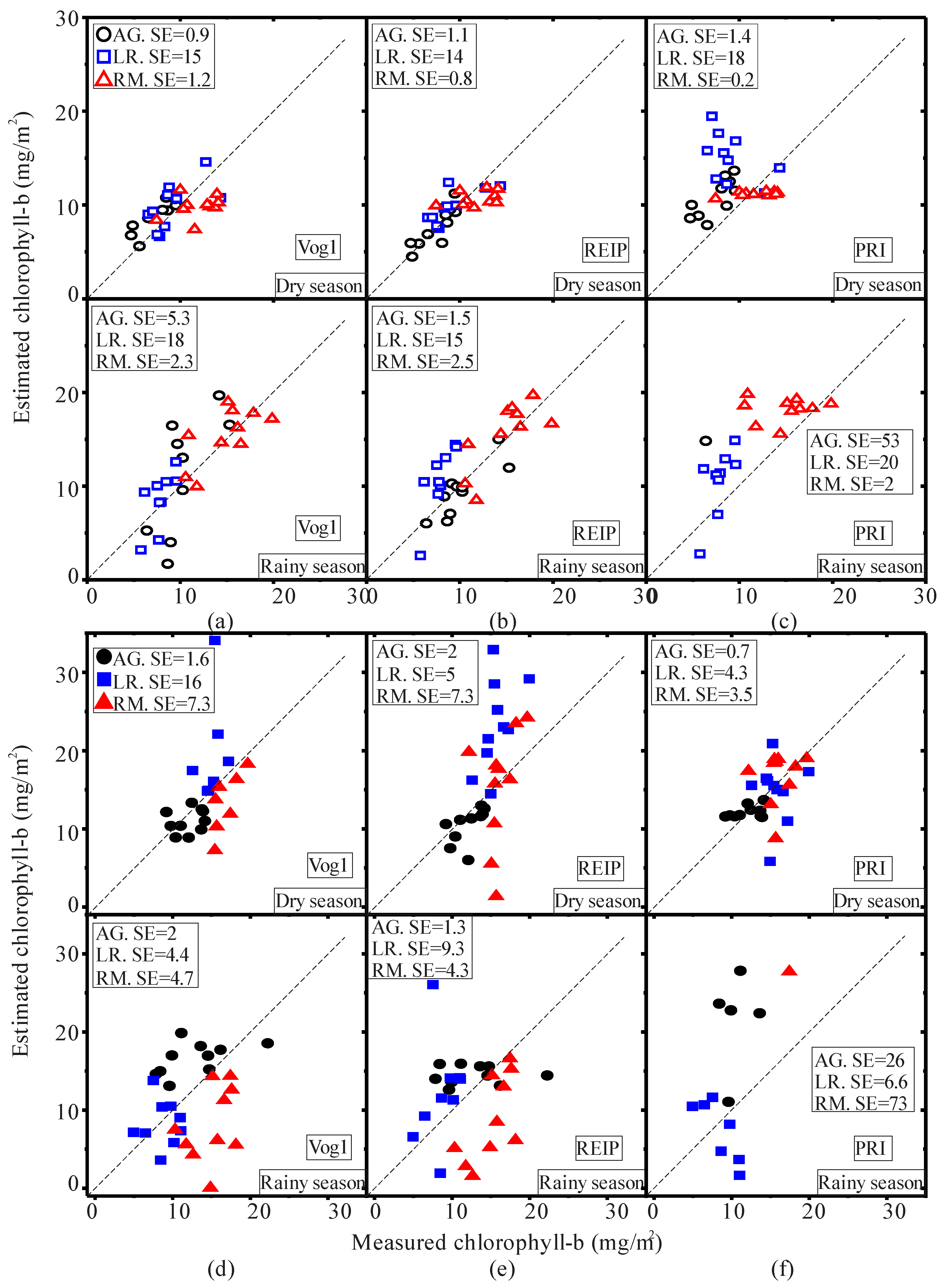
2.4. Statistical Analysis
3. Results
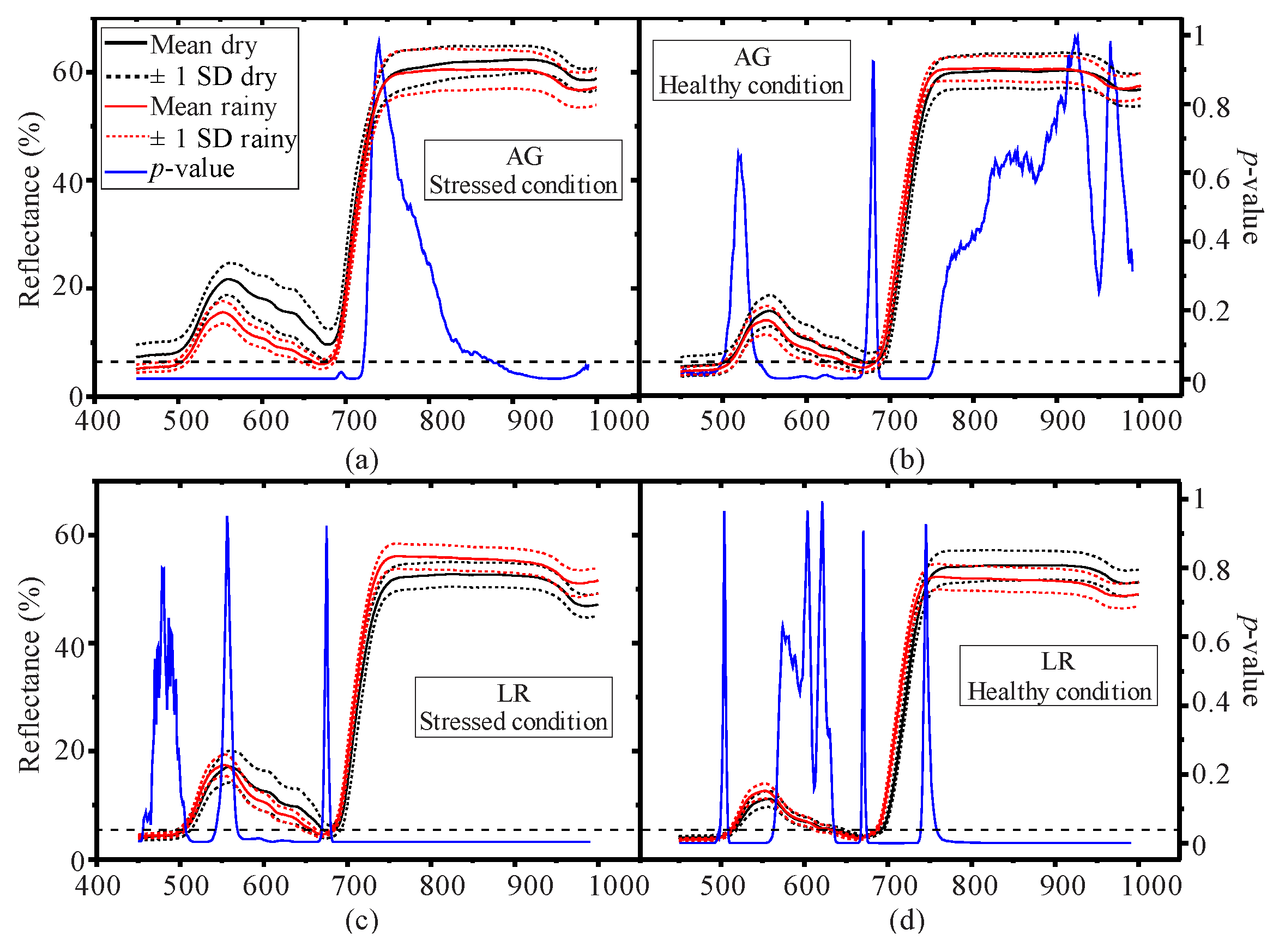
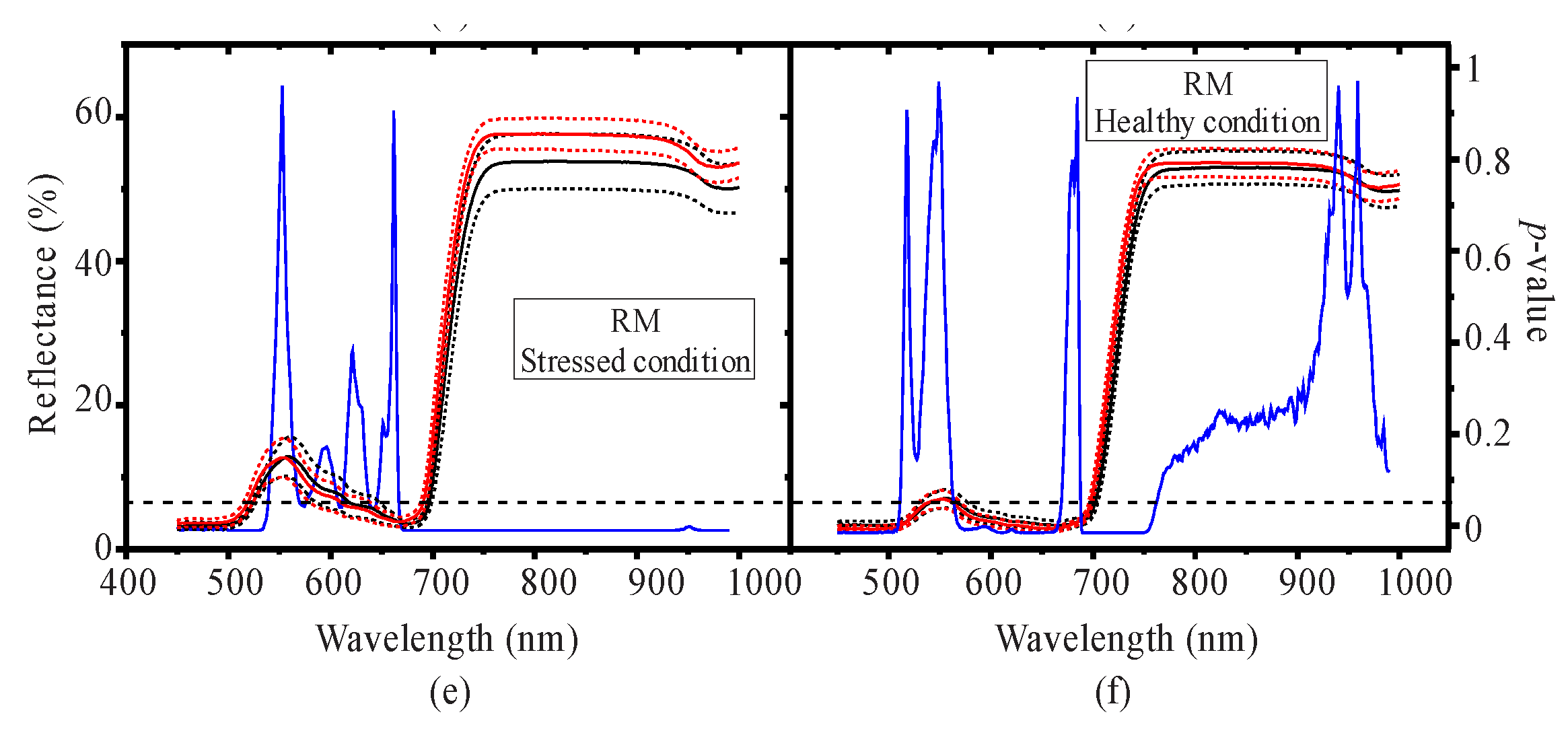
3.1. Seasonal Assessment of the Spectroscopy Data and Mangrove Leaf Pigments
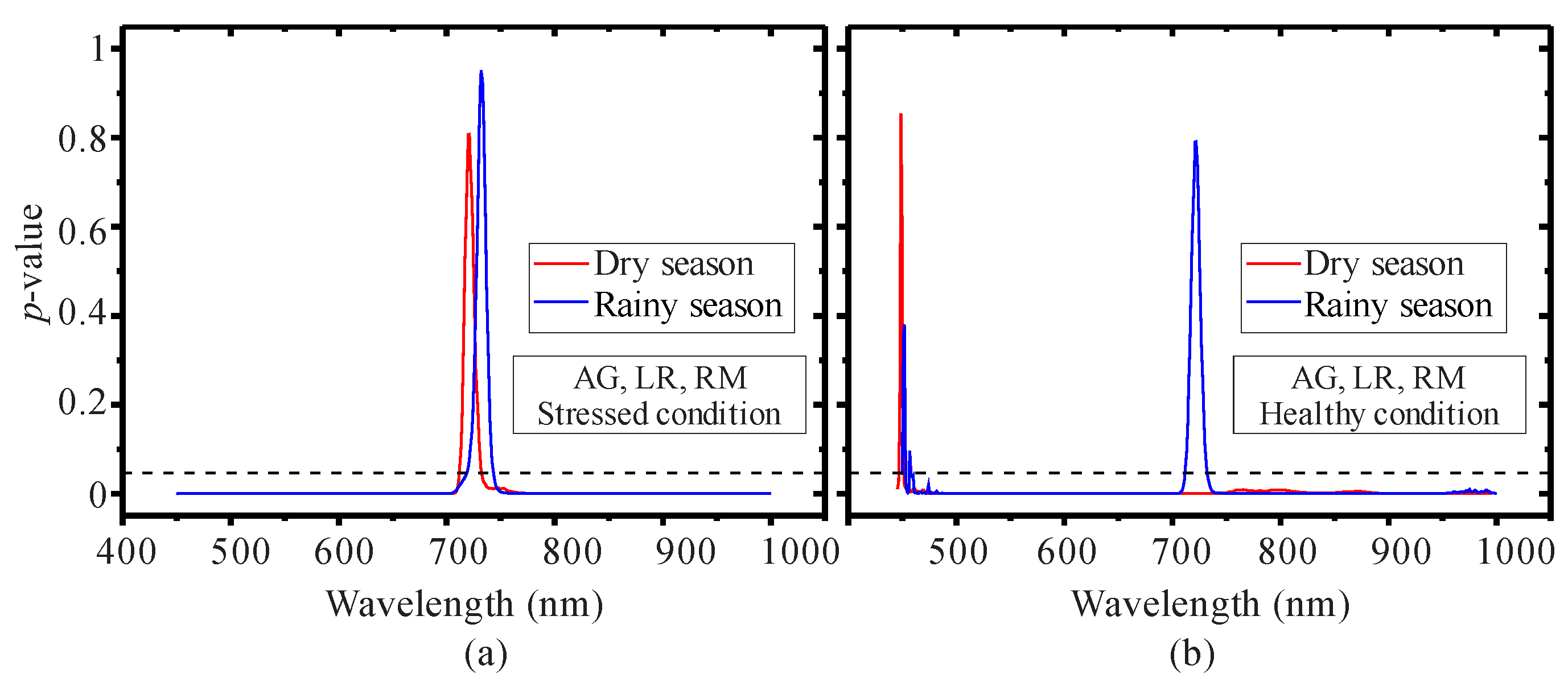
3.2. Seasonal Assessment of the Spectroscopy Correlations
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Blasco, F.; Saenger, P.; Janodet, E. Mangroves as indicators of coastal change. Catena 1996, 27, 167–178. [Google Scholar] [CrossRef]
- Saenger, P. Mangrove Ecology, Silviculture, and Conservation, 1st ed.; Kluwer Academic Publisher: London, UK, 2002. [Google Scholar]
- Walters, B.B.; Rönnbäck, P.; Kovacs, J.M.; Crona, B.; Hussain, A.; Badola, R.; Dahdouh-Guebas, F.; Barbier, E. Ethnobiology, socio-economics and management of mangrove forests: A review. Aquat. Bot. 2008, 89, 220–236. [Google Scholar] [CrossRef]
- Flores-Verdugo, F.; Day, J.W.; Briseño-Dueñas, R. Structure, litter fall, decomposition, and detritus dynamics of mangroves in a Mexican coastal lagoon with an ephemeral inlet. Mar. Ecol. Prog. Ser. 1987, 35, 83–90. [Google Scholar] [CrossRef]
- Bashan, Y.; Holguin, G. Plant growth-promoting bacteria: A potential tool for arid mangrove reforestation. Trees 2002, 16, 159–166. [Google Scholar] [CrossRef]
- Kristensen, E.; Bouillon, S.; Dittmar, T.; Marchand, C. Organic carbon dynamics in mangrove ecosystems: A review. Aquat. Bot. 2008, 89, 201–219. [Google Scholar] [CrossRef]
- Dittmar, T.; Hertkorn, N.; Kattner, G.; Lara, R.J. Mangroves, a major source of dissolved organic carbon to the oceans. Global Biogeochem. Cycles 2006, 20, GB1012. [Google Scholar] [CrossRef]
- Raven, P.H.; Evert, R.F.; Eichhorn, S.E. Biology of Plants, 8th ed.; Worth Publishers: New York, NY, USA, 2012. [Google Scholar]
- Feller, I.C.; Whigham, D.F.; O’Neill, J.P.; McKee, K.L. Effects of nutrient enrichment on within-stand cycling in a mangrove forest. Ecology 1999, 80, 2193–2205. [Google Scholar] [CrossRef]
- Gilman, E.L.; Ellison, J.; Duke, N.C.; Field, C. Threats to mangroves from climate change and adaptation options: A review. Aquat. Bot. 2008, 89, 237–250. [Google Scholar] [CrossRef]
- Valiela, I.; Bowen, J.L.; York, J.K. Mangrove forest: One of the worlds threatened major tropical environments. BioScience 2001, 51, 807–815. [Google Scholar] [CrossRef]
- Polidoro, B.A.; Carpenter, K.E.; Collis, L.; Duke, N.C.; Ellison, A.M.; Ellison, J.C.; Farnsworth, E.J.; Fernando, E.S.; Kathiresan, K.; Koedam, N.E.; et al. The loss of species: Mangrove extinction risk and geographic areas of global concern. PLoS ONE 2010, 5, e10095. [Google Scholar] [CrossRef] [PubMed]
- Duke, N.C.; Meynecke, J.O.; Dittman, S.; Ellison, A.M.; Anger, K.; Berger, U.; Cannicci, S.; Diele, K.; Ewel, K.C.; Field, C.D.; et al. A world without mangroves? Science 2007, 317, 41–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heumann, B.W. Satellite remote sensing of mangrove forests: Recent advances and future opportunities. Prog. Phys. Geog. 2011, 35, 87–108. [Google Scholar] [CrossRef]
- Kuenzer, C.; Bluemel, A.; Gebhardt, S.; Quoc, T.V.; Dech, S. Remote sensing of mangrove ecosystems: A review. Remote Sens. 2011, 3, 878–928. [Google Scholar] [CrossRef]
- Flores-de-Santiago, F.; Kovacs, J.M.; Lafrance, P. An object-oriented classification method for mapping mangroves in Guinea, West Africa, using multipolarized ALOS PALSAR L-band data. Int. J. Remote Sens. 2013, 34, 563–586. [Google Scholar] [CrossRef]
- Kovacs, J.M.; Jiao, X.; Flores-de-Santiago, F.; Zhang, C.; Flores-Verdugo, F. Assessing relationships between Radarsat-2 C-band and structural parameters of a degraded mangrove forest. Int. J. Remote Sens. 2013, 34, 7002–7019. [Google Scholar] [CrossRef]
- Kovacs, J.M.; Lu, X.X.; Flores-Verdugo, F.; Zhang, C.; Flores-de-Santiago, F.; Jiao, X. Applications of ALOS PALSAR for monitoring biophysical parameters of a degraded black mangrove (Avicennia germinans) forest. ISPRS J. Photogramm. Remote Sens. 2013, 82, 102–111. [Google Scholar] [CrossRef]
- Souza-Filho, P.W.M.; Paradella, W.R. Recognition of the main geobotanical features along the Braganca mangrove coast (Brazilian Amazon region) from Landsat TM and RADARSAT-a data. Wetlands Ecol. Manag. 2002, 10, 121–130. [Google Scholar] [CrossRef]
- Sulong, I.; Mohd-Lokman, H.; Mohd-Tarmizi, K.; Ismail, A. Mangrove mapping using Landsat imagery and aerial photographs: Kemaman district, Terengganu, Malaysia. Environ. Dev. Sustain. 2002, 4, 135–152. [Google Scholar] [CrossRef]
- Wang, Y.; Bonynge, G.; Nugranad, J.; Traber, M.; Ngusaru, A.; Tobey, J.; Hale, L.; Bowen, R.; Makota, V. Remote sensing of mangrove change along the Tanzania coast. Mar. Geod. 2003, 26, 35–48. [Google Scholar] [CrossRef]
- Godstime, K.J.; Adegoke, J.O.; Saba, E.; Nwilo, P.; Akinyede, J. Satellite-based assessment of the extent and changes in the mangrove ecosystem of the Niger delta. Mar. Geod. 2007, 30, 249–267. [Google Scholar]
- Satyanarayana, B.; Mohamad, K.A.; Idris, I.F.; Husain, M.L.; Dahdouh-Guebas, F. Assessment of mangrove vegetation based on remote sensing and ground-truth measurements at Tumpat, Kelantan Delta, East Coast of Peninsular Malaysia. Int. J. Remote Sens. 2011, 32, 1635–1650. [Google Scholar] [CrossRef]
- Vaiphasa, C.; Ongsomwang, S.; Vaiphasa, T.; Skidmore, A.K. Tropical mangrove species discrimination using hyperspectral data: A laboratory study. Estuar. Coast. Shelf Sci. 2005, 65, 371–379. [Google Scholar] [CrossRef]
- Wang, L.; Sousa, W.P. Distinguishing mangrove species with laboratory measurements of hyperspectral leaf reflectance. Int. J. Remote Sens. 2009, 30, 1267–1281. [Google Scholar] [CrossRef]
- Panigrahy, S.; Kumar, T.; Manjunath, K.R. Hyperspectral leaf signature as an added dimension for species discrimination: Case of study of four tropical mangroves. Wetlands Ecol. Manag. 2012, 20, 101–110. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, Y.; Kovacs, J.M.; Flores-Verdugo, F.; Flores-de-Santiago, F.; Chen, K. Spectral response to varying levels of leaf pigments collected from a degraded mangrove forest. J. Appl. Remote Sens. 2012, 6, 063501. [Google Scholar]
- Zhang, C.; Kovacs, J.M.; Wachowiak, M.P.; Flores-Verdugo, F. Relationship between hyperspectral measurements and mangrove leaf nitrogen concentrations. Remote Sens. 2013, 5, 891–908. [Google Scholar] [CrossRef]
- Flores-de-Santiago, F.; Kovacs, J.M.; Flores-Verdugo, F. The influence of seasonality in estimating mangrove leaf chlorophyll-a content from hyperspectral data. Wetlands Ecol. Manag. 2013, 21, 193–207. [Google Scholar] [CrossRef]
- Zhang, C.; Kovacs, J.M.; Liu, Y.; Flores-Verdugo, F.; Flores-de-Santiago, F. Separating mangrove species and conditions using laboratory hyperspectral data: A case of study of a degraded mangrove forest of the Mexican Pacific. Remote Sens. 2014, 6, 11673–11688. [Google Scholar] [CrossRef]
- Cochrane, M.A. Using vegetation reflectance variability for species level classification of hyperspectral data. Int. J. Remote Sens. 2000, 21, 2075–2087. [Google Scholar] [CrossRef]
- Vaiphasa, C.; Skidmore, A.K.; de Boer, W.F.; Vaiphasa, T. A hyperspectral band selector for plant species discrimination. ISPRS J. Photogramm. Remote Sens. 2007, 62, 225–235. [Google Scholar] [CrossRef]
- Hopkins, W.G.; Hüner, N.P.A. Introduction to Plant Physiology, 4th ed.; John Wiley & Sons: New York, NY, USA, 2008. [Google Scholar]
- Jensen, J.R. Introductory Digital Image Processing, 3rd ed.; Pearson Education, Inc.: New York, NY, USA, 2005. [Google Scholar]
- Sims, D.A.; Gamon, J.A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Peñuelas, J.; Filella, I. Visible and near-infrared reflectance techniques for diagnosing plant physiological status. Trends Plant Sci. 1998, 3, 151–156. [Google Scholar] [CrossRef]
- Blackburn, G.A. Relationships between spectral reflectance and pigment concentrations in stack of deciduous broadleaves. Remote Sens. Environ. 1999, 70, 224–237. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Miller, J.R.; Noland, T.L.; Mohammed, G.H.; Sampson, P.H. Scaling-up and model inversion methods with narrowband optical indices for chlorophyll content estimation in closed forest canopies with hyperspectral data. IEEE Trans. Geosci. Remote Sens. 2001, 39, 1491–1506. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Pushnik, J.C.; Dobrowski, S.; Ustin, S.L. Steady state chlorophyll a fluorescence detection from canopy derivative reflectance and double-peak red edge effects. Remote Sens. Environ. 2003, 84, 283–294. [Google Scholar] [CrossRef]
- Wu, C.; Niu, Z.; Tang, Q.; Huang, W. Estimating chlorophyll content from hyperspectral vegetation indices: Modeling and validation. Agric. For. Meteorol. 2008, 148, 1230–1241. [Google Scholar] [CrossRef]
- Blackburn, G.A. Quantifying chlorophylls and carotenoids at leaf level and canopy scales: An evaluation of some hyperspectral approaches. Remote Sens. Environ. 1998, 66, 273–285. [Google Scholar] [CrossRef]
- Merzlyak, M.N.; Solovchenko, A.E.; Gitelson, A.A. Reflectance spectral features and non-destructive estimation of chlorophyll, carotenoid and anthocyanin content in apple fruit. Postharvest Biol. Technol. 2003, 27, 197–211. [Google Scholar] [CrossRef]
- Vittorio, A.V.D. Enhancing leaf radiative transfer model to estimate concentrations and in vivo specific absorption coefficients of total carotenoids and chlorophylls a and b from single-needle reflectance and transmittance. Remote Sens. Environ. 2009, 113, 1948–1966. [Google Scholar] [CrossRef]
- Garrity, S.R.; Eitel, J.U.H.; Vierling, L.A. Disentangling the relationships between plant pigments and the photochemical reflectance index reveals a new approach for remote estimation of carotenoid content. Remote Sens. Environ. 2011, 115, 628–635. [Google Scholar] [CrossRef]
- Dale, M.P.; Causton, D.R. Use of the chlorophyll a/b ratio as a bioassay for the light environment of a plant. Funct. Ecol. 1992, 6, 190–196. [Google Scholar] [CrossRef]
- Li, G.; Wan, S.; Zhou, J.; Yang, Z.; Qin, P. Leaf chlorophyll fluorescence, hyperspectral reflectance, pigments content, malondialde-hyde and proline accumulation responses of castor bean (Ricinus communis L.) seedlings to salt stress levels. Ind. Crop. Prod. 2010, 31, 13–19. [Google Scholar] [CrossRef]
- Main, R.; Cho, M.A.; Mathieu, R.; O’Kennedy, M.M.; Ramoelo, A.; Koch, S. An investigation into robust spectral indices for leaf chlorophyll estimation. ISPRS J. Photogramm. Remote Sens. 2011, 66, 751–761. [Google Scholar] [CrossRef]
- Flores-de-Santiago, F.; Kovacs, J.M.; Flores-Verdugo, F. Seasonal changes in leaf chlorophyll a content and morphology in a sub-tropical mangrove forest of the Mexican Pacific. Mar. Ecol. Prog. Ser. 2012, 444, 57–68. [Google Scholar] [CrossRef]
- Villalba, L.A. Descripción general del estero de Urías, Mazatlán, Sinaloa. Cienc. Mar. 1986, 8, 32–37. [Google Scholar]
- Arreola-Lizarraga, J.A.; Flores-Verdugo, F.J.; Ortega-Rubio, A. Structure and litterfall of an arid mangrove stand on the Gulf of California, Mexico. Aquat. Bot. 2004, 79, 137–143. [Google Scholar] [CrossRef]
- Kovacs, J.M.; Liu, Y.; Zhang, C.; Flores-Verdugo, F.; Flores-de-Santiago, F. A field based statistical approach for validating a remotely sensed mangrove forest classification scheme of Isla La Palma, Sinaloa, Mexico. Wetlands Ecol. Manag. 2011, 19, 409–421. [Google Scholar] [CrossRef]
- Flores-de-Santiago, F.; Kovacs, J.M.; Flores-Verdugo, F. Assessing the utility of a portable pocket instrument for estimating seasonal mangrove leaf chlorophyll contents. Bull. Mar. Sci. 2013, 89, 621–633. [Google Scholar] [CrossRef]
- Biber, P.D. Evaluating a chlorophyll content meter on three coastal wetland plant species. Agric. Food Environ. Sci. 2007, 1, 1–11. [Google Scholar]
- Lichtenthaler, H.K.; Wellburn, A.R. Determination of total carotenoids and chlorophylls a and b in leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Chatfield, C. Fitting time-series models in the time domain. In The Analysis of Time Series an Introduction, 6th ed.; Chatfield, C., Ed.; Chapman & Hall: London, UK, 1996; Volume 4, pp. 49–53. [Google Scholar]
- Uraibi, H.S.; Midi, H.; Talib, B.A.; Yousif, J.B. Linear regression model selection based on robust bootstrapping technique. Am. J. Appl. Sci. 2009, 6, 1191–1198. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B.; Mittra, B. Effects of salt on growth, ion accumulation, photosynthesis and leaf anatomy of the mangrove, Bruguiera parviflora. Trees 2004, 18, 167–174. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N. Spectral reflectance changes associated with autumn senescence of Aesculus hippocastanum L. and Acer platanoides L. leaves. Spectral features and relation to chlorophyll estimation. J. Plant Physiol. 1994, 143, 286–292. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N.; Grits, Y. Novel algorithms for remote sensing of chlorophyll content in higher plants. IEEE Trans. Geosci. Remote Sens. 1996, 4, 2355–2357. [Google Scholar]
- Gitelson, A.A.; Kaufman, Y.J.; Merzlyak, M.N. Use of a green channel in remote sensing of global vegetation from EOS-MODIS. Remote Sens. Environ. 1996, 58, 289–298. [Google Scholar] [CrossRef]
- Gitelson, A.; Merzlyak, M.N.; Lichtenthaler, H.K. Detection of red edge position and chlorophyll content by reflectance measurements near 700 nm. J. Plant Physiol. 1996, 148, 501–508. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Gitelson, A.; Lang, M. Nondestructive determination of chlorophyll content of leaves of a green and an aurea mutant of tobacco by reflectance measurements. J. Plant Physiol. 1996, 148, 483–493. [Google Scholar] [CrossRef]
- Datt, B. Remote sensing of chlorophyll a, chlorophyll b, chlorophyll a + b, and total carotenoid content in Eucalyptus leaves. Remote Sens. Environ. 1998, 66, 111–121. [Google Scholar] [CrossRef]
- Held, A.; Ticehurst, C.; Lymburner, L.; Williams, N. High resolution mapping of tropical mangrove ecosystems using hyperspectral and radar remote sensing. Int. J. Remote Sens. 2003, 24, 2739–2759. [Google Scholar] [CrossRef]
- Hirano, A.; Madden, M.; Welch, R. Hyperspectral image data for mapping wetland vegetation. Wetlands 2003, 23, 436–448. [Google Scholar] [CrossRef]
- Yang, C.; Everitt, J.H.; Fletcher, R.S.; Jensen, R.R.; Mausel, P.W. Evaluating AISA + hyperspectral imagery for mapping black mangrove along the South Texas gulf coast. Photogramm. Eng. Remote Sens. 2009, 75, 425–435. [Google Scholar] [CrossRef]
- Kamal, M.; Phinn, S. Hyperspectral data for mangrove species mapping: A comparison of pixel-based and object-based approach. Remote Sens. 2011, 3, 2222–2242. [Google Scholar] [CrossRef]
- Das, A.B.; Parida, A.; Basak, U.C.; Das, P. Studies on pigments, proteins and photosynthetic rates in some mangroves and mangrove associates from Bhitarkanika, Orissa. Mar. Biol. 2002, 141, 415–422. [Google Scholar]
- Snedaker, S.C.; Araujo, R.J. Stomatal conductance and gas exchange in four species of Caribbean mangrove exposed to ambient and increased CO2. Mar. Freshw. Res. 1998, 49, 325–327. [Google Scholar] [CrossRef]
- Tomlinson, P.B. The Botany of Mangrove; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- Wooller, M.; Smallwood, B.; Jacobson, M.; Fogel, M. Carbon and nitrogen stable isotopic variation in Laguncularia racemosa (L.) (white mangrove) from Florida and Belize: Implications for trophic level studies. Hydrobiologia 2003, 499, 13–23. [Google Scholar] [CrossRef]
- Sobrado, M.A. Leaf characteristics and gas exchange of the mangrove Laguncularia racemosa as affected by salinity. Photosynthetica 2005, 43, 217–221. [Google Scholar] [CrossRef]
- Beneragama, C.K.; Goto, K. Chlorophyll a:b ratio increases under low-light in “shade-tolerant” Euglena gracilis. Trop. Agric. Res. 2010, 22, 12–25. [Google Scholar]
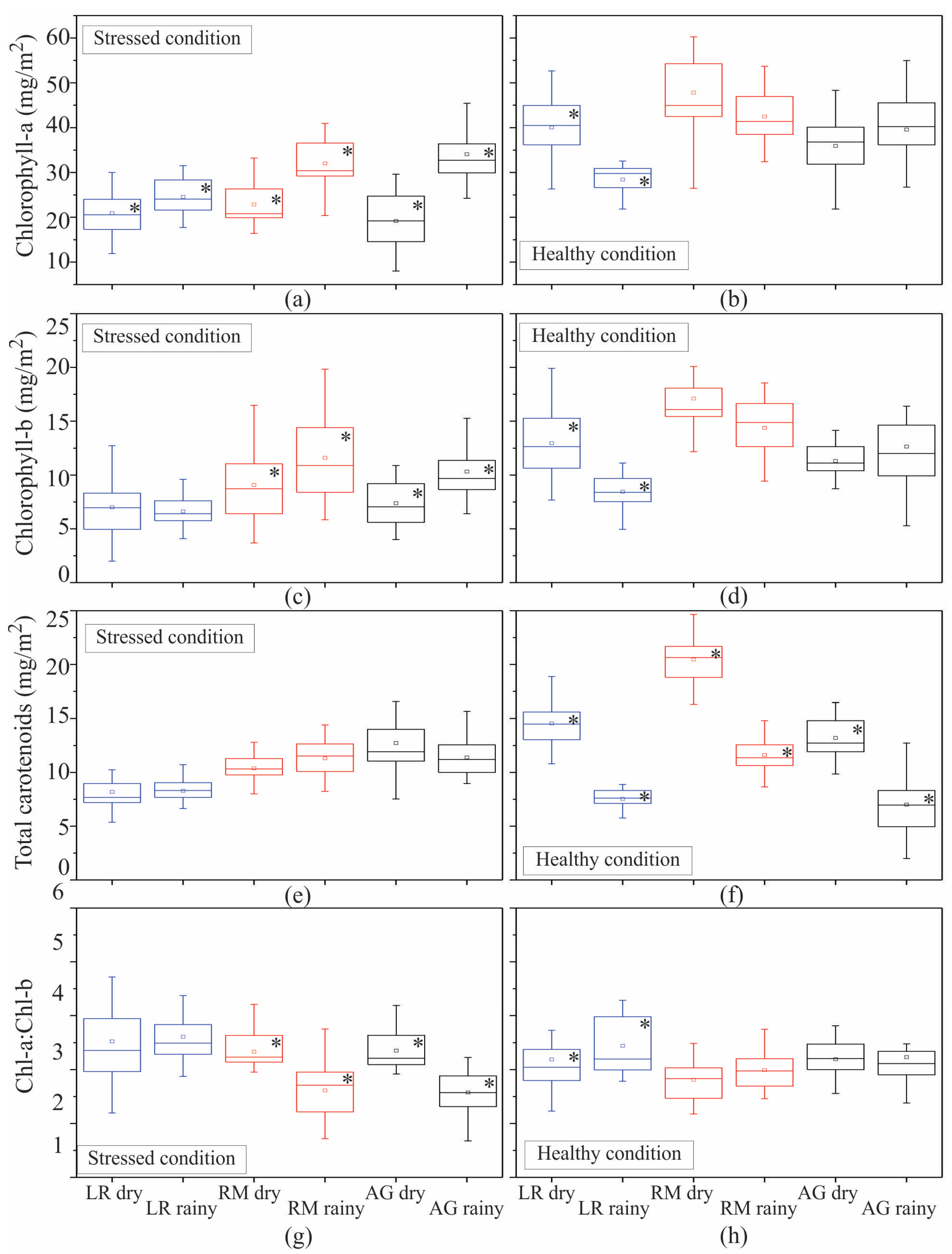
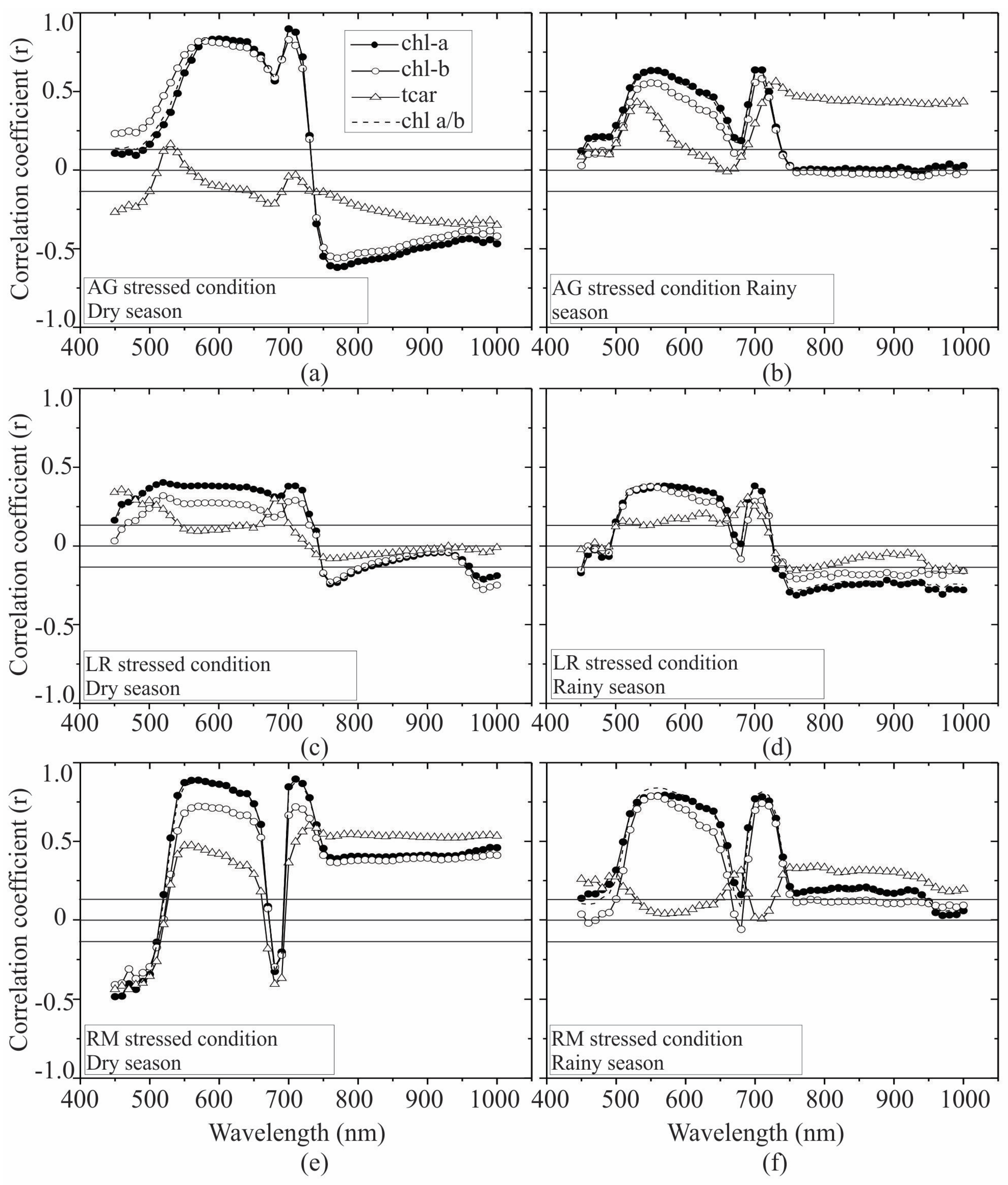
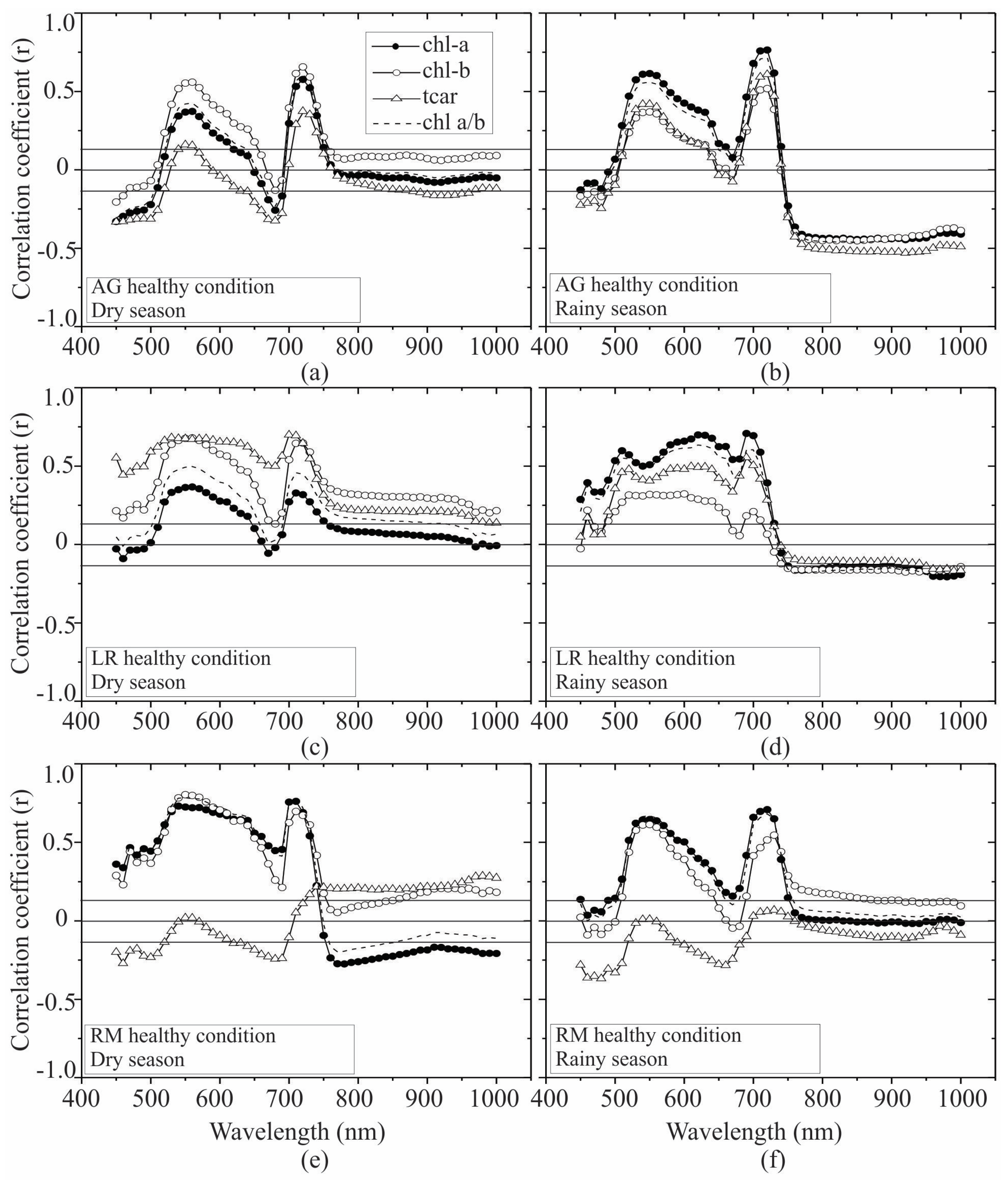
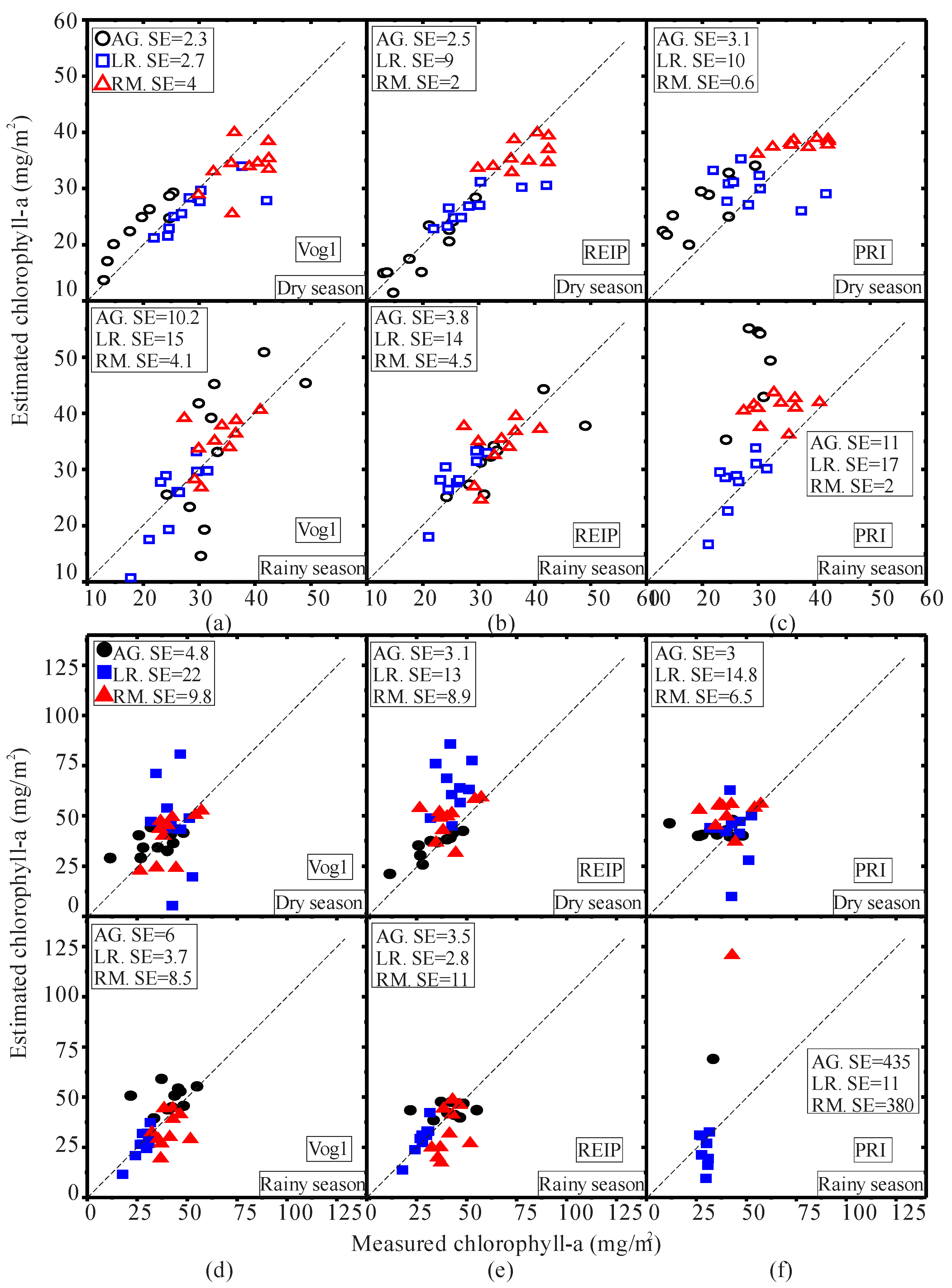
| Pigment | Condition | H | p |
|---|---|---|---|
| Chl-a | stressed | 19.6 * | 0 |
| Chl-b | stressed | 7.1 * | 0.03 |
| Tcar | stressed | 45.5 * | 0 |
| Chl a/b | stressed | 40.1 * | 0 |
| Chl-a | healthy | 26.2 * | 0 |
| Chl-b | healthy | 42.9 * | 0 |
| Tcar | healthy | 58.7 * | 0 |
| Chl a/b | healthy | 13.5 * | 0.001 |
| Pigment | Condition | H | p |
|---|---|---|---|
| Chl-a | stressed | 42.9 * | 0 |
| Chl-b | stressed | 43 * | 0 |
| Tcar | stressed | 48 * | 0 |
| Chl a/b | stressed | 43.4 * | 0 |
| Chl-a | healthy | 50 * | 0 |
| Chl-b | healthy | 46.3 * | 0 |
| Tcar | healthy | 51 * | 0 |
| Chl a/b | healthy | 8.7 * | 0.013 |
| Pigment | Season | Species | VI | Regression Equation | R2 |
|---|---|---|---|---|---|
| AG | Vog1 | 0.9483 − 0.0122X | 0.85 * | ||
| Dry | AG | REIP | −6.56 + 0.732X | 0.66 * | |
| AG | PRI | 0.15 − 0.0037X | 0.51 * | ||
| LR | Vog1 | 0.9672 − 0.0116X | 0.30 * | ||
| Dry | LR | REIP | −6.981 + 0.614X | 0.35 * | |
| LR | PRI | 0.1293 − 0.0029X | 0.32 * | ||
| RM | Vog1 | 0.8316 − 0.0064X | 0.53 * | ||
| Dry | RM | REIP | −4.139 + 0.5007X | 0.82 * | |
| chl-a | RM | PRI | 0.21 − 0.0048X | 0.55 * | |
| AG | Vog1 | 0.763 − 0.003X | 0.10 | ||
| Rainy | AG | REIP | −13.26 + 0.44X | 0.47 * | |
| AG | PRI | 0.029 − 0.0003X | 0.01 | ||
| LR | Vog1 | 0.8738 − 0.0067X | 0.30 * | ||
| Rainy | LR | REIP | −24.38 + 0.786X | 0.40 * | |
| LR | PRI | 0.028 − 0.0012X | 0.22 * | ||
| RM | Vog1 | 0.868 − 0.0066X | 0.54 * | ||
| Rainy | RM | REIP | −28.96 + 0.95X | 0.61 * | |
| RM | PRI | 0.06 − 0.0017X | 0.33 * | ||
| AG | Vog1 | 0.9784 − 0.035X | 0.68 * | ||
| Dry | AG | REIP | −6.47 + 1.849X | 0.40 * | |
| AG | PRI | 0.15 − 0.009X | 0.29 * | ||
| LR | Vog1 | 0.8444 − 0.019X | 0.15 | ||
| Dry | LR | REIP | −0.84 + 1.05X | 0.20 | |
| LR | PRI | 0.09 − 0.0034X | 0.13 | ||
| RM | Vog1 | 0.831 − 0.02196X | 0.48 * | ||
| Dry | RM | REIP | −3.418 + 1.623X | 0.67 * | |
| chl-b | RM | PRI | 0.21 − 0.016X | 0.52 * | |
| AG | Vog1 | 0.7096 − 0.0058X | 0.06 | ||
| Rainy | AG | REIP | −7.88 + 0.94X | 0.40 * | |
| AG | PRI | 0.019 − 0.00006X | 0 | ||
| LR | Vog1 | 0.7906 − 0.0113X | 0.19 | ||
| Rainy | LR | REIP | −12.89 + 1.015X | 0.18 | |
| LR | PRI | 0.013 − 0.0017X | 0.09 | ||
| RM | Vog1 | 0.792 − 0.01X | 0.39 * | ||
| Rainy | RM | REIP | −16.24 + 1.27X | 0.34 * | |
| RM | PRI | 0.046 − 0.003X | 0.33 * | ||
| AG | Vog1 | 0.8558 − 0.0097X | 0.06 | ||
| Dry | AG | REIP | 0.825 + 0.4549X | 0.03 | |
| AG | PRI | 0.079 + 0.00045X | 0.01 | ||
| LR | Vog1 | 0.7469 − 0.0007X | 0.01 | ||
| Dry | LR | REIP | 5.089 − 0.0237X | 0.01 | |
| LR | PRI | 0.062 + 0.0012X | 0.02 | ||
| RM | Vog1 | 0.7103 − 0.0037X | 0.02 | ||
| Dry | RM | REIP | 6.706 + 0.1631X | 0.01 | |
| tcar | RM | PRI | 0.043 + 0.0043X | 0.06 | |
| AG | Vog1 | 0.478 + 0.01X | 0.19 | ||
| Rainy | AG | REIP | −0.57 + 0.212X | 0.01 | |
| AG | PRI | −0.031 + 0.004X | 0.30 | ||
| LR | Vog1 | 0.7705 − 0.0059X | 0.05 | ||
| Rainy | LR | REIP | −14.5 + 0.942X | 0.16 | |
| LR | PRI | 0.0022 − 0.00002X | 0 | ||
| RM | Vog1 | 0.704 − 0.001X | 0.02 | ||
| Rainy | RM | REIP | −6.32 + 0.25X | 0.05 | |
| RM | PRI | 0.006 + 0.0009X | 0.01 |
| Pigment | Season | Species | VI | Regression Equation | R2 |
|---|---|---|---|---|---|
| AG | Vog1 | 0.7499 − 0.0043X | 0.47 * | ||
| Dry | AG | REIP | −1.235 + 0.4X | 0.68 * | |
| AG | PRI | 0.086 − 0.001X | 0.47 * | ||
| LR | Vog1 | 0.6255 − 0.0009X | 0.05 | ||
| Dry | LR | REIP | 11.24 + 0.0648X | 0.31 * | |
| LR | PRI | 0.0322 − 0.00016X | 0.11 | ||
| RM | Vog1 | 0.6875 − 0.0027X | 0.53 * | ||
| Dry | RM | REIP | 8.445 + 0.1774X | 0.73 * | |
| chl-a | RM | PRI | 0.089 − 0.0013X | 0.68 * | |
| AG | Vog1 | 0.765 − 0.0038X | 0.61 * | ||
| Rainy | AG | REIP | −17.92 + 0.547X | 0.72 * | |
| AG | PRI | −0.0036 + 0.00001X | 0 | ||
| LR | Vog1 | 0.82 − 0.0047X | 0.66 * | ||
| Rainy | LR | REIP | −13.66 + 0.5X | 0.54 * | |
| LR | PRI | −0.002 − 0.0003X | 0.15 | ||
| RM | Vog1 | 0.727 − 0.003X | 0.37 * | ||
| Rainy | RM | REIP | −1.37 + 0.28X | 0.57 * | |
| RM | PRI | −0.01 + 0.000009X | 0 | ||
| AG | Vog1 | 0.7565 − 0.0148X | 0.55 * | ||
| Dry | AG | REIP | −0.041 + 1.219X | 0.62 * | |
| AG | PRI | 0.092 − 0.0053X | 0.61 * | ||
| LR | Vog1 | 0.6058 − 0.00129X | 0.02 | ||
| Dry | LR | REIP | 12.07 + 0.1436X | 0.21 * | |
| LR | PRI | 0.035 − 0.0007X | 0.29 * | ||
| RM | Vog1 | 0.6021 − 0.0031X | 0.20 * | ||
| Dry | RM | REIP | 13.75 + 0.214X | 0.31 * | |
| chl-b | RM | PRI | 0.062 − 0.0024X | 0.62 * | |
| AG | Vog1 | 0.759 − 0.011X | 0.63 * | ||
| Rainy | AG | REIP | −16.2 + 1.5X | 0.69 * | |
| AG | PRI | −0.0007 − 0.0002X | 0.05 | ||
| LR | Vog1 | 0.75 − 0.0076X | 0.26 * | ||
| Rainy | LR | REIP | −2.47 + 0.39X | 0.05 | |
| LR | PRI | −0.006 − 0.0005X | 0.07 | ||
| RM | Vog1 | 0.665 − 0.0056X | 0.21 * | ||
| Rainy | RM | REIP | 2.6 + 0.59X | 0.46 * | |
| RM | PRI | −0.01 + 0.000048X | 0.01 | ||
| AG | Vog1 | 0.6834 − 0.0068X | 0.08 | ||
| Dry | AG | REIP | −1.856 + 1.15X | 0.36 | |
| AG | PRI | 0.053 − 0.0015X | 0.03 | ||
| LR | Vog1 | 0.6347 − 0.0033X | 0.03 | ||
| Dry | LR | REIP | 13.12 + 0.046X | 0.06 | |
| LR | PRI | 0.029 − 0.00021X | 0.07 | ||
| RM | Vog1 | 0.5874 − 0.002X | 0.04 | ||
| Dry | RM | REIP | 15.03 + 0.126X | 0.05 | |
| tcar | RM | PRI | 0.042 − 0.0011X | 0.05 | |
| AG | Vog1 | 0.729 − 0.01X | 0.2 | ||
| Rainy | AG | REIP | −14.99 + 1.76X | 0.10 | |
| AG | PRI | −0.018 + 0.002X | 0.14 | ||
| LR | Vog1 | 0.78 − 0.013X | 0.2 | ||
| Rainy | LR | REIP | −9.46 + 1.39X | 0.02 | |
| LR | PRI | −0.009 − 0.0002X | 0.06 | ||
| RM | Vog1 | 0.548 + 0.003X | 0.02 | ||
| Rainy | RM | REIP | 9.15 + 0.15X | 0.01 | |
| RM | PRI | −0.036 + 0.0023X | 0.04 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores-de-Santiago, F.; Kovacs, J.M.; Wang, J.; Flores-Verdugo, F.; Zhang, C.; González-Farías, F. Examining the Influence of Seasonality, Condition, and Species Composition on Mangrove Leaf Pigment Contents and Laboratory Based Spectroscopy Data. Remote Sens. 2016, 8, 226. https://doi.org/10.3390/rs8030226
Flores-de-Santiago F, Kovacs JM, Wang J, Flores-Verdugo F, Zhang C, González-Farías F. Examining the Influence of Seasonality, Condition, and Species Composition on Mangrove Leaf Pigment Contents and Laboratory Based Spectroscopy Data. Remote Sensing. 2016; 8(3):226. https://doi.org/10.3390/rs8030226
Chicago/Turabian StyleFlores-de-Santiago, Francisco, John M. Kovacs, Jinfei Wang, Francisco Flores-Verdugo, Chunhua Zhang, and Fernando González-Farías. 2016. "Examining the Influence of Seasonality, Condition, and Species Composition on Mangrove Leaf Pigment Contents and Laboratory Based Spectroscopy Data" Remote Sensing 8, no. 3: 226. https://doi.org/10.3390/rs8030226







