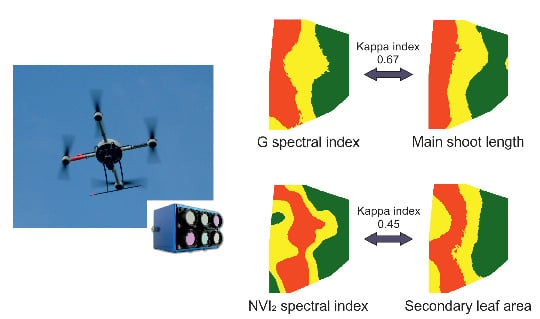
Using RPAS Multi-Spectral Imagery to Characterise Vigour, Leaf Development, Yield Components and Berry Composition Variability within a Vineyard
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
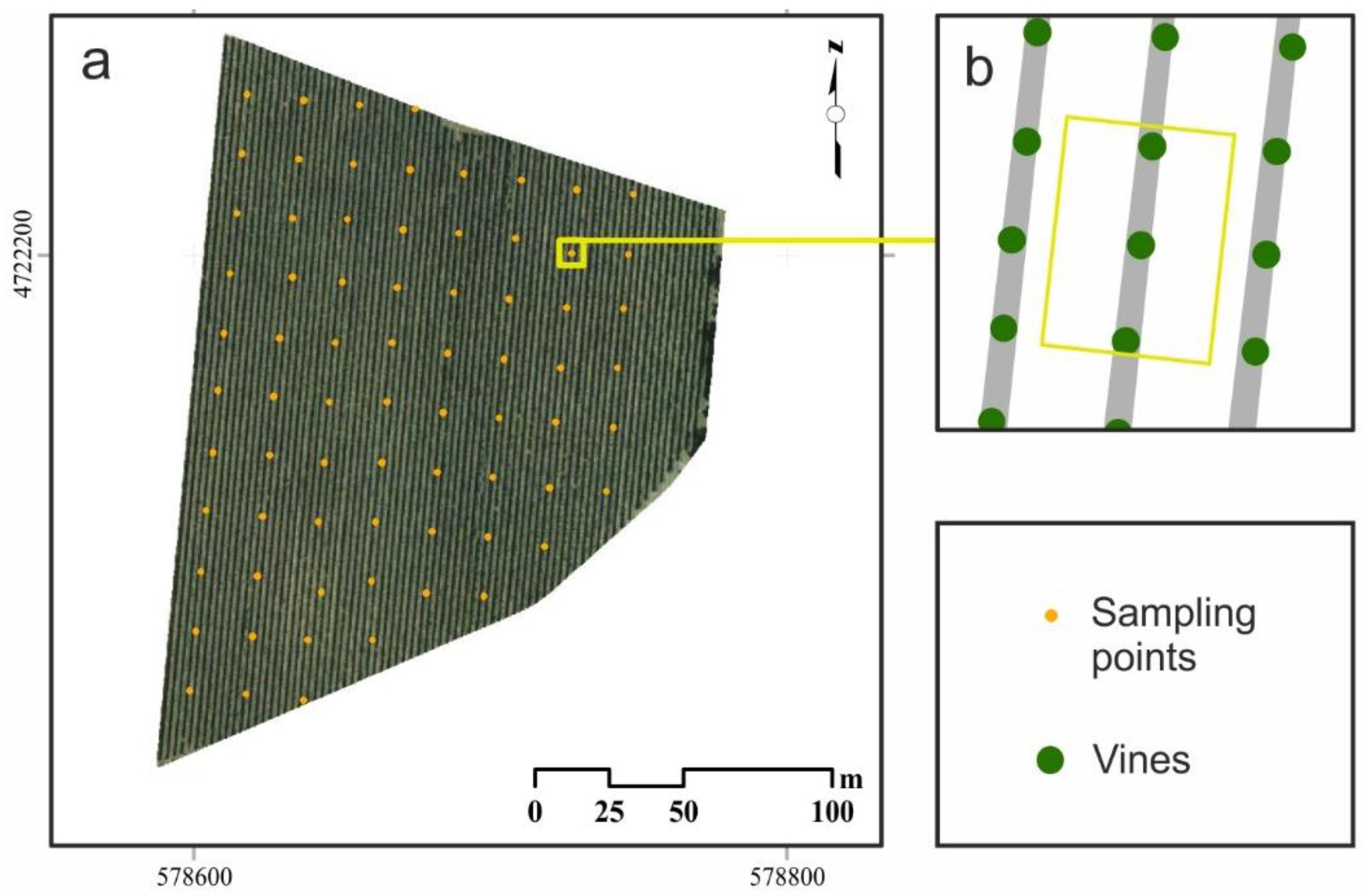
2.2. Field Data Acquisition of Vegetative and Yield Components. Berry Composition Analysis.
2.3. RPAS Multi-Spectral Images

2.4. Image Processing
2.5. Statistical Analysis
| Spectral Index | Reference |
|---|---|
| Normalized Difference Vegetation Index (NDVI) | (Rouse et al. 1974) [34] |
| Modified Simple Ratio (MSR) | (Chen 1996) [35] |
| Modified Triangular Vegetation Index (MTVI1) | (Haboudane et al. 2004) [36] |
| Renormalized Difference Vegetation Index (RDVI) | (Roujean and Breon 1995) [37] |
| Greenness Index (G) | - |
| Modified SAVI (MSAVI) | (Qi et al. 1994) [38] |
| Optimized Soil Adjusted Vegetation Index (OSAVI) | (Rondeaux et al. 1996) [39] |
| Modified Cab Absorption in Reflectance Index (MCARI) | (Daughtry et al. 2000) [40] |
| Transformed CARI (TCARI) | (Haboudane et al. 2002) [41] |
| TCARI/OSAVI | (Haboudane et al. 2002) [41] |
| Plant Cell Density Index (PCD) or Ratio Vegetation Index (RVI) | (Jordan 1969) [42] |
3. Results and Discussion
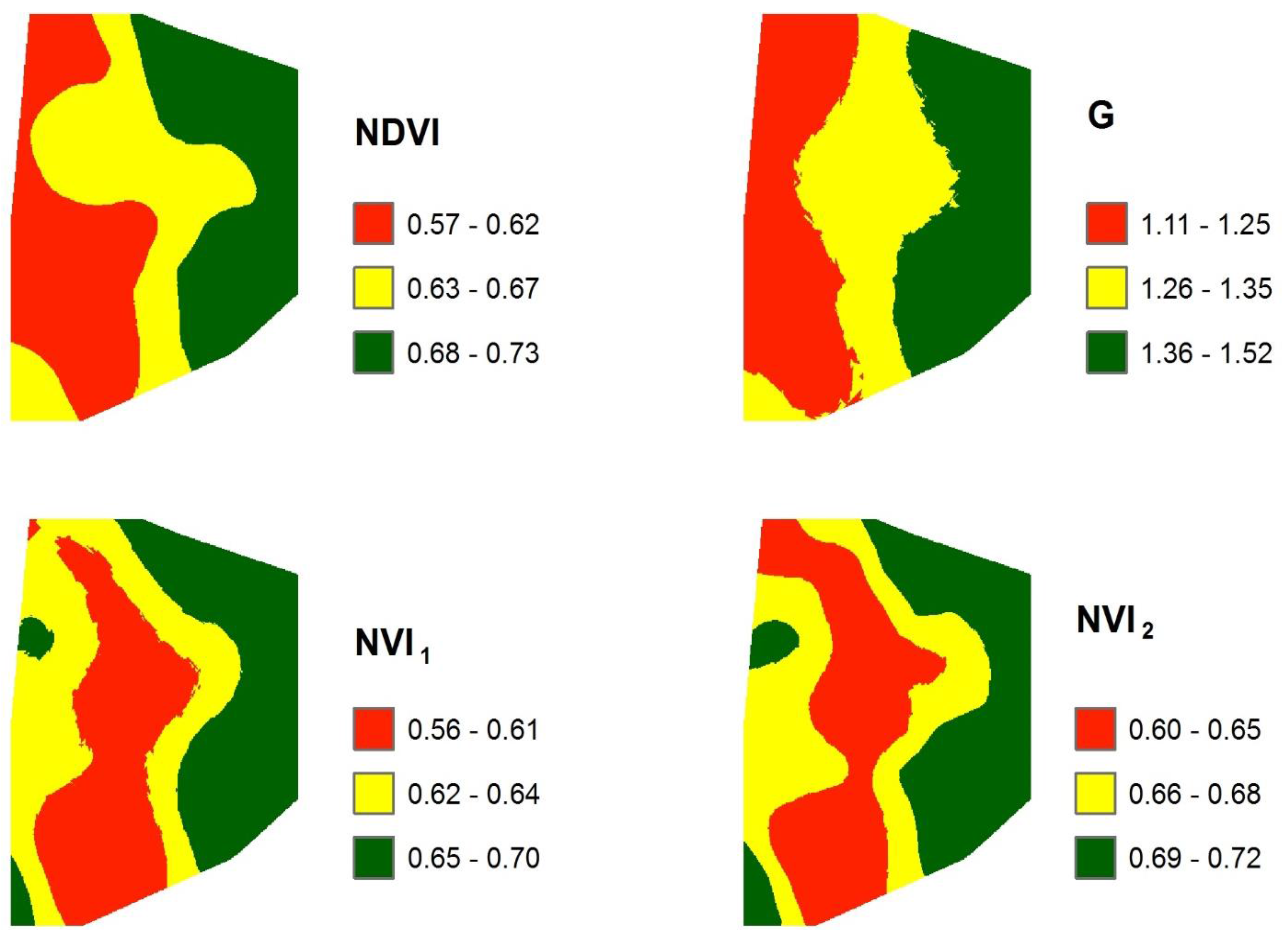
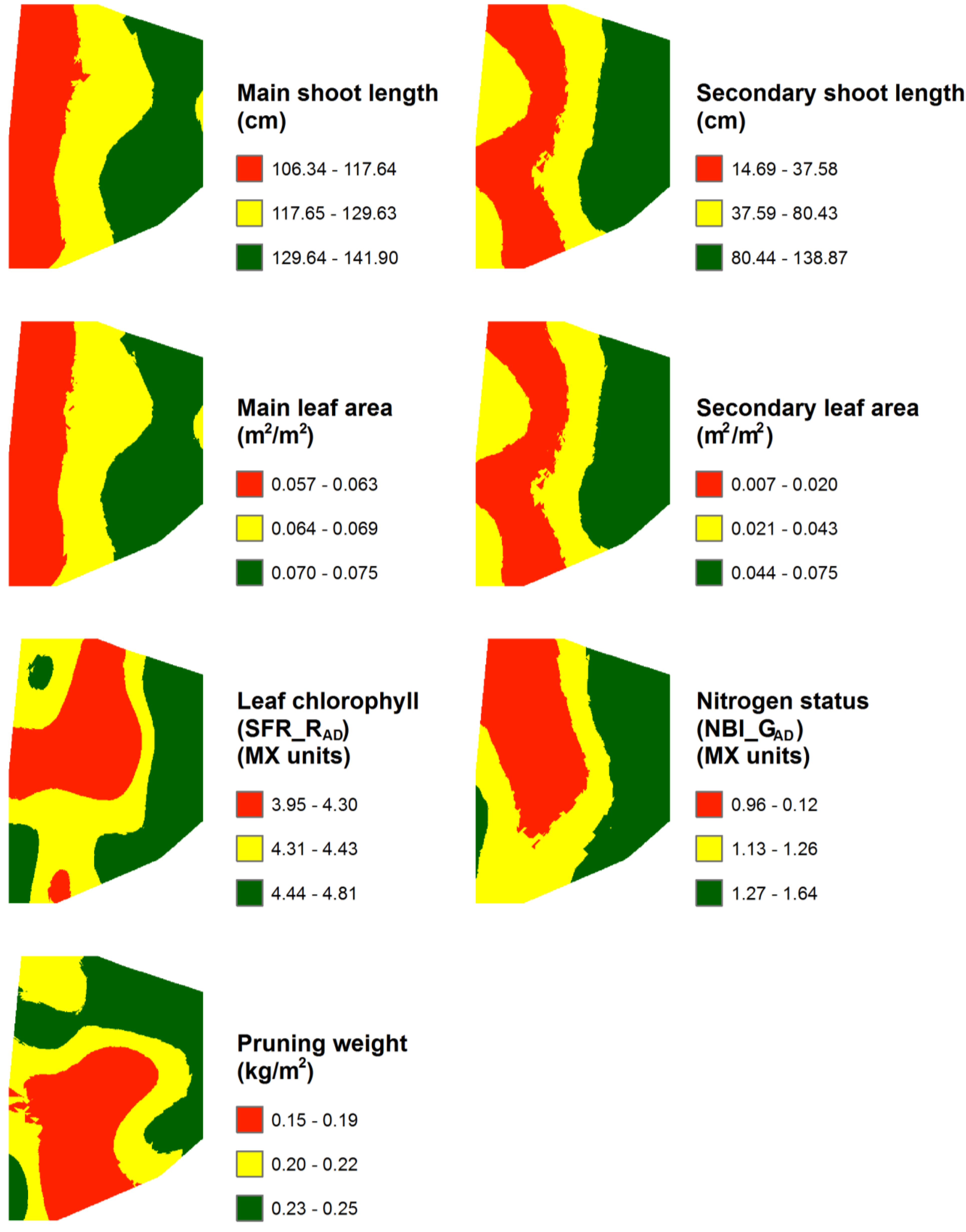
3.1. Spatial Variability of Spectral Indices, Field Variables, and Berry Composition
| Mean | Min. | Max. | SD | CV (%) | Spread (%) | % of Nugget | Range | |
|---|---|---|---|---|---|---|---|---|
| Spectral indices | ||||||||
| NDVI | 0.65 | 0.51 | 0.76 | 0.06 | 10.01 | 38.11 | 29 | 111.41 |
| G | 1.31 | 1.00 | 1.66 | 0.15 | 11.62 | 50.69 | 28 | 202.01 |
| NVI1 | 0.63 | 0.51 | 0.72 | 0.05 | 7.42 | 32.90 | 7 | 59.15 |
| NVI2 | 0.67 | 0.57 | 0.74 | 0.04 | 6.45 | 25.82 | 13 | 57.73 |
| Vegetative variables | ||||||||
| Pruning weight (kg/m2) | 0.20 | 0.12 | 0.34 | 0.05 | 25.21 | 116.96 | 70 | 52.65 |
| Main shoot length (cm) | 123.22 | 95.83 | 172.17 | 15.36 | 12.47 | 61.44 | 46 | 126.33 |
| Secondary shoot length (cm) | 63.95 | 6.67 | 189.83 | 49.99 | 78.17 | 379.61 | 33 | 105.14 |
| Main leaf area (m2/m2) | 0.07 | 0.05 | 0.09 | 0.01 | 12.87 | 60.00 | 46 | 122.55 |
| Secondary leaf area (m2/m2) | 0.03 | 0.003 | 0.10 | 0.03 | 83.80 | 475.00 | 34 | 98.91 |
| Leaf chlorophyll - SFR_RAD(Mx units) | 4.37 | 3.55 | 5.04 | 0.35 | 7.92 | 34.17 | 66 | 65.00 |
| Nitrogen status - NBI_GAD(Mx units) | 1.21 | 0.69 | 1.94 | 0.32 | 26.89 | 108.70 | 43 | 152.55 |
| Yield variables | ||||||||
| Cluster number/vine | 6.06 | 1.50 | 14.00 | 3.14 | 51.82 | 250.00 | 62 | 80.79 |
| Cluster weight (g) | 284.28 | 147.50 | 520.00 | 73.01 | 25.68 | 133.15 | 44 | 156.42 |
| Yield/vine (kg/m2) | 0.44 | 0.10 | 1.11 | 0.25 | 57.19 | 279.95 | – | – |
| Berry weight (g) | 2.29 | 1.84 | 2.91 | 0.22 | 9.47 | 46.75 | 34 | 48.82 |
| Grape composition variables | ||||||||
| pH | 3.99 | 3.63 | 4.29 | 0.14 | 3.45 | 16.53 | 53 | 117.54 |
| Total soluble solids (ºBrix) | 24.61 | 22.03 | 26.87 | 1.15 | 4.67 | 19.73 | 52 | 72.69 |
| Titratable acidity (g/l tartaric acid) | 2.95 | 2.30 | 3.67 | 0.28 | 9.57 | 46.96 | 59 | 137.22 |
| Anthocyanins (mg/ g berry) | 0.95 | 0.38 | 1.50 | 0.26 | 27.65 | 120.33 | – | – |
| Total phenols (AU/g berry) | 1.26 | 0.60 | 1.85 | 0.30 | 23.77 | 99.97 | 52 | 38 |
3.2. Correlation Analysis between Spectral Indices, Field Variables, and Berry Composition
| NDVI | G | NVI1 | NVI2 | SFR_RAD | NBI_GAD | Pruning Weight | Mean Shoot Length | Secondary Shoot Length | Main Leaf Area | Secondary Leaf Area | Cluster Number | Cluster Weight | Yield | Berry Weight | pH | Total Soluble Solids | Titratabe Acidity | Anthocyanins | Total Phenols | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NDVI | 1 | |||||||||||||||||||
| G | 0.84 | 1 | ||||||||||||||||||
| NVI1 | 0.81 | 0.54 | 1 | |||||||||||||||||
| NVI2 | 0.79 | 0.56 | 0.98 | 1 | ||||||||||||||||
| SFR_RAD | 0.37 | NS | 0.54 | 0.53 | 1 | |||||||||||||||
| NBI_GAD | 0.43 | 0.32 | 0.58 | 0.56 | 0.58 | 1 | ||||||||||||||
| Pruning weight | 0.55 | 0.36 | 0.58 | 0.58 | NS | 0.36 | 1 | |||||||||||||
| Mean shoot length | 0.49 | 0.54 | 0.34 | 0.32 | NS | NS | NS | 1 | ||||||||||||
| Secondary shoot length | 0.49 | 0.47 | 0.52 | 0.50 | NS | 0.52 | 0.32 | 0.61 | 1 | |||||||||||
| Main leaf area | 0.49 | 0.53 | 0.35 | 0.33 | NS | NS | NS | 1.00 | 0.59 | 1 | ||||||||||
| Secondary leaf area | 0.49 | 0.47 | 0.52 | 0.50 | NS | 0.52 | 0.33 | 0.61 | 1.00 | 0.59 | 1 | |||||||||
| Cluster number | NS | NS | NS | NS | NS | −0.34 | NS | −0.37 | −0.50 | −0.35 | −0.50 | 1 | ||||||||
| Cluster weight | 0.59 | 0.51 | 0.65 | 0.69 | 0.45 | 0.56 | 0.59 | NS | 0.45 | NS | 0.45 | NS | 1 | |||||||
| Yield | NS | NS | NS | NS | NS | NS | 0.35 | NS | −0.31 | NS | NS | 0.90 | NS | 1 | ||||||
| Berry weight | 0.41 | 0.33 | 0.38 | 0.42 | NS | NS | 0.52 | NS | NS | NS | NS | NS | 0.45 | 0.35 | 1 | |||||
| pH | NS | NS | NS | NS | NS | NS | −0.40 | 0.37 | NS | 0.37 | NS | −0.44 | NS | −0.53 | −0.44 | 1 | ||||
| Total soluble solids | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | 0.52 | 1 | |||
| Titratable acidity | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | 0.32 | −0.62 | −0.58 | 1 | ||
| Anthocyanins | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | −0.33 | 1 | |
| Total phenols | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | 0.94 | 1 |
3.3. Mapping and Spatial Agreement between Spectral Indices and Field Variables
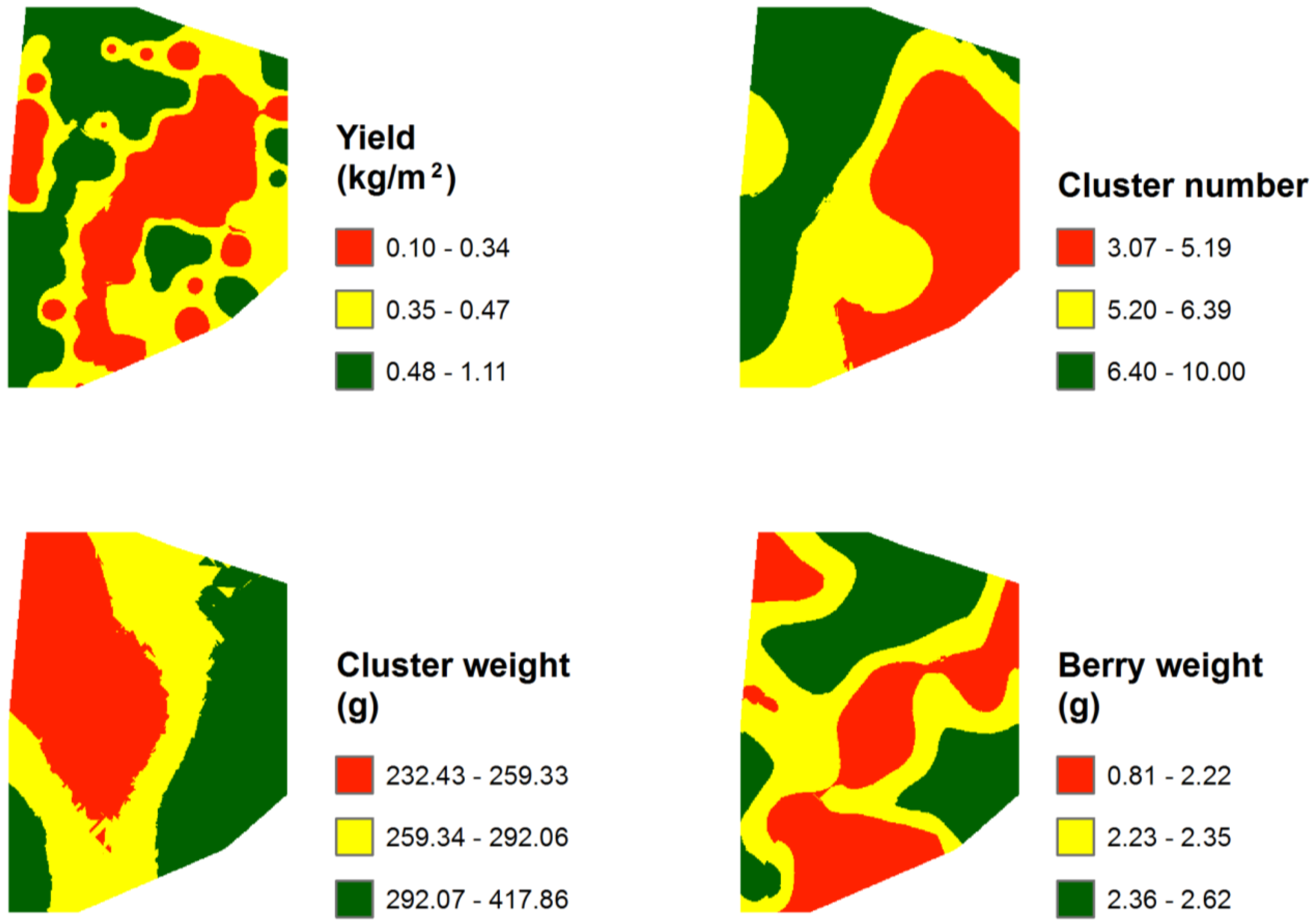
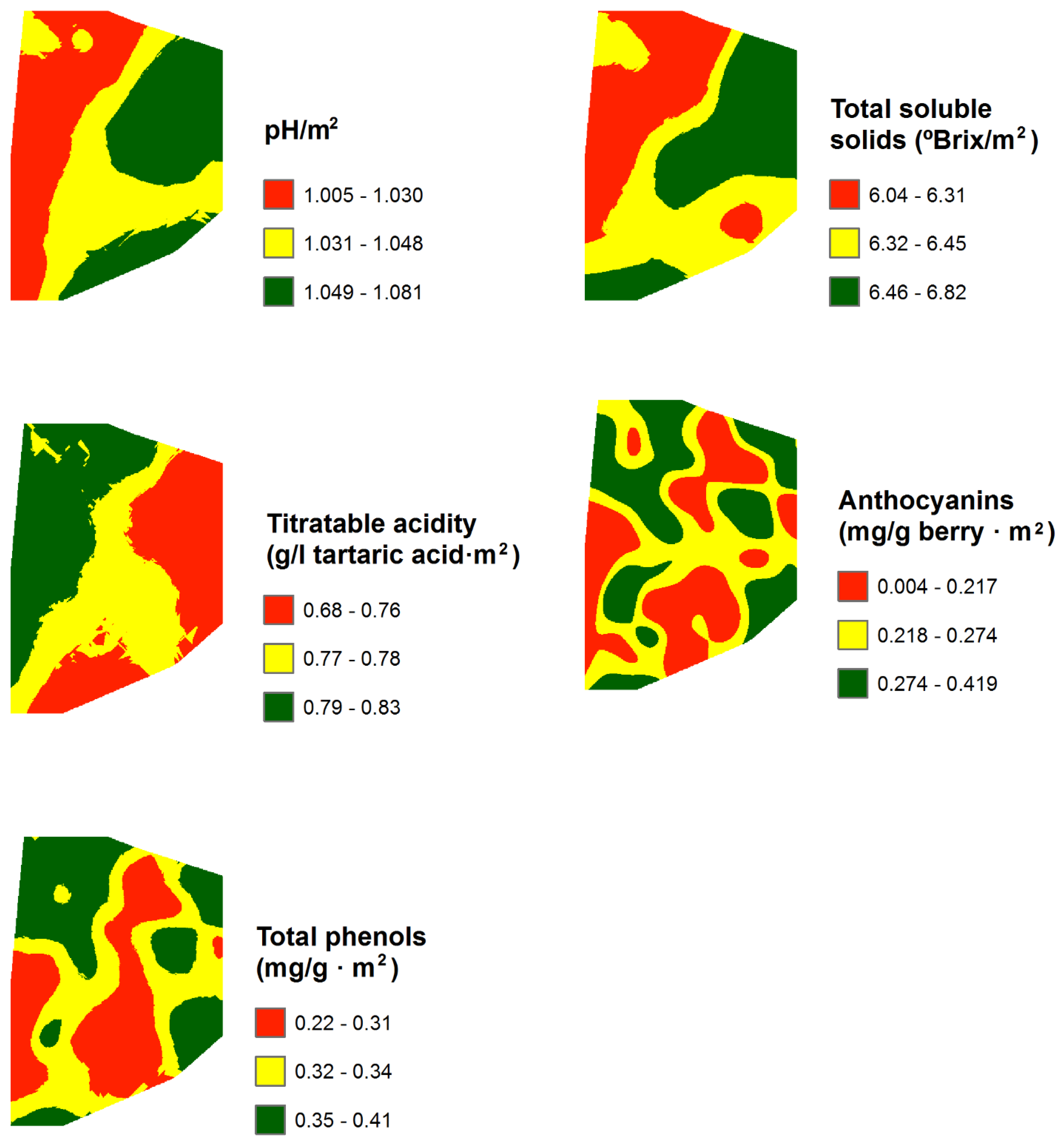
| NDVI | G | NVI1 | NVI2 | |
|---|---|---|---|---|
| Main shoot length | 0.41 | 0.67 | 0.18 | 0.17 |
| Secondary shoot length | 0.47 | 0.59 | 0.45 | 0.44 |
| Main leaf area | 0.43 | 0.70 | 0.19 | 0.16 |
| Secondary leaf area | 0.47 | 0.57 | 0.46 | 0.45 |
| Pruning weight | 0.32 | 0.08 | 0.39 | 0.35 |
| SFR_R | 0.11 | 0.10 | 0.39 | 0.30 |
| NBI_G | 0.32 | 0.36 | 0.38 | 0.35 |
| Cluster number | −0.26 | −0.27 | −0.17 | −0.15 |
| Mean cluster weight | 0.35 | 0.35 | 0.37 | 0.40 |
| Yield | 0.01 | −0.14 | 0.01 | −0.01 |
| Mean berry weight | 0.12 | 0.11 | 0.13 | 0.12 |
| pH | 0.14 | 0.35 | −0.05 | −0.05 |
| Total soluble solids | 0.11 | 0.25 | −0.06 | −0.04 |
| Titratable acidity | −0.16 | −0.15 | −0.24 | −0.23 |
| Anthocyanins | −0.01 | 0.00 | −0.04 | −0.07 |
| Total phenols | 0.00 | −0.02 | −0.09 | −0.08 |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tey, Y.S.; Brindal, M. Factors influencing the adoption of precision agricultural technologies: A review for policy implications. Precis. Agric. 2012, 13, 713–730. [Google Scholar] [CrossRef]
- Schieffer, J.; Dillon, C. The economic and environmental impacts of precision agriculture and interactions with agro-environmental policy. Precis. Agric. 2015, 16, 46–61. [Google Scholar] [CrossRef]
- Hall, A.; Lamb, D.W.; Holzapfel, B.; Louis, J. Optical remote sensing applications in viticulture-a review. Aust. J. Grape Wine Res. 2002, 8, 36–47. [Google Scholar] [CrossRef]
- Acevedo-Opazo, C.; Tisseyre, B.; Guillaume, S.; Ojeda, H. The potential of high spatial resolution information to define within-vineyard zones related to vine water status. Precis. Agric. 2008, 9, 285–302. [Google Scholar] [CrossRef]
- Baluja, J.; Diago, M.P.; Balda, P.; Zorer, R.; Meggio, F.; Morales, F.; Tardaguila, J. Assessment of vineyard water status variability by thermal and multispectral imagery using an unmanned aerial vehicle (UAV). Irrig. Sci. 2012, 30, 511–522. [Google Scholar] [CrossRef]
- Martin, P.; Zarco-Tejada, P.J.; Gonzalez, M.R.; Berjón, A. Using hyperspectral remote sensing to map grape quality in “tempranillo” vineyards affected by iron deficiency chlorosis. VITIS 2007, 46, 7–14. [Google Scholar]
- Zarco-Tejada, P.J.; Guillén-Climent, M.L.; Hernández-Clemente, R.; Catalina, A.; González, M.R.; Martín, P. Estimating leaf carotenoid content in vineyards using high resolution hyperspectral imagery acquired from an unmanned aerial vehicle (UAV). Agric. For. Meteorol. 2013, 171–172, 281–294. [Google Scholar] [CrossRef]
- Hall, A.; Louis, J.P.; Lamb, D.W. Low-resolution remotely sensed images of winegrape vineyards map spatial variability in planimetric canopy area instead of leaf area index. Aust. J. Grape Wine Res. 2008, 14, 9–17. [Google Scholar] [CrossRef]
- Mathews, A.; Jensen, J. Visualizing and quantifying vineyard canopy LAI using an unmanned aerial vehicle (UAV) collected high density structure from motion point cloud. Remote Sens. 2013, 5, 2164–2183. [Google Scholar] [CrossRef]
- Lamb, D.W.; Weedon, M.M.; Bramley, R.G.V. Using remote sensing to predict grape phenolics and colour at harvest in a cabernet sauvignon vineyard: Timing observations against vine phenology and optimising image resolution. Aust. J. Grape Wine Res. 2004, 10, 46–54. [Google Scholar] [CrossRef]
- Matese, A.; Capraro, F.; Primicerio, J.; Gualato, G.; Gennaro, S.F.D.; Agati, G. Mapping of vine vigor by UAV and anthocyanin content by a non-destructive fluorescence technique. In Precision Agriculture’13; Stafford, J., Ed.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2013; pp. 201–208. [Google Scholar]
- Serrano Porta, L.; Zarco Tejada, P.; González Flor, C.; Pons Valls, J.M.; Gorchs Altarriba, G. Assessment of berry quality using airborne derived NDVI and PRI in rainfed vineyards. In Proceedings of the 9th European Conference on Precision Agriculture, Lleida, Spain, 7–11 July 2013.
- Turner, D; Lucieer, A.; Watson, C. Development of an unmanned aerial vehicle (UAV) for hyper resolution vineyard mapping based on visible, multispectral, and thermal imagery. In Proceedings of the 34th International Symposium on Remote Sensing of Environment, Sydney, NSW, Australia, 10–15 April 2011; p. 4.
- Matese, A.; Toscano, P.; Di Gennaro, S.; Genesio, L.; Vaccari, F.; Primicerio, J.; Belli, C.; Zaldei, A.; Bianconi, R.; Gioli, B. Intercomparison of uav, aircraft and satellite remote sensing platforms for precision viticulture. Remote Sens. 2015, 7, 2971–2990. [Google Scholar] [CrossRef]
- Berni, J.; Zarco-Tejada, P.J.; Suárez, L.; Fereres, E. Thermal and narrowband multispectral remote sensing for vegetation monitoring from an unmanned aerial vehicle. IEEE Trans. Geosci. Remote Sens. 2009, 47, 722–738. [Google Scholar] [CrossRef]
- Zhang, C.; Kovacs, J. The application of small unmanned aerial systems for precision agriculture: A review. Precis. Agric. 2012, 13, 693–712. [Google Scholar] [CrossRef]
- Jenkins, D.; Vasigh, B. The Economic Impact of Unmanned Aircraft Systems Integration in the United States. Available online: http://www.auvsi.org/econreport (accessed on 10 August 2015).
- Sepulcre-Cantó, G.; Diago, M.P.; Balda, P.; Martínez de Toda, F.; Morales, F.; Tardáguila, J. Monitoring vineyard spatial variability of vegetative growth and physiological status using an unmanned aerial vehicle (UAV). In Proceedings of the 16th International Symposium of GiESCO, Davis, CA, USA, 16–20 July 2009.
- Gamon, J.A.; Peņuelas, J.; Field, C.B. A narrow-waveband spectral index that tracks diurnal changes in photosynthetic efficiency. Remote Sens. Environ. 1992, 41, 35–44. [Google Scholar] [CrossRef]
- Martínez de Toda, F.; Tardáguila, J.; Sancha, J.C. Estimation of grape quality in vineyards using a new viticultural index. VITIS—J. Grapevine Res. 2007, 46, 168–173. [Google Scholar]
- Smart, R.; Robinson, M. Sunlight into Wine: A Handbook for Winegrape Canopy Management; Winetitles: Adelaida, SA, Australia, 1991. [Google Scholar]
- Ben Ghozlen, N.; Moise, N.; Latouche, G.; Martinon, V.; Mercier, L.; Besancon, E.; Cerovic, Z. Assessment of grapevine maturity using a new portable sensor: Non destructive quantification of anthocyanins. J. Int. Sci. Vigne Vin 2010, 44, 1–8. [Google Scholar]
- Ben Ghozlen, N.; Cerovic, Z.G.; Germain, C.; Toutain, S.; Latouche, G. Non-destructive optical monitoring of grape maturation by proximal sensing. Sensors 2010, 10, 10040–10068. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, N.; Wang, Z.; Cerovic, Z. Sensing crop nitrogen status with fluorescence indicators. A review. Agron. Sustain. Dev. 2012, 32, 451–464. [Google Scholar] [CrossRef]
- Rey-Caramés, C. Assessment of the Spatial Variability of Vegetative Status in Vineyards Using Non-Destructive sensors. Application of Remote and Proximal Sensing Technologies in Precision Viticulture. Ph.D. Thesis, University of La Rioja, Logroño, Spain, 10 July 2015. [Google Scholar]
- OIV. Compendium of International Methods of Wine and Must Analysis; International Organisation of Vine and Wine: Paris, France, 2012. [Google Scholar]
- Iland, P.; Bruer, N.; Edwards, G.; Weeks, S.; Wilkes, E. Chemical Analysis of Grapes and Wine: Techniques and Concepts; Patrick Iland Wine Promotions: Campbelltown, SA, Australia, 2004. [Google Scholar]
- Sánchez-de-Miguel, P.; Baeza, P.; Junquera, P.; Lissarrague, J. Vegetative development: Total leaf area and surface area indexes. In Methodologies and Results in Grapevine Research; Delrot, S., Medrano, H., Or, E., Bavaresco, L., Grando, S., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2010; pp. 31–44. [Google Scholar]
- Laliberte, A.S.; Goforth, M.A.; Steele, C.M.; Rango, A. Multispectral remote sensing from unmanned aircraft: Image processing workflows and applications for rangeland environments. Remote Sens. 2011, 3, 2529–2551. [Google Scholar] [CrossRef]
- Inglada, J.; Christophe, E. The orfeo toolbox remote sensing image processing software. In Proceedings of the IEEE International Geoscience and Remote Sensing Symposium (IGARSS’09), Cape Town, South Africa, 12–17 July 2009; pp. 733–736.
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2012. [Google Scholar]
- Keitt, T.H.; Bivand, R.; Pebesma, E.; Rowlingson, B. Rgdal: Bindings for the Geospatial Data Abstraction Library. Available online: http://CRAN.R-project.org/package=rgdal (accessed on 10 August 2015).
- Hijmans, R.J.; van Etten, J. Raster: Geographic Analysis and Modeling with Raster Data. Available online: http://CRAN.R-project.org/package=raster (accessed on 10 August 2015).
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W.; Harlan, J.C. Monitoring the Vernal Advancements and Retrogradation of Natural Vegetation; NASA/GSFC Final Report; NASA: Greenbelt, MD, USA, 1974; pp. 1–371.
- Chen, J.M. Evaluation of vegetation indices and a modified simple ratio for boreal applications. Can. J. Remote Sens. 1996, 22, 229–242. [Google Scholar] [CrossRef]
- Haboudane, D.; Miller, J.R.; Pattey, E.; Zarco-Tejada, P.J.; Strachan, I.B. Hyperspectral vegetation indices and novel algorithms for predicting green LAI of crop canopies: Modeling and validation in the context of precision agriculture. Remote Sens. Environ. 2004, 90, 337–352. [Google Scholar] [CrossRef]
- Roujean, J.L.; Breon, F.M. Estimating par absorbed by vegetation from bidirectional reflectance measurements. Remote Sens. Environ. 1995, 51, 375–384. [Google Scholar] [CrossRef]
- Qi, J.; Chehbouni, A.; Huete, A.; Kerr, Y.; Sorooshian, S. A modified soil adjusted vegetation index. Remote Sens. Environ. 1994, 48, 119–126. [Google Scholar] [CrossRef]
- Rondeaux, G.; Steven, M.; Baret, F. Optimization of soil-adjusted vegetation indices. Remote Sens. Environ. 1996, 55, 95–107. [Google Scholar] [CrossRef]
- Daughtry, C.S.T.; Walthall, C.L.; Kim, M.S.; De Colstoun, E.B.; McMurtrey, J.E. Estimating corn leaf chlorophyll concentration from leaf and canopy reflectance. Remote Sens. Environ. 2000, 74, 229–239. [Google Scholar] [CrossRef]
- Haboudane, D.; Miller, J.R.; Tremblay, N.; Zarco-Tejada, P.J.; Dextraze, L. Integrated narrow-band vegetation indices for prediction of crop chlorophyll content for application to precision agriculture. Remote Sens. Environ. 2002, 81, 416–426. [Google Scholar] [CrossRef]
- Jordan, C.F. Derivation of leaf-area index from quality of light on the forest floor. Ecology 1969, 50, 663–666. [Google Scholar] [CrossRef]
- Bramley, R.G.V. Understanding variability in winegrape production systems 2. Within vineyard variation in quality over several vintages. Aust. J. Grape Wine Res. 2005, 11, 33–42. [Google Scholar] [CrossRef]
- Pebesma, E.J. Multivariable geostatistics in S: The gstat package. Comput. Geosci. 2004, 30, 683–691. [Google Scholar] [CrossRef]
- Hudson, W.D.; Ramm, C.V. Correct formulation of the kappa coefficient of agreement. Photogramm. Eng. Remote Sens. 1987, 53, 421–422. [Google Scholar]
- Gitelson, A.A.; Kaufman, Y.J.; Merzlyak, M.N. Use of a green channel in remote sensing of global vegetation from EOS-MODIS. Remote Sens. Environ. 1996, 58, 289–298. [Google Scholar] [CrossRef]
- Baluja, J.; Diago, M.P.; Goovaerts, P.; Tardaguila, J. Assessment of the spatial variability of anthocyanins in grapes using a fluorescence sensor: Relationships with vine vigour and yield. Precis. Agric. 2012, 13, 457–472. [Google Scholar] [CrossRef]
- Bramley, R.; Lamb, D. Making sense of vineyard variability in Australia. In Proceedings of the International Symposium on Precision Viticulture, Ninth Latin American Congress on Viticulture and Oenology, Santiago, Chile, 24–28 November 2003; pp. 35–54.
- Bramley, R.G.V.; Hamilton, R.P. Understanding variability in winegrape production systems 1. Within vineyard variation in yield over several vintages. Aust. J. Grape Wine Res. 2004, 10, 32–45. [Google Scholar] [CrossRef]
- Agati, G.; Foschi, L.; Grossi, N.; Guglielminetti, L.; Cerovic, Z.G.; Volterrani, M. Fluorescence-based versus reflectance proximal sensing of nitrogen content in Paspalum vaginatum and Zoysia matrella turfgrasses. Eur. J. Agron. 2013, 45, 39–51. [Google Scholar] [CrossRef]
- Cartelat, A.; Cerovic, Z.G.; Goulas, Y.; Meyer, S.; Lelarge, C.; Prioul, J.L.; Barbottin, A.; Jeuffroy, M.H.; Gate, P.; Agati, G.; et al. Optically assessed contents of leaf polyphenolics and chlorophyll as indicators of nitrogen deficiency in wheat (Triticum aestivum L.). Field Crops Res. 2005, 91, 35–49. [Google Scholar] [CrossRef]
- Cerovic, Z.G.; Goutouly, J.-P.; Hilbert, G.; Destrac-Irvine, A.; Martinon, V.; Moise, N. Mapping winegrape quality attributes using portable fluorescence-based sensors. Frutic 2009, 9, 301–310. [Google Scholar]
- Oliver, M.A. Geostatistical Applications for Precision Agriculture; Springer: London, UK, 2010. [Google Scholar]
- Taylor, J.A.; Bates, T.R. Sampling and estimating average pruning weights in concord grapes. Am. J. Enol. Vitic. 2012, 63, 559–563. [Google Scholar] [CrossRef]
- Cambardella, C.A.; Moorman, T.B.; Parkin, T.B.; Karlen, D.L.; Novak, J.M.; Turco, R.F.; Konopka, A.E. Field-scale variability of soil properties in central Iowa soils. Soil Sci. Soc. Am. J. 1994, 58, 1501–1511. [Google Scholar] [CrossRef]
- Bonilla, I.; Martinez de Toda, F.; Martínez-Casasnovas, J.A. Vine vigor, yield and grape quality assessment by airborne remote sensing over three years: Analysis of unexpected relationships in cv. Tempranillo. Span. J. Agric. Res. 2015, 13, 1–8. [Google Scholar] [CrossRef]
- Dobrowski, S.Z.; Ustin, S.L.; Wolpert, J.A. Grapevine dormant pruning weight prediction using remotely sensed data. Aust. J. Grape Wine Res. 2003, 9, 177–182. [Google Scholar] [CrossRef]
- Hall, A.; Lamb, D.W.; Holzapfel, B.P.; Louis, J.P. Within-season temporal variation in correlations between vineyard canopy and winegrape composition and yield. Precis. Agric. 2011, 12, 103–117. [Google Scholar] [CrossRef]
- Proffitt, T.; Malcolm, A. Implementing zonal vineyard management through airborne remote sensing. Aust. N. Z. Grapegrow. Winemak. 2005, 502, 22–27. [Google Scholar]
- Keller, M.; Tarara, J.M.; Mills, L.J. Spring temperatures alter reproductive development in grapevines. Aust. J. Grape Wine Res. 2010, 16, 445–454. [Google Scholar] [CrossRef]
- Martínez de Toda, F. Claves de la Viticultura de Calidad, Nuevas Técnicas de Estimación y Control de la Calidad de la Uva en el Viñedo. Mundi-Prensa: Madrid, Spain, 2011. [Google Scholar]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Congalton, R.G.; Green, K. Assessing the Accuracy of Remotely Sensed Data; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Tisseyre, B.; Mazzoni, C.; Fonta, H. Whithin-field temporal stability of some parameters in viticulture: Potential toward a site specific management. J. Int. Sci. Vigne Vin. 2008, 42, 27. [Google Scholar]
- Serrano, L.; González-Flor, C.; Gorchs, G. Assessment of grape yield and composition using the reflectance based water index in Mediterranean rainfed vineyards. Remote Sens. Environ. 2012, 118, 249–258. [Google Scholar] [CrossRef] [Green Version]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rey-Caramés, C.; Diago, M.P.; Martín, M.P.; Lobo, A.; Tardaguila, J. Using RPAS Multi-Spectral Imagery to Characterise Vigour, Leaf Development, Yield Components and Berry Composition Variability within a Vineyard. Remote Sens. 2015, 7, 14458-14481. https://doi.org/10.3390/rs71114458
Rey-Caramés C, Diago MP, Martín MP, Lobo A, Tardaguila J. Using RPAS Multi-Spectral Imagery to Characterise Vigour, Leaf Development, Yield Components and Berry Composition Variability within a Vineyard. Remote Sensing. 2015; 7(11):14458-14481. https://doi.org/10.3390/rs71114458
Chicago/Turabian StyleRey-Caramés, Clara, María P. Diago, M. Pilar Martín, Agustín Lobo, and Javier Tardaguila. 2015. "Using RPAS Multi-Spectral Imagery to Characterise Vigour, Leaf Development, Yield Components and Berry Composition Variability within a Vineyard" Remote Sensing 7, no. 11: 14458-14481. https://doi.org/10.3390/rs71114458