Selecting Canopy Zones and Thresholding Approaches to Assess Grapevine Water Status by Using Aerial and Ground-Based Thermal Imaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Design
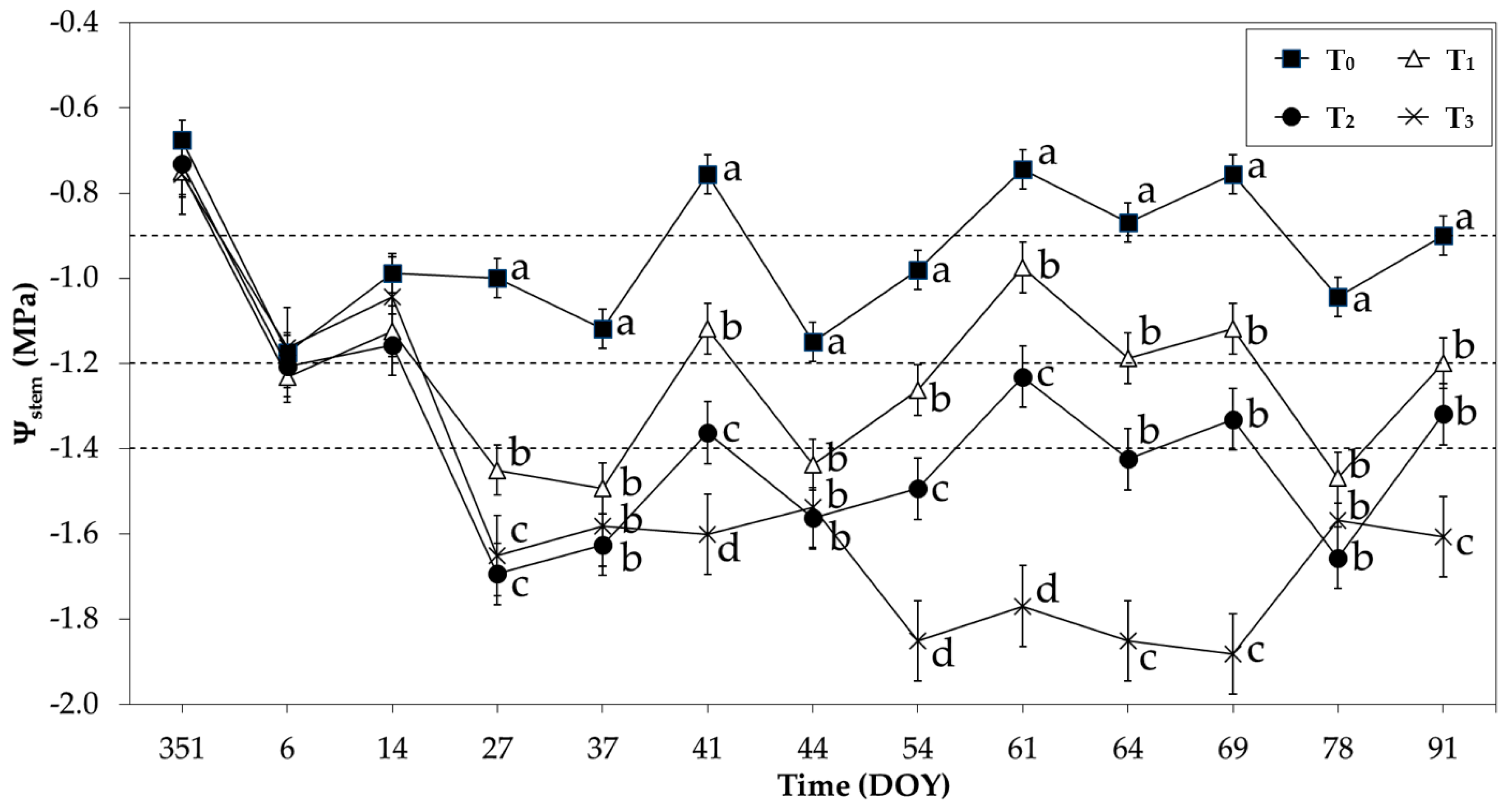
2.3. Plant-Based Variables
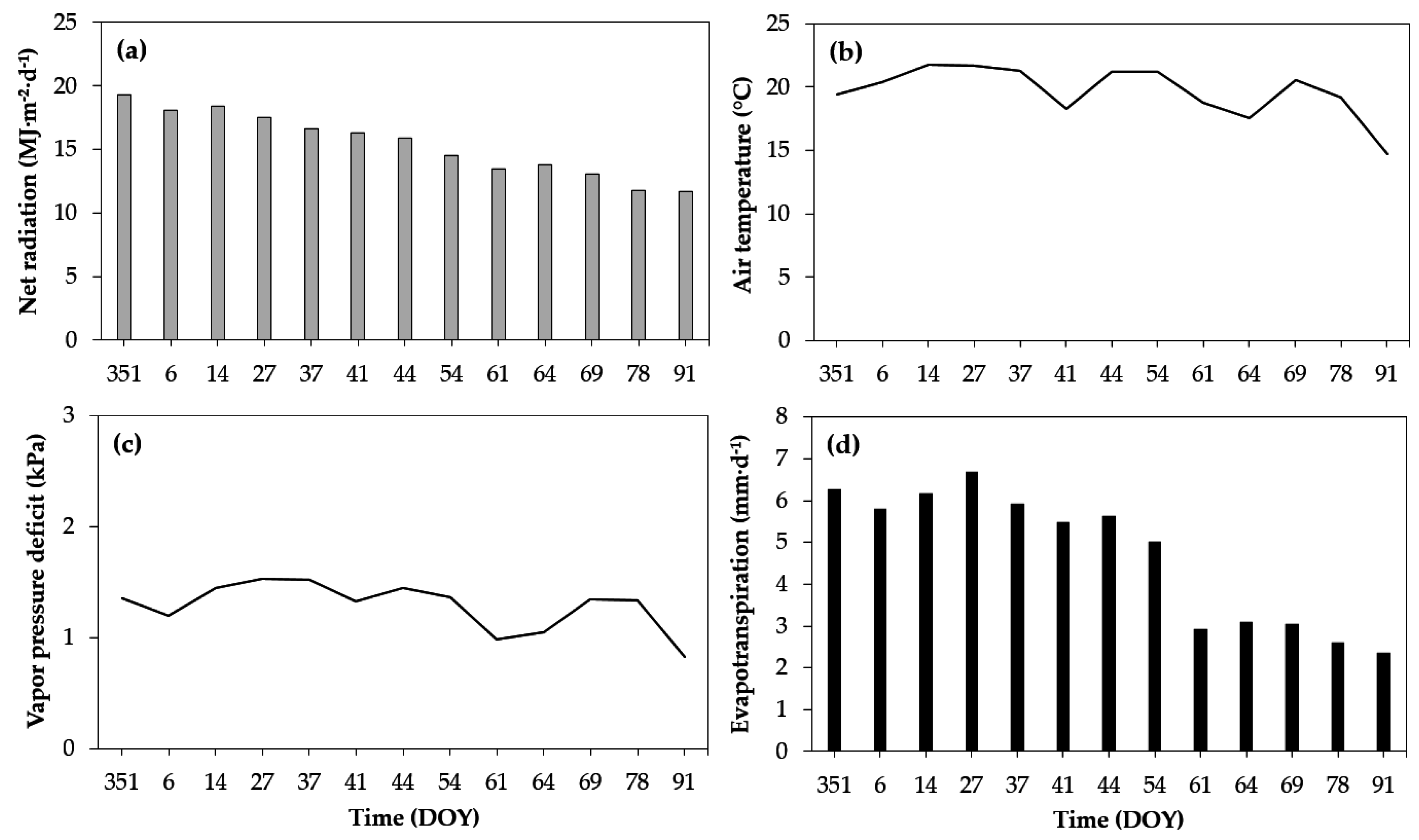
2.4. Meteorological Data
2.5. Aerial and Ground-Based Thermal Imaging
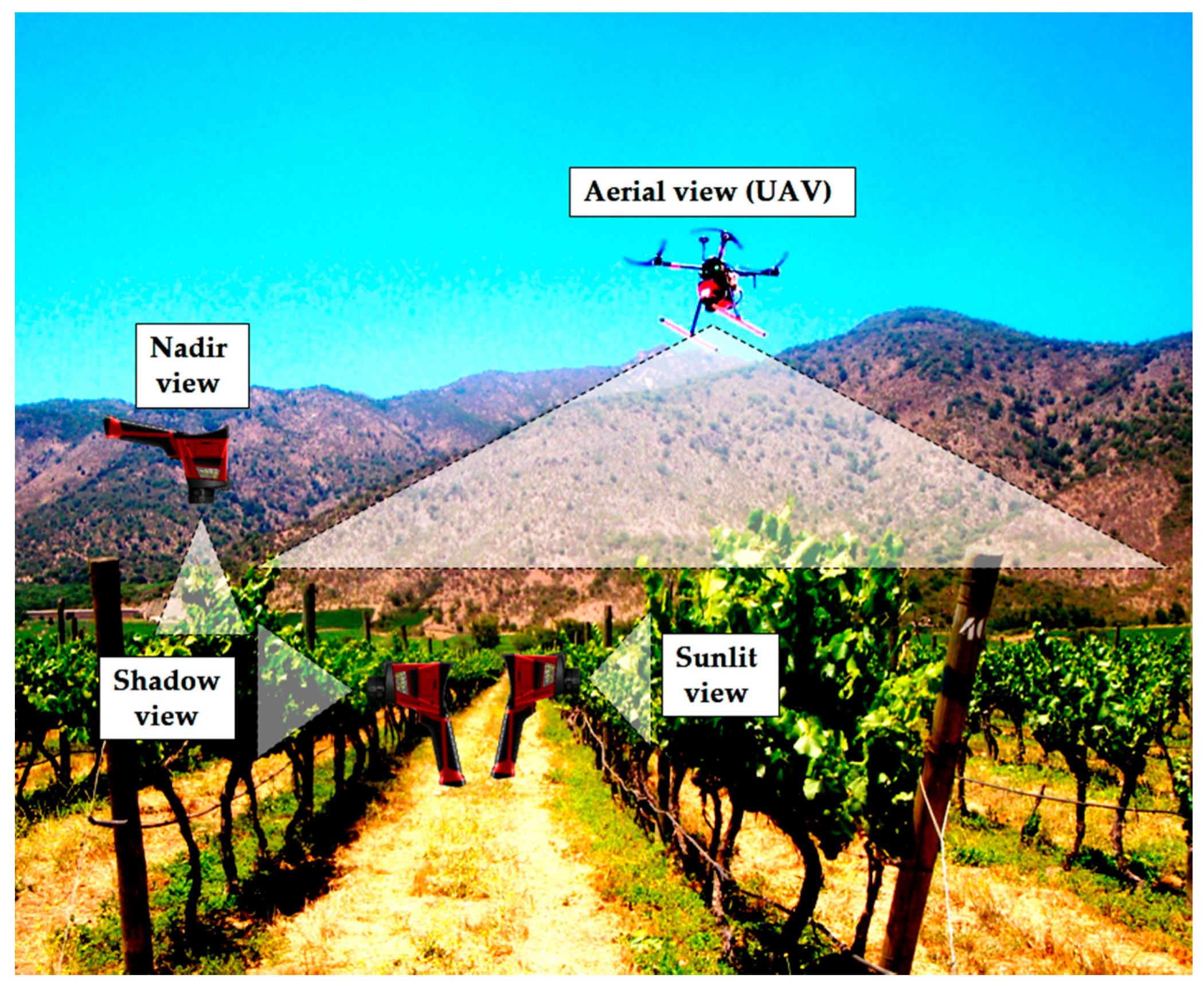
2.6. Canopy Zones and Views
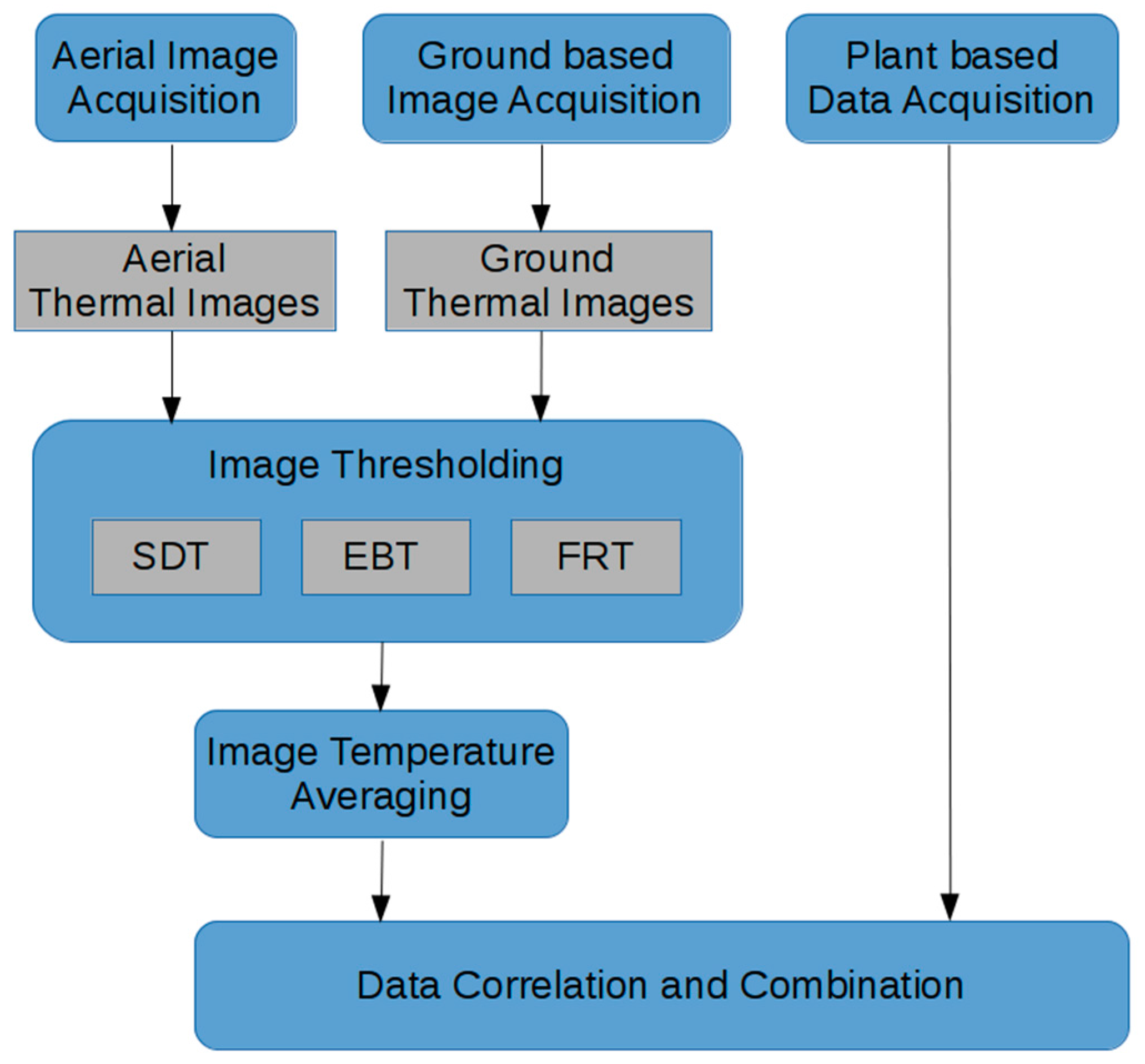
2.7. Image Analysis Processing
Thresholding Approaches
- (1)
- Standard deviation technique (SDT): In this technique we used the thermal data file with its unfiltered mean temperature (Tnf) and standard deviation (SD). In each thermal data file the lower temperature was obtained by subtracting a specific multiplier of SD from Tnf and an upper temperature adding another specific multiplier of SD from Tnf. The averages of lower and upper temperatures were calculated to estimate threshold temperature from each batch for a particular date. Specific multiplier values used for each canopy side were estimated using a relationship with threshold values obtained with the methodology described in Field Reference Temperature (see below).
- (2)
- Energy balance technique (EBT): In this case, temperature threshold values were determined using Equations (2) and (3) described below [23]:where, Ta is the air temperature (°C), rHR is the resistance to the radiative heat transfer (s∙m−1), raW is the boundary layer resistance for water vapor (s∙m−1), γ is the psychometric constant (Pa∙K−1), Rni is the net isothermal radiation (W∙m−2), ρa is the density of the air (Kg∙m−3), Cp is the specific heat of air (J∙kg−1∙K−1), and Δ is the slope of the saturation vapor pressure curve (Pa∙K−1). Ancillary information was obtained from an automatic micro-climatic station located near the experimental plot (described in Section 2.4). In this study, net isothermal radiation (Rni) was assumed equal to the net radiation [30,31]. Net radiation (W∙m−2), air temperature (°C), relative humidity (%), and wind velocity (m∙s−1) were used to estimate upper and lower temperatures. This method enabled the estimation of threshold temperatures for each acquisition image time.
- (3)
- Field reference temperature technique (FRT): This technique was proposed by Jones et al. [21]. Two mature and non-detached leaves were selected from canopy side (sunlit and shaded zone) to obtain upper and lower baseline temperatures. One leaf was painted with a solution of water and detergent (dish-washing soap) to obtain a Twet reference and the other leaf was painted with liquid petroleum jelly (Vaseline) to obtain a Tdry reference. Reference values from sunlit view were used for images captured from nadir view. These threshold temperatures were obtained with IrAnalyzer software (Wuhan Guide Infrared Co., Ltd., Wuhan, China) selecting an area of interest for reference leaves.
2.8. Statistical Analysis
3. Results
3.1. Description of Climatic Conditions
3.2. Results of Plant-Based Variables
3.3. Results of Thresholding Approaches
3.4. Relationship between CWSIs and Plant-Based Variables
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jones, H.G. Irrigation scheduling: Advantages and pitfalls of plant-based methods. J. Exp. Bot. 2004, 55, 2427–2436. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.G. The effect of transient water deficit on berry development of cv. Shiraz (Vitis vinifera L.). Aust. J. Grape Wine Res. 1997, 3, 2–8. [Google Scholar] [CrossRef]
- Peterlunger, E.; Sivilotti, P.; Bonetto, C.; Paladin, M. Water stress induces changes in polyphenol concentration in Merlot grapes and wines. Riv. Vitic. Enol. 2002, 1, 51–66. [Google Scholar]
- Acevedo, C.; Ortega-Farías, S.; Moreno, Y.; Córdova, F. Effects of different levels of water application in pre- and post-veraison on must composition and wine color (cv. Cabernet sauvignon). ISHS Acta Hortic. 2004, 664, 483–489. [Google Scholar] [CrossRef]
- Meza, L.; Corso, S.; Soza, S. Gestión del Riesgo de Sequía y Otros Eventos Climáticos Extremos en Chile; Organización de las Naciones Unidas para la Alimentación y la Agricultura (FAO): Santiago, Chile, 2010; p. 117. [Google Scholar]
- Romero, P.; Fernandez-Fernandez, J.I.; Martinez-Cutillas, A. Physiological thresholds for efficient regulated deficit-irrigation management in winegrapes grown under semiarid conditions. Am. J. Enol. Vitic. 2010, 61, 300–312. [Google Scholar]
- Fuentes, S.; Bei, R.; Pech, J.; Tyerman, S. Computational water stress indices obtained from thermal image analysis of grapevine canopies. Irrig. Sci. 2012, 30, 523–536. [Google Scholar] [CrossRef]
- Ballester, C.; Castel, J.; Jiménez-Bello, M.A.; Castel, J.R.; Intrigliolo, D.S. Thermographic measurement of canopy temperature is a useful tool for predicting water deficit effects on fruit weight in citrus trees. Agric. Water Manag. 2013, 122, 1–6. [Google Scholar] [CrossRef]
- Costa, J.M.; Grant, O.M.; Chaves, M.M. Thermography to explore plant–environment interactions. J. Exp. Bot. 2013, 64, 3937–3949. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.G. Use of thermography for quantitative studies of spatial and temporal variation of stomatal conductance over leaf surfaces. Plant Cell Environ. 1999, 22, 1043–1055. [Google Scholar] [CrossRef]
- Poblete-Echeverría, C.; Ortega-Farías, S.; Zuñiga, M.; Lobos, G.A.; Romero, S.; Estrada, F.; Fuentes, S. Use of infrared thermography on canopies as indicator of water stress in ‘Arbequina’ olive orchards. ISHS Acta Hortic. 2014, 1057, 399–403. [Google Scholar] [CrossRef]
- Poblete-Echeverría, C.; Sepulveda-Reyes, D.; Ortega-Farias, S.; Zuñiga, M.; Fuentes, S. Plant water stress detection based on aerial and terrestrial infrared thermography: A study case from vineyard and olive orchard. ISHS Acta Hortic. 2016, 1112, 141–146. [Google Scholar] [CrossRef]
- Idso, S.B.; Jackson, R.D.; Pinter, P.J., Jr.; Reginato, R.J.; Hatfield, J.L. Normalizing the stress-degree-day parameter for environmental variability. Agric. Meteorol. 1981, 24, 45–55. [Google Scholar] [CrossRef]
- Jackson, R.D.; Idso, S.B.; Reginato, R.J.; Pinter, P.J. Canopy temperature as a crop water stress indicator. Water Resour. Res. 1981, 17, 1133–1138. [Google Scholar] [CrossRef]
- Wang, X.; Yang, W.; Wheaton, A.; Cooley, N.; Moran, B. Automated canopy temperature estimation via infrared thermography: A first step towards automated plant water stress monitoring. Comput. Electron. Agric. 2010, 73, 74–83. [Google Scholar] [CrossRef]
- Grant, O.M.; Tronina, L.; Jones, H.G.; Chaves, M.M. Exploring thermal imaging variables for the detection of stress responses in grapevine under different irrigation regimes. J. Exp. Bot. 2007, 58, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.G.; Serraj, R.; Loveys, B.R.; Xiong, L.; Wheaton, A.; Price, A.H. Thermal infrared imaging of crop canopies for the remote diagnosis and quantification of plant responses to water stress in the field. Funct. Plant Biol. 2009, 36, 978–989. [Google Scholar] [CrossRef]
- Baluja, J.; Diago, M.; Balda, P.; Zorer, R.; Meggio, F.; Morales, F.; Tardaguila, J. Assessment of vineyard water status variability by thermal and multispectral imagery using an unmanned aerial vehicle (UAV). Irrig. Sci. 2012, 30, 511–522. [Google Scholar] [CrossRef]
- Ochagavía, H. Application of Thermography for the Assessment of Vineyard Water Status. Master’s Thesis, Universidad de la Rioja, Logroño, España, 2012. [Google Scholar]
- Zarco-Tejada, P.J.; Gonzalez-Dugo, V.; Berni, J.A.J. Fluorescence, temperature and narrow-band indices acquired from a UAV platform for water stress detection using a micro-hyperspectral imager and a thermal camera. Remote Sens. Environ. 2012, 117, 322–337. [Google Scholar] [CrossRef]
- Jones, H.G.; Stoll, M.; Santos, T.; de Sousa, C.; Chaves, M.M.; Grant, O.M. Use of infrared thermography for monitoring stomatal closure in the field: Application to grapevine. J. Exp. Bot. 2002, 53, 2249–2260. [Google Scholar] [CrossRef] [PubMed]
- Idso, S.B.; Reginato, R.J.; Jackson, R.D.; Pinter, P.J., Jr. Foliage and air temperatures: Evidence for a dynamic “equivalence point”. Agric. Meteorol. 1981, 24, 223–226. [Google Scholar] [CrossRef]
- Jones, H.G. Use of infrared thermometry for estimation of stomatal conductance as a possible aid to irrigation scheduling. Agric. For. Meteorol. 1999, 95, 139–149. [Google Scholar] [CrossRef]
- Reinert, S.; Bögelein, R.; Thomas, F.M. Use of thermal imaging to determine leaf conductance along a canopy gradient in European beech (Fagus sylvatica). Tree Physiol. 2012, 32, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Pou, A.; Diago, M.P.; Medrano, H.; Baluja, J.; Tardaguila, J. Validation of thermal indices for water status identification in grapevine. Agric. Water Manag. 2014, 134, 60–72. [Google Scholar] [CrossRef]
- Moller, M.; Alchanatis, V.; Cohen, Y.; Meron, M.; Tsipris, J.; Naor, A.; Ostrovsky, V.; Sprintsin, M.; Cohen, S. Use of thermal and visible imagery for estimating crop water status of irrigated grapevine. J. Exp. Bot. 2007, 58, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuwen, C.; Tregoat, O.; Chone, X.; Bois, B.; Pernet, D.; Gaudillere, J.P. Vine water status is a key factor in grape ripening and vintage quality for red bordeaux wine. How can it be assessed for vineyard management purposes? J. Int. Sci. Vigne Vin 2009, 43, 121–134. [Google Scholar] [CrossRef]
- Chone, X.; van Leeuwen, C.; Dubourdieu, D.; Gaudillere, J.P. Stem water potential is a sensitive indicator of grapevine water status. Ann. Bot. 2001, 87, 477–483. [Google Scholar] [CrossRef]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration-Guidelines for Computing Crop Water Requirements—FAO Irrigation and Drainage Paper 56; Food and Agriculture Organization (FAO): Rome, Italy, 1998. [Google Scholar]
- Leinonen, I.; Grant, O.M.; Tagliavia, C.P.P.; Chaves, M.M.; Jones, H.G. Estimating stomatal conductance with thermal imagery. Plant. Cell Environ. 2006, 29, 1508–1518. [Google Scholar] [CrossRef] [PubMed]
- Agam, N.; Cohen, Y.; Alchanatis, V.; Ben-Gal, A. How sensitive is the CWSI to changes in solar radiation? Int. J. Remote Sens. 2013, 34, 6109–6120. [Google Scholar] [CrossRef]
- Sibille, I.; Ojeda, H.; Prieto, J.; Maldonado, S.; Lacapere, J.N.; Carbonneau, A. Relation between the values of three pressure chamber modalities (midday leaf, midday stem and predawn water potential) of 4 grapevine cultivars in drought situation of the southern of France. Applications for the irrigation control. In Proceedings of the XVth International Symposium (GESCO), Porec, Croacia, 20–23 June 2007; pp. 685–695.
- Williams, L.E.; Araujo, F.J. Correlations among Predawn Leaf, Midday Leaf, and Midday Stem Water Potential and their Correlations with other Measures of Soil and Plant Water Status in Vitis vinifera. J. Am. Soc. Hortic. Sci. 2002, 127, 448–454. [Google Scholar]
- Cifre, J.; Bota, J.; Escalona, J.M.; Medrano, H.; Flexas, J. Physiological tools for irrigation scheduling in grapevine (Vitis vinifera L.): An open gate to improve water-use efficiency? Agric. Ecosyst. Environ. 2005, 106, 159–170. [Google Scholar]
- Jara-Rojas, F.; Ortega-Farías, S.; Valdés-Gómez, H.; Acevedo-Opazo, C. Gas exchange relations of ungrafted grapevines (cv. Carménère) growing under irrigated field conditions. S. Afr. J. Enol. Vitic. 2015, 36, 231–242. [Google Scholar]
- Leinonen, I.; Jones, H.G. Combining thermal and visible imagery for estimating canopy temperature and identifying plant stress. J. Exp. Bot. 2004, 55, 1423–1431. [Google Scholar] [CrossRef] [PubMed]
- Berni, J.A.J.; Zarco-Tejada, P.J.; Sepulcre-Canto, G.; Fereres, E.; Villalobos, F. Mapping canopy conductance and CWSI in olive orchards using high resolution thermal remote sensing imagery. Remote Sens. Environ. 2009, 113, 2380–2388. [Google Scholar] [CrossRef]
- Ben-Gal, A.; Agam, N.; Alchanatis, V.; Cohen, Y.; Yermiyahu, U.; Zipori, I.; Presnov, E.; Sprintsin, M.; Dag, A. Evaluating water stress in irrigated olives: Correlation of soil water status, tree water status, and thermal imagery. Irrig. Sci. 2009, 27, 367–376. [Google Scholar] [CrossRef]
- Bellvert, J.; Marsal, J.; Girona, J.; Zarco-Tejada, P.J. Seasonal evolution of crop water stress index in grapevine varieties determined with high-resolution remote sensing thermal imagery. Irrig. Sci. 2014, 33, 81–93. [Google Scholar] [CrossRef]
- Ballester, C.; Jiménez-Bello, M.A.; Castel, J.R.; Intrigliolo, D.S. Usefulness of thermography for plant water stress detection in citrus and persimmon trees. Agric. For. Meteorol. 2013, 168, 120–129. [Google Scholar] [CrossRef]
- Bellvert, J.; Marsal, J.; Girona, J.; Gonzalez-Dugo, V.; Fereres, E.; Ustin, S.L.; Zarco-Tejada, P.J. Airborne thermal imagery to detect the seasonal evolution of crop water status in peach, nectarine and Saturn peach orchards. Remote Sens. 2016, 8, 39–55. [Google Scholar] [CrossRef]


| Treatments | Water Stress Conditions | * Stem Water Potential (MPa) |
|---|---|---|
| T0 | Null-Slight | 0 to −0.8 |
| T1 | Slight-Moderate | −0.8 to −1.1 |
| T2 | Moderate-Severe | −1.1 to −1.4 |
| T3 | Severe | Less than −1.4 |
| Zone | Technique | Code | n | Treatments | |||
|---|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T3 | ||||
| NADIR | SDT | NAD1 | 351 | 0.18 (0.09) | 0.29 (0.15) | 0.33 (0.13) | 0.44 (0.18) |
| SUNLIT | SDT | SUN1 | 384 | 0.20 (0.13) | 0.31 (0.15) | 0.31 (0.16) | 0.43 (0.18) |
| SHADOW | SDT | SHAD1 | 365 | 0.47 (0.24) | 0.66 (0.25) | 0.70 (0.22) | 0.83 (0.16) |
| AERIAL | SDT | AER1 | 248 | 0.21 (0.06) | 0.24 (0.06) | 0.29 (0.07) | 0.36 (0.08) |
| NADIR | EBT | NAD2 | 351 | 0.35 (0.18) | 0.46 (0.18) | 0.51 (0.17) | 0.59 (0.16) |
| SUNLIT | EBT | SUN2 | 384 | 0.37 (0.18) | 0.49 (0.20) | 0.49 (0.18) | 0.57 (0.19) |
| SHADOW | EBT | SHAD2 | 382 | 0.27 (0.16) | 0.38 (0.19) | 0.40 (0.17) | 0.49 (0.17) |
| AERIAL | EBT | AER2 | 256 | 0.52 (0.09) | 0.58 (0.11) | 0.62 (0.10) | 0.69 (0.09) |
| NADIR | FRT | NAD3 | 348 | 0.44 (0.20) | 0.58 (0.23) | 0.63 (0.21) | 0.72 (0.20) |
| SUNLIT | FRT | SUN3 | 367 | 0.50 (0.21) | 0.62 (0.22) | 0.63 (0.20) | 0.73 (0.18) |
| SHADOW | FRT | SHAD3 | 309 | 0.67 (0.25) | 0.74 (0.24) | 0.80 (0.21) | 0.91 (0.11) |
| AERIAL | FRT | AER3 | 248 | 0.49 (0.15) | 0.58 (0.16) | 0.63 (0.17) | 0.69 (0.14) |
| Statistical Parameters | NAD1 | SUN1 | SHAD1 | AER1 | NAD2 | SUN2 | SHAD2 | AER2 | NAD3 | SUN3 | SHAD3 | AER3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ψstem | ||||||||||||
| r2 | 0.34 | 0.32 | 0.46 | 0.36 | 0.40 | 0.36 | 0.33 | 0.22 | 0.26 | 0.21 | 0.36 | 0.20 |
| RMSE | 0.30 | 0.32 | 0.29 | 0.25 | 0.28 | 0.31 | 0.31 | 0.30 | 0.30 | 0.32 | 0.30 | 0.27 |
| MAE | 0.23 | 0.26 | 0.23 | 0.19 | 0.22 | 0.24 | 0.23 | 0.24 | 0.23 | 0.26 | 2.47 | 0.22 |
| gs | ||||||||||||
| r2 | 0.33 | 0.30 | 0.42 | 0.26 | 0.57 | 0.52 | 0.51 | 0.28 | 0.43 | 0.38 | 0.47 | 0.27 |
| RMSE | 0.084 | 0.089 | 0.082 | 0.075 | 0.073 | 0.075 | 0.077 | 0.078 | 0.077 | 0.080 | 0.077 | 0.074 |
| MAE | 0.065 | 0.071 | 0.065 | 0.052 | 0.053 | 0.057 | 0.060 | 0.060 | 0.060 | 0.063 | 0.060 | 0.052 |
| DOY | NAD1 | SUN1 | SHAD1 | AER1 | NAD2 | SUN2 | SHAD2 | AER2 | NAD3 | SUN3 | SHAD3 | AER3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ψstem | ||||||||||||
| 351 | n.s. | n.s. | n.s. | - | n.s. | n.s. | n.s. | - | n.s. | n.s. | n.s. | - |
| 6 | n.s. | 0.35 | n.s. | - | n.s. | n.s. | n.s. | - | n.s. | n.s. | n.s. | - |
| 14 | 0.13 | 0.11 | n.s. | - | n.s. | 0.13 | n.s. | - | n.s. | 0.12 | 0.16 | - |
| 27 | - | n.s. | n.s. | 0.59 | - | n.s. | n.s. | 0.50 | - | n.s. | n.s. | 0.55 |
| 37 | 0.44 | 0.53 | 0.63 | - | 0.65 | 0.66 | 0.63 | - | 0.68 | 0.65 | 0.68 | - |
| 41 | 0.74 | 0.61 | 0.76 | 0.68 | 0.82 | 0.72 | 0.78 | 0.68 | 0.71 | 0.70 | 0.76 | 0.55 |
| 44 | 0.28 | 0.31 | 0.37 | n.s. | 0.42 | 0.51 | 0.38 | n.s. | 0.42 | 0.47 | 0.40 | n.s. |
| 54 | 0.70 | 0.62 | 0.70 | 0.46 | 0.77 | 0.68 | 0.80 | 0.53 | 0.77 | 0.68 | 0.72 | 0.36 |
| 61 | 0.30 | 0.21 | 0.31 | 0.28 | 0.39 | 0.29 | 0.17 | 0.16 | 0.39 | 0.30 | 0.31 | 0.24 |
| 64 | 0.27 | 0.24 | 0.57 | 0.48 | 0.33 | 0.32 | 0.38 | 0.61 | 0.35 | 0.33 | 0.43 | 0.61 |
| 69 | 0.22 | 0.27 | 0.22 | 0.57 | 0.25 | 0.36 | 0.14 | 0.52 | 0.27 | 0.35 | 0.17 | 0.61 |
| 78 | n.s. | n.s. | n.s. | 0.19 | 0.15 | n.s. | n.s. | 0.34 | 0.13 | n.s. | n.s. | 0.39 |
| 91 | n.s. | 0.13 | n.s. | - | n.s. | 0.23 | 0.13 | - | n.s. | 0.22 | n.s. | - |
| gs | ||||||||||||
| 351 | n.s. | n.s. | n.s. | - | n.s. | n.s. | n.s. | - | n.s. | n.s. | n.s. | - |
| 6 | n.s. | n.s. | n.s. | - | n.s. | n.s. | n.s. | - | n.s. | n.s. | n.s. | - |
| 14 | n.s. | n.s. | n.s. | - | n.s. | n.s. | n.s. | - | n.s. | n.s. | n.s. | - |
| 27 | - | n.s. | n.s. | n.s. | - | n.s. | n.s. | n.s. | - | n.s. | 0.15 | n.s. |
| 37 | 0.49 | 0.51 | 0.68 | - | 0.62 | 0.63 | 0.66 | - | 0.60 | 0.46 | 0.71 | - |
| 41 | 0.63 | 0.56 | 0.70 | 0.71 | 0.73 | 0.67 | 0.77 | 0.75 | 0.58 | 0.60 | 0.69 | 0.54 |
| 44 | 0.51 | 0.54 | 0.43 | 0.24 | 0.59 | 0.54 | 0.46 | 0.22 | 0.65 | 0.57 | 0.32 | 0.40 |
| 54 | 0.62 | 0.40 | 0.37 | 0.48 | 0.66 | 0.48 | 0.51 | 0.32 | 0.64 | 0.49 | 0.38 | 0.41 |
| 61 | 0.40 | 0.28 | 0.26 | 0.32 | 0.48 | 0.39 | 0.32 | 0.17 | 0.48 | 0.40 | 0.28 | 0.26 |
| 64 | 0.29 | 0.37 | 0.64 | 0.45 | 0.43 | 0.59 | 0.67 | 0.48 | 0.43 | 0.61 | 0.54 | 0.61 |
| 69 | 0.33 | 0.15 | 0.12 | 0.31 | 0.33 | 0.26 | 0.20 | 0.26 | 0.32 | 0.24 | n.s. | 0.47 |
| 78 | 0.17 | n.s. | 0.17 | 0.23 | 0.14 | n.s. | n.s. | 0.72 | 0.13 | n.s. | n.s. | 0.51 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sepúlveda-Reyes, D.; Ingram, B.; Bardeen, M.; Zúñiga, M.; Ortega-Farías, S.; Poblete-Echeverría, C. Selecting Canopy Zones and Thresholding Approaches to Assess Grapevine Water Status by Using Aerial and Ground-Based Thermal Imaging. Remote Sens. 2016, 8, 822. https://doi.org/10.3390/rs8100822
Sepúlveda-Reyes D, Ingram B, Bardeen M, Zúñiga M, Ortega-Farías S, Poblete-Echeverría C. Selecting Canopy Zones and Thresholding Approaches to Assess Grapevine Water Status by Using Aerial and Ground-Based Thermal Imaging. Remote Sensing. 2016; 8(10):822. https://doi.org/10.3390/rs8100822
Chicago/Turabian StyleSepúlveda-Reyes, Daniel, Benjamin Ingram, Matthew Bardeen, Mauricio Zúñiga, Samuel Ortega-Farías, and Carlos Poblete-Echeverría. 2016. "Selecting Canopy Zones and Thresholding Approaches to Assess Grapevine Water Status by Using Aerial and Ground-Based Thermal Imaging" Remote Sensing 8, no. 10: 822. https://doi.org/10.3390/rs8100822







