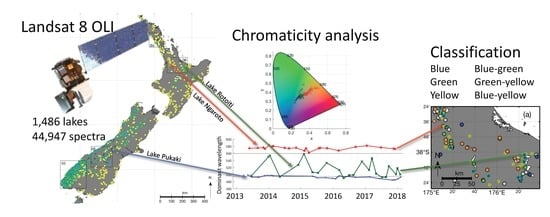
Colour Classification of 1486 Lakes across a Wide Range of Optical Water Types
Abstract
:1. Introduction
2. Materials and Methods
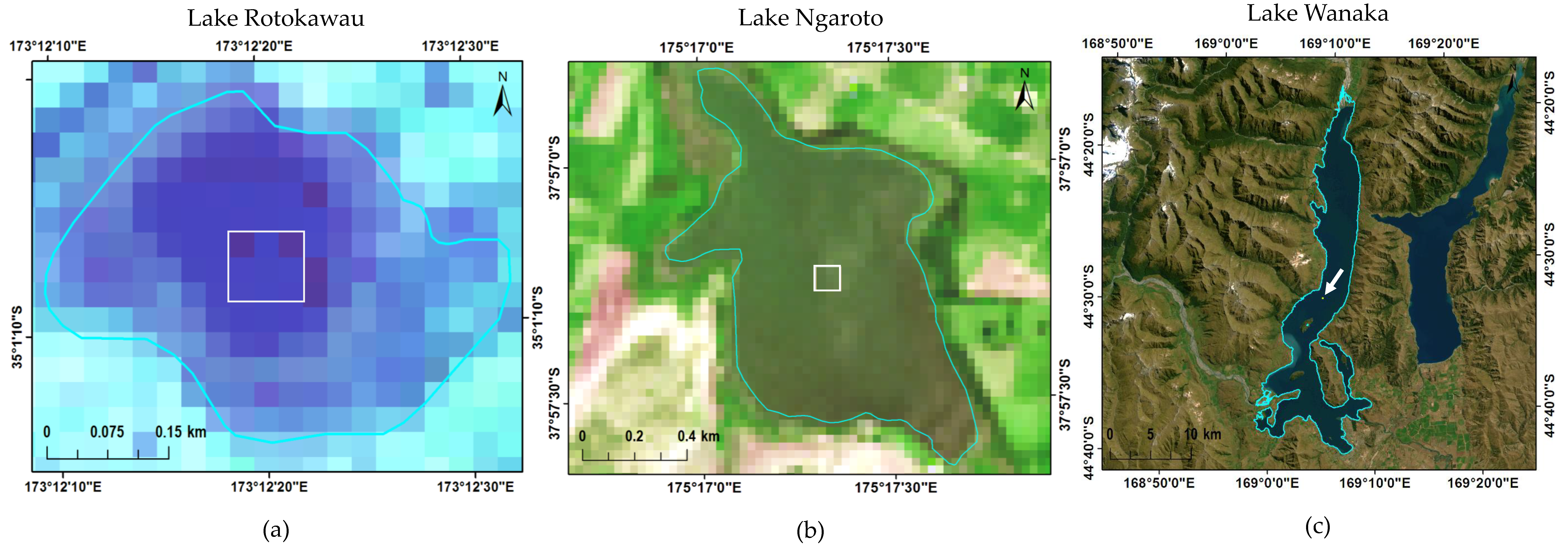
2.1. Study Area
2.2. Satellite Imagery Acquisition and Processing
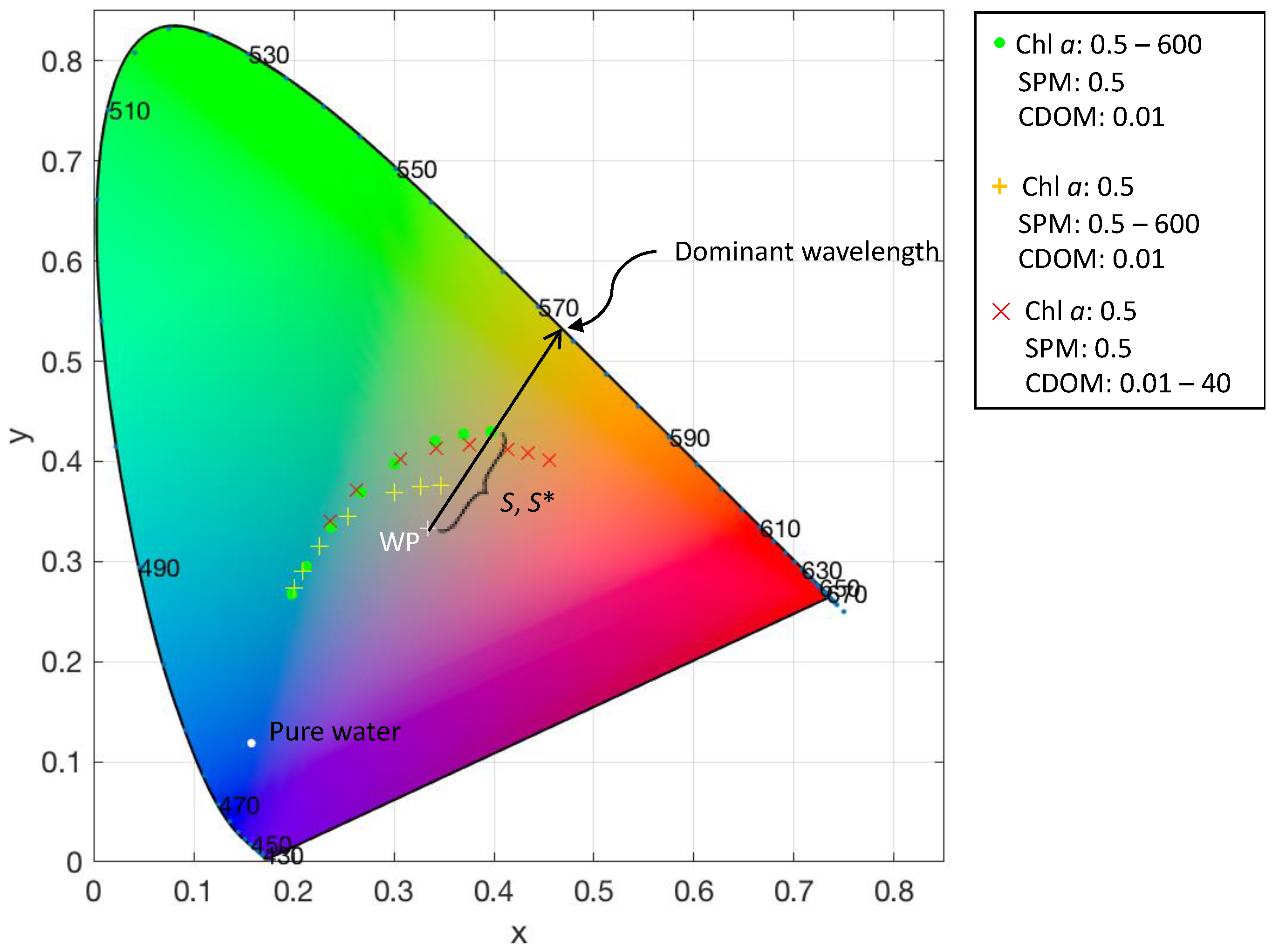
2.3. Calculation of Chromaticity
3. Results
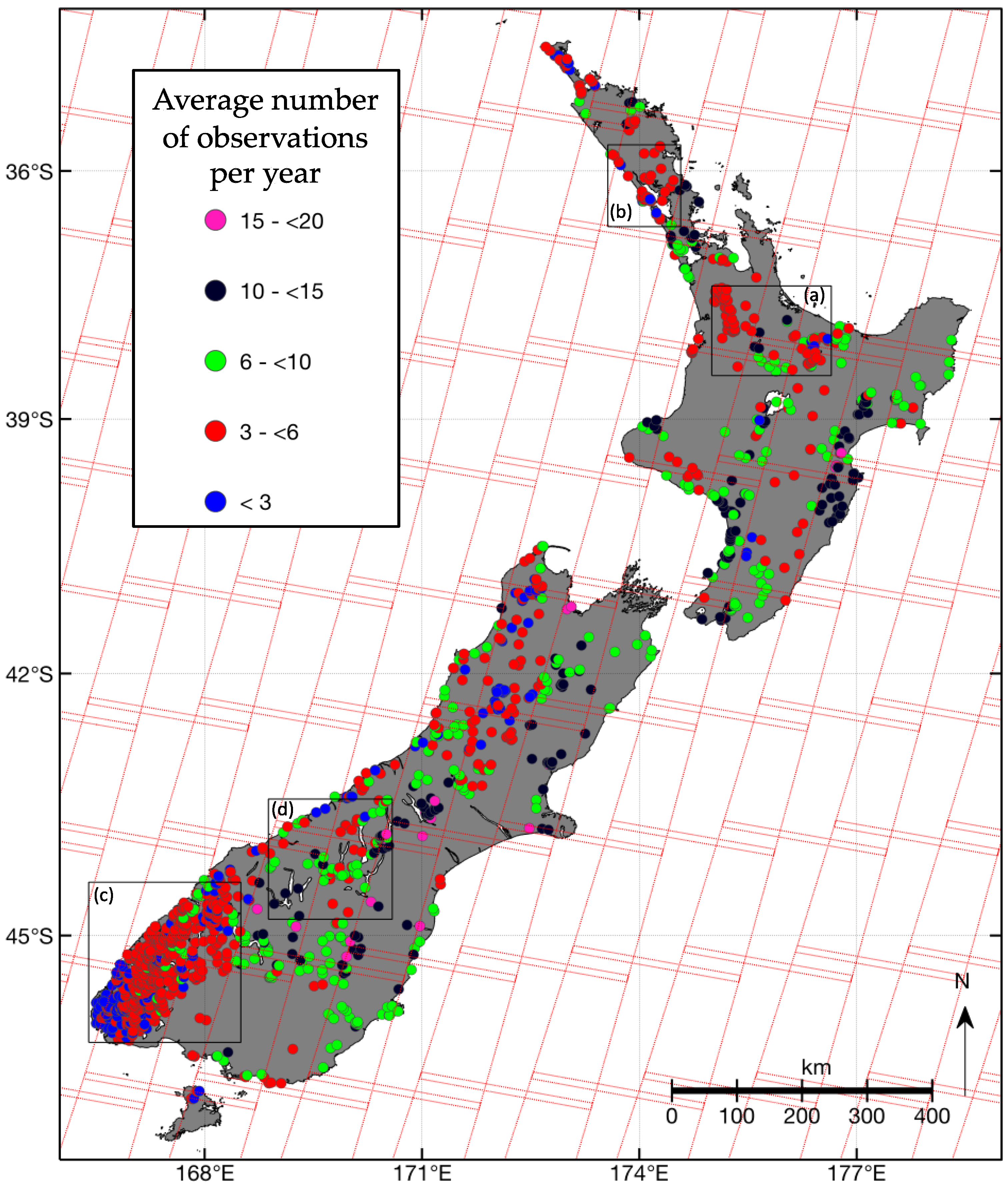
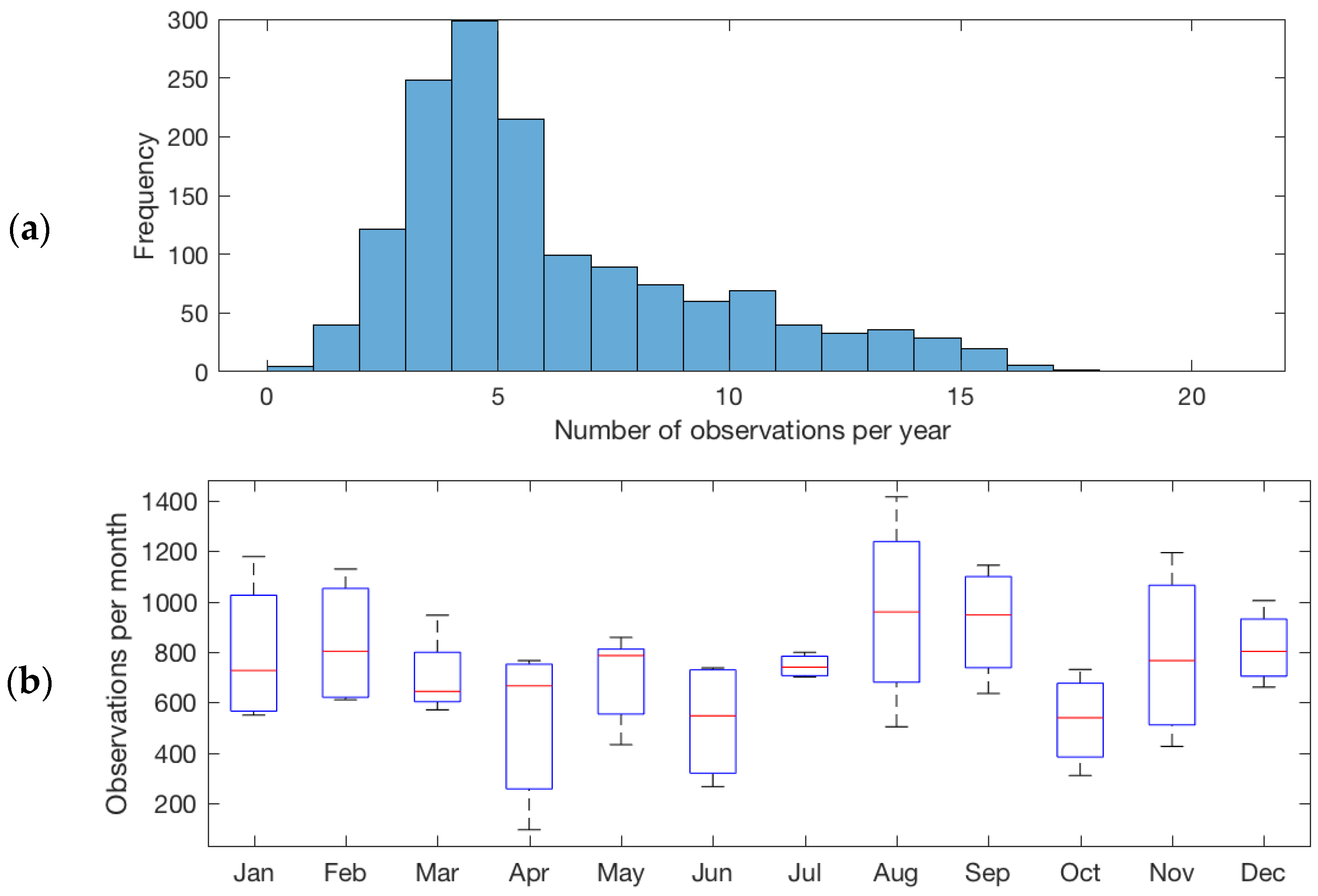
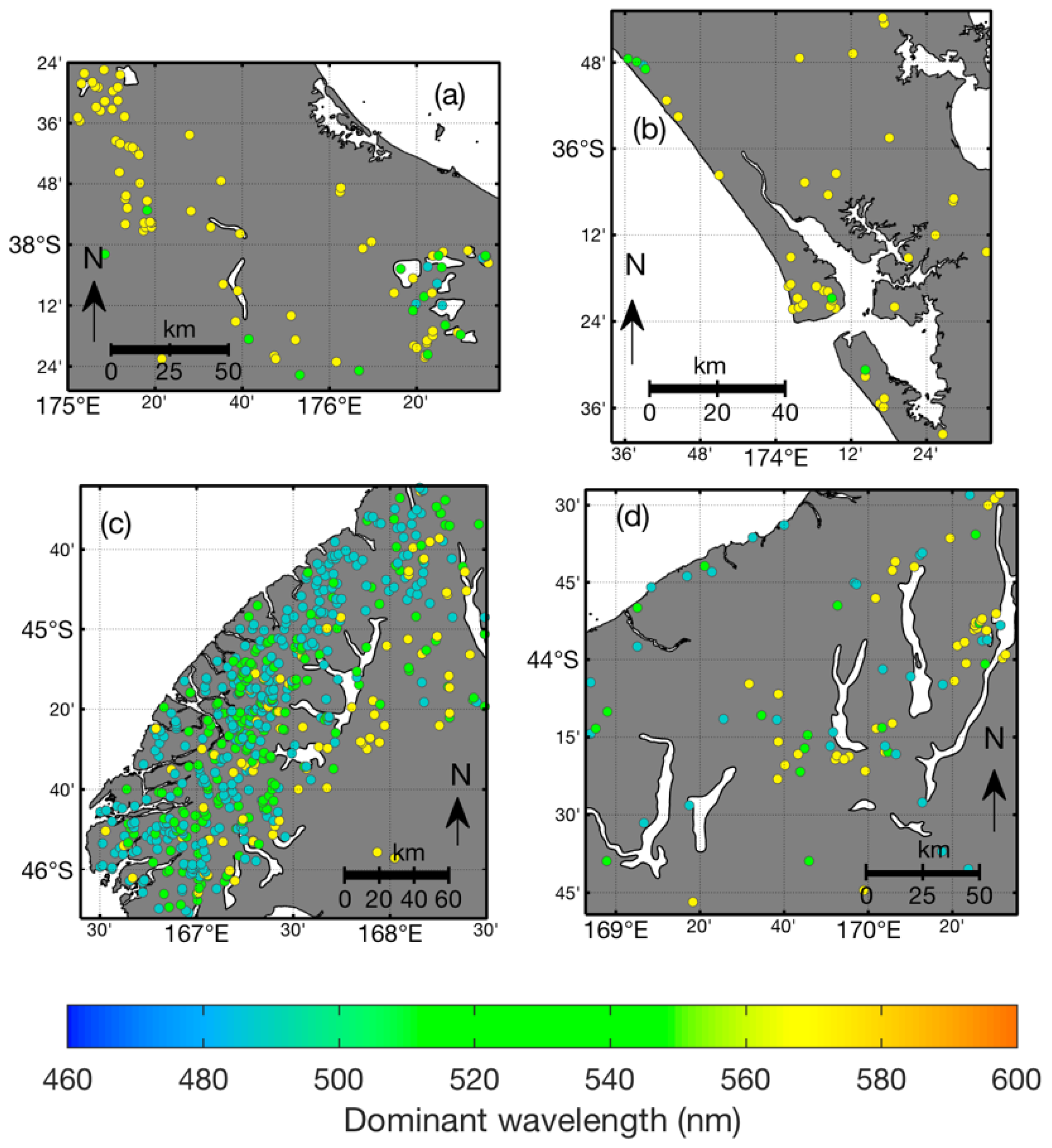
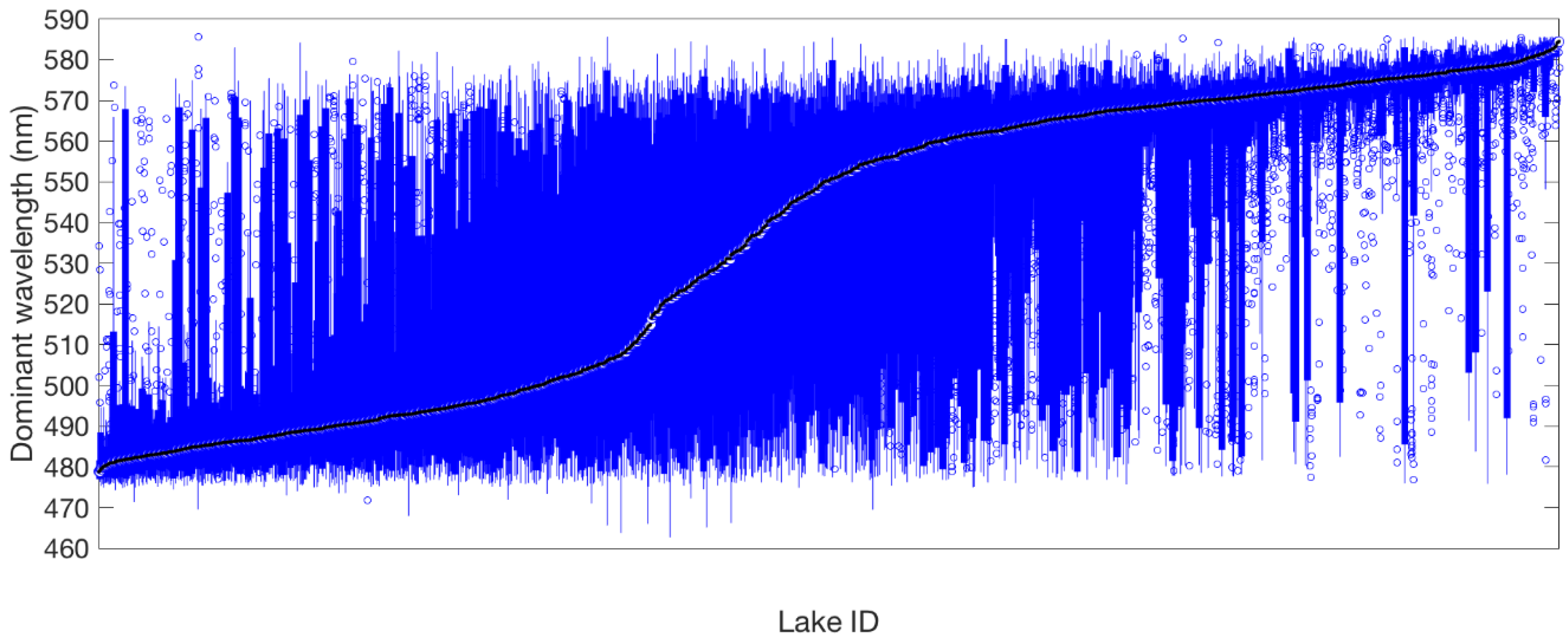
3.1. Observation Frequency of New Zealand Lakes
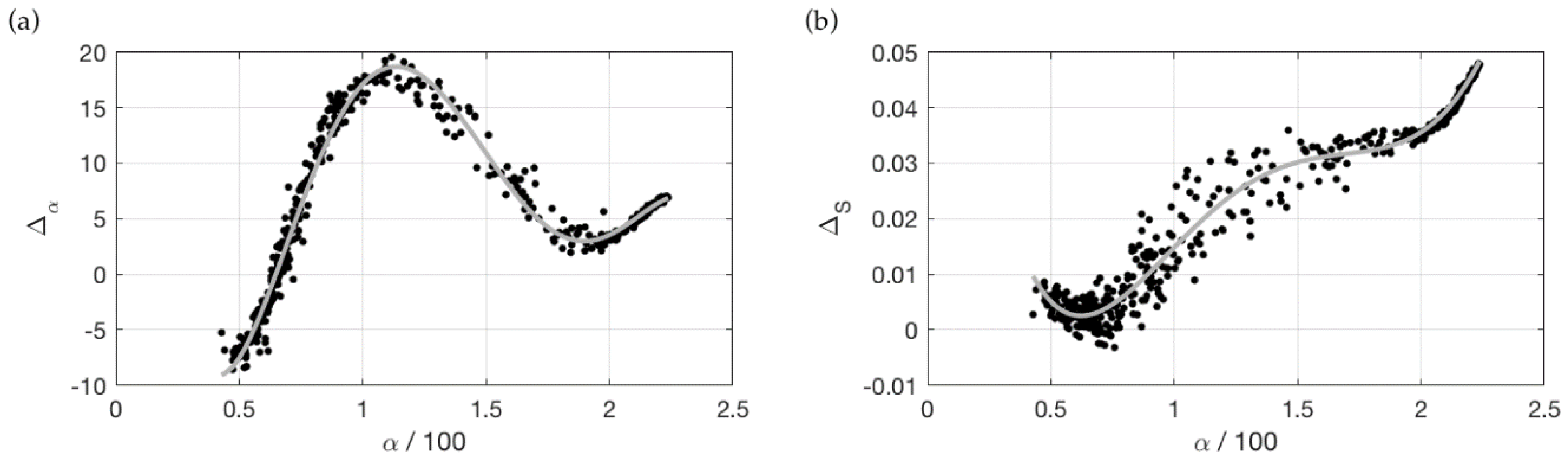
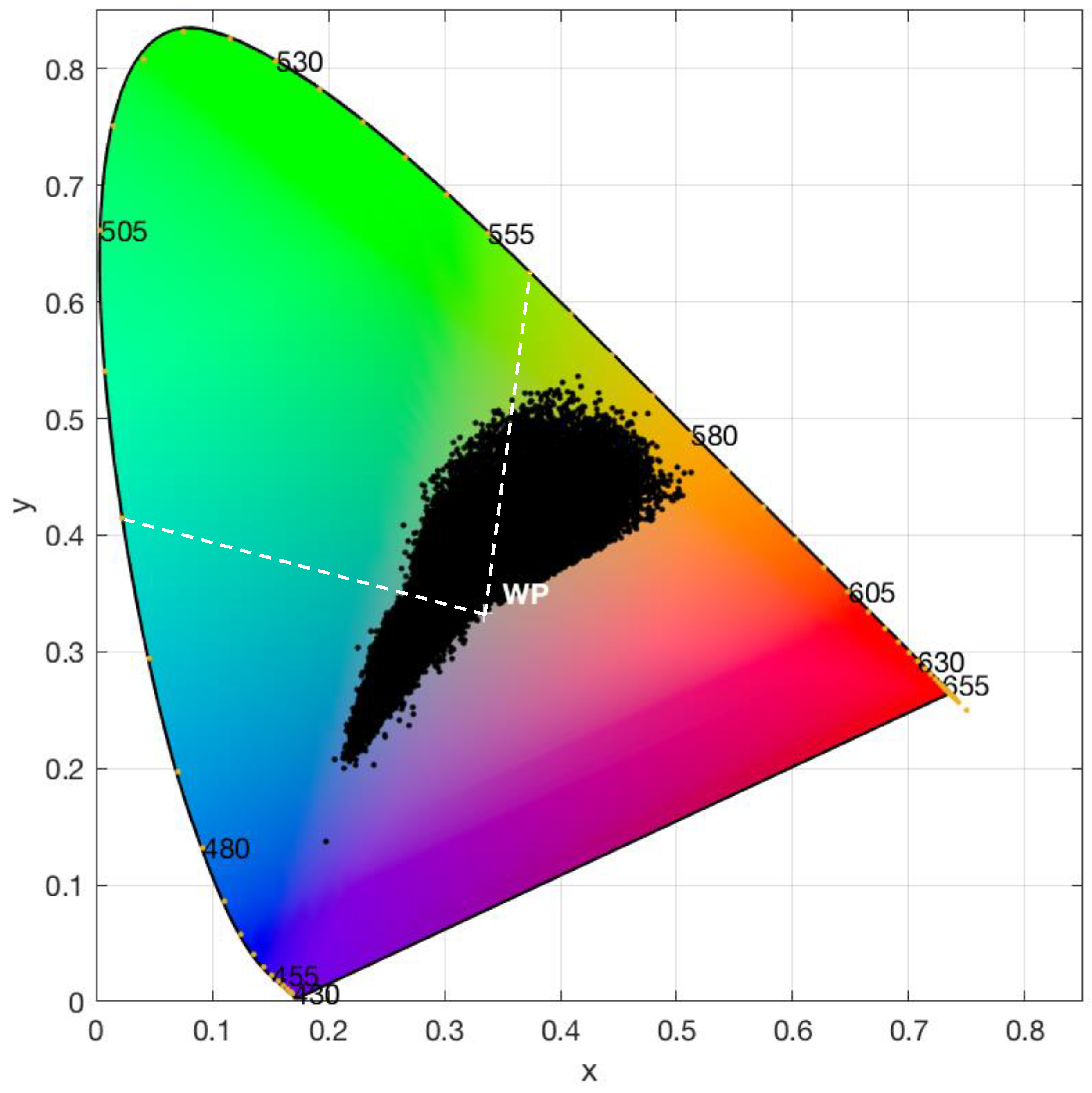
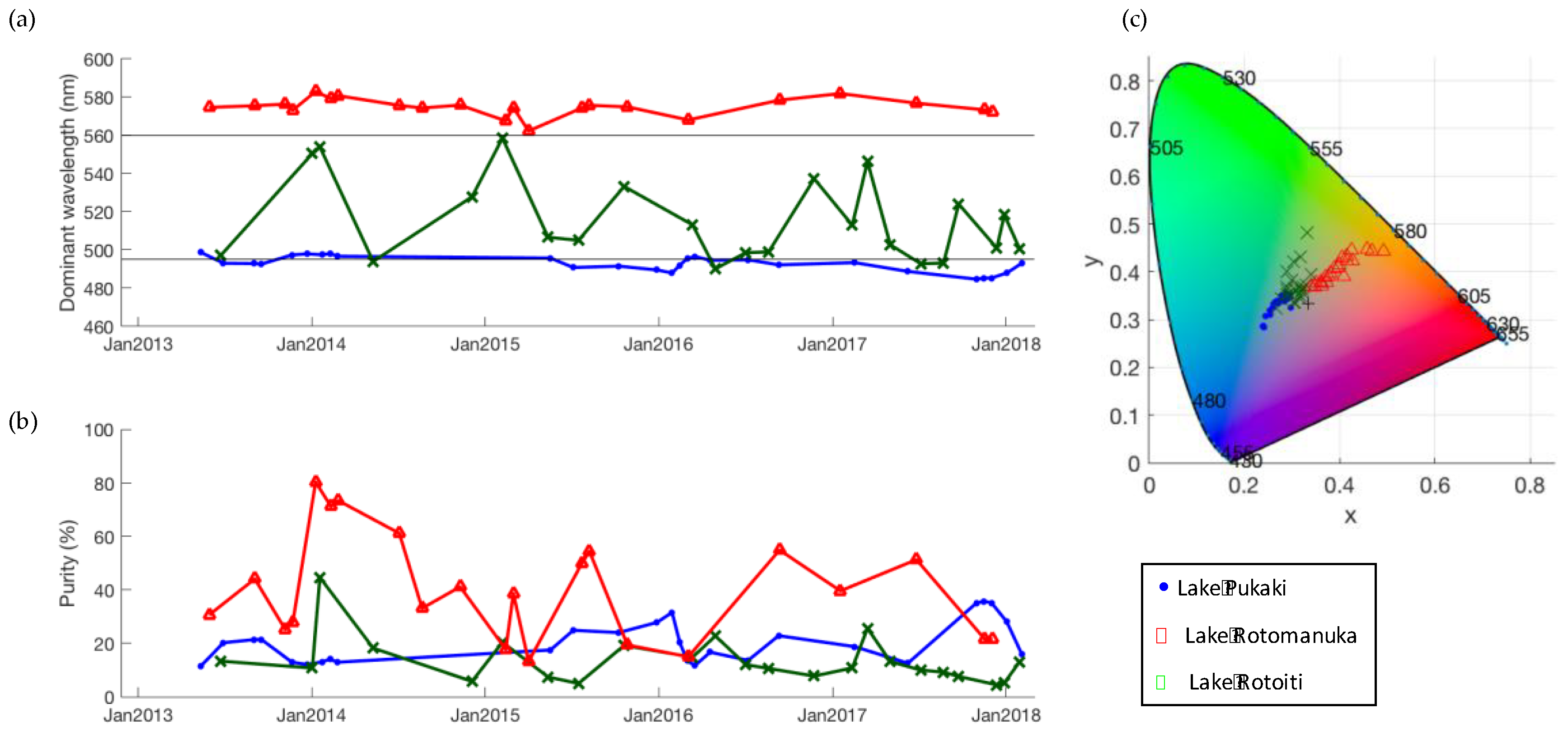
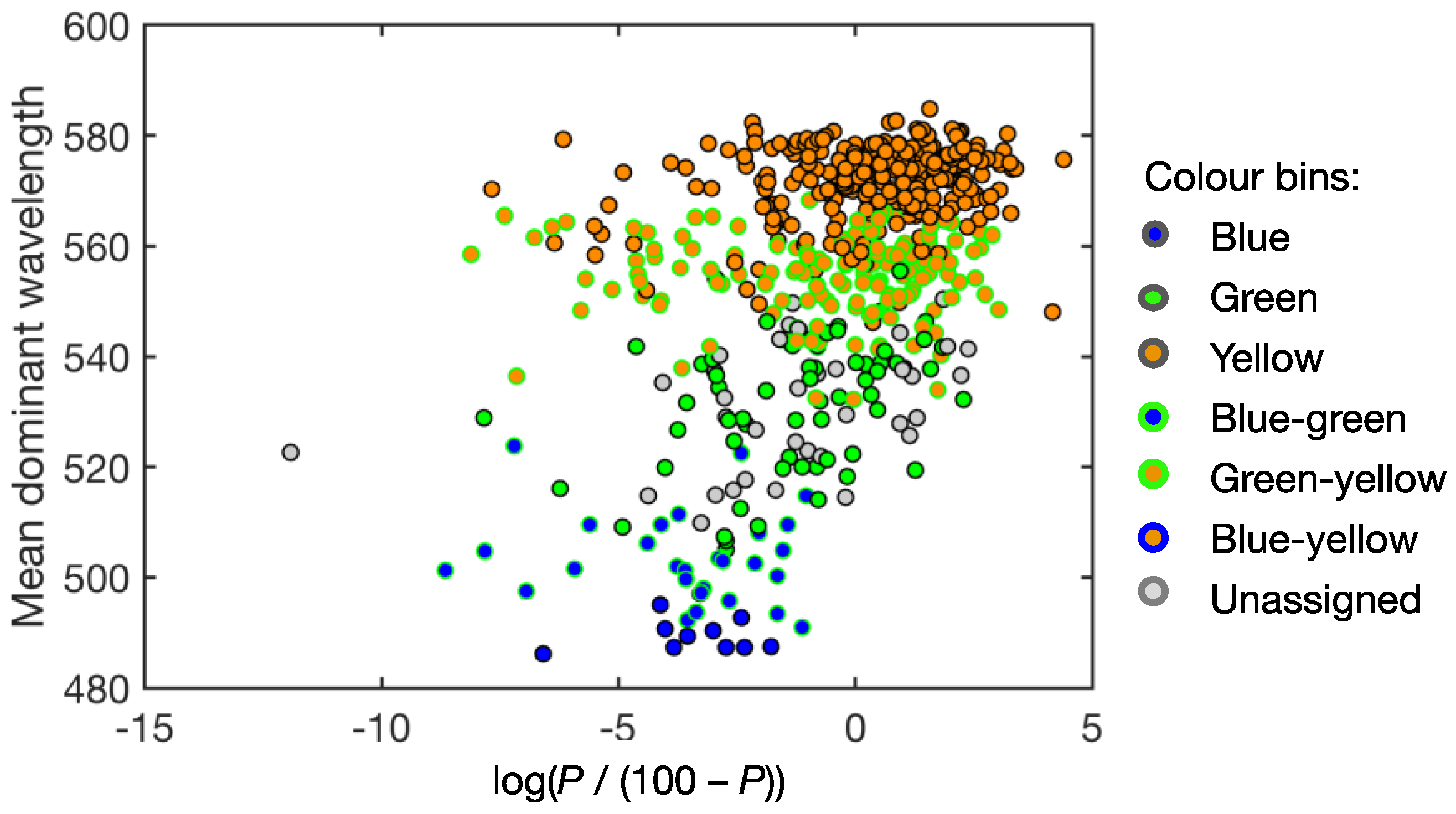
3.2. Correction for Purity
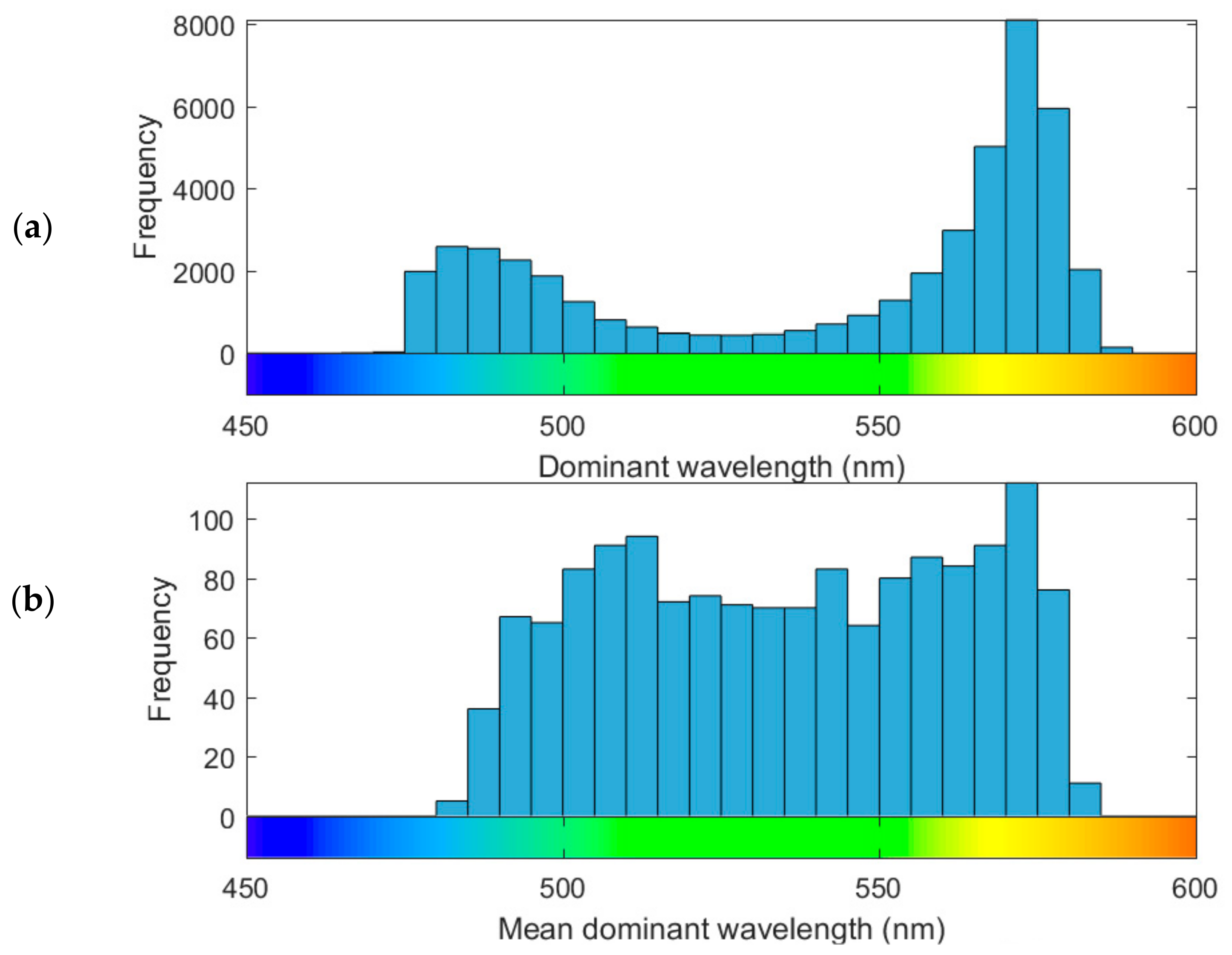
3.3. Lake Colour and Purity Distribution
4. Discussion
Limitations of Our Approach
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Davies-Colley, R.J.; Vant, W.N.; Smith, D.G. Colour and Clarity of Natural Waters—Science and Management of Optical Water Quality; The Blackburn Press: Caldwell, NJ, USA, 1993. [Google Scholar]
- Smith, D.G.; Davies-Colley, R.J. Perception of water clarity and colour in terms of suitability for recreational use. J. Environ. Manag. 1992, 36, 225–235. [Google Scholar] [CrossRef]
- West, A.O.; Nolan, J.M.; Scott, J.T. Optical water quality and human perceptions of rivers: An ethnohydrology study. Ecosyst. Health Sustain. 2016, 2. [Google Scholar] [CrossRef]
- Kirk, J.T.O. Optical Water-Quality—What Does It Mean and How Should We Measure It. J. Water Pollut. Con. F 1988, 60, 194–197. [Google Scholar]
- Wyszecki, G.; Stiles, W.S. Color Science: Concepts and Methods, Quantitative Data, and Formulae, 2nd ed.; Wiley-Interscience: Hoboken, NJ, USA, 2000. [Google Scholar]
- CIE. Commission Internationale de l’Éclairage Proceedings; Cambridge University Press: Cambridge, UK, 1932. [Google Scholar]
- Kirk, J.T.O. Light & Photosynthesis in Aquatic Ecosystems, 2nd ed.; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- Mobley, C.D. Light and Water: Radiative Transfer in Natural Waters; Academic Press: Cambridge, MA, USA, 1994. [Google Scholar]
- IOCCG. Remote Sensing of Inherent Optical Properties: Fundamentals, Tests of Algorithms, and Applications. In Reports of the International Ocean-Colour Coordinating Group (IOCCG); IOCCG: Dartmouth, NS, Canada, 2006. [Google Scholar]
- Pope, R.M.; Fry, E.S. Absorption spectrum (380–700 nm) of pure water. II. Integrating cavity measurements. Appl. Opt. 1997, 36, 8710–8723. [Google Scholar] [CrossRef] [PubMed]
- Bukata, R.P.; Bruton, J.E.; Jerome, J.H. Use of Chromaticity in Remote Measurements of Water-Quality. Remote Sens. Environ. 1983, 13, 161–177. [Google Scholar] [CrossRef]
- Bukata, P.R.; Jerome, J.H.; Kondratyev, K.Y.; Pozdnyakov, D. Optical Properties and Remote Sensing of Inland and Coastal Waters; CRC Press: Boca Raton, FL, USA, 1995. [Google Scholar]
- Dekker, A.G.; Peters, S.W.M.; Vos, R.J.; Rijkeboer, M. Remote sensing for inland water quality detection and monitoring: State-of-the-art application in Friesland. In GIS and Remote Sensing techniques in Land-and Water-Management; Dijk, A.v., Bos, M.G., Eds.; Kluwer Academic Publishers: Alphen aan den Rijn, The Netherlands, 2001; pp. 17–38. [Google Scholar]
- Julian, J.P.; Davies-Colley, R.J.; Gallegos, C.L.; Tran, T.V. Optical water quality of inland waters: A landscape perspective. Ann. Assoc. Am. Geogr. 2013, 103, 309–318. [Google Scholar] [CrossRef]
- Gholizadeh, M.H.; Melesse, A.M.; Reddi, L. A Comprehensive Review on Water Quality Parameters Estimation Using Remote Sensing Techniques. Sensors 2016, 16, 1298. [Google Scholar] [CrossRef] [PubMed]
- Palmer, S.C.J.; Kutser, T.; Hunter, P.D. Remote sensing of inland waters: Challenges, progress and future directions. Remote Sens. Environ. 2015, 157, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Tyler, A.N.; Hunter, P.D.; Spyrakos, E.; Groom, S.; Constantinescu, A.M.; Kitchen, J. Developments in Earth observation for the assessment and monitoring of inland, transitional, coastal and shelf-sea waters. Sci. Total Environ. 2016, 572, 1307–1321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allan, M.G. Remote Sensing of Water Quality in Rotorua and Waikato Lakes. Master’s Thesis, University of Waikato, Hamilton, New Zealand, 2008. [Google Scholar]
- Odermatt, D.; Gitelson, A.; Brando, V.E.; Schaepman, M. Review of constituent retrieval in optically deep and complex waters from satellite imagery. Remote Sens. Environ. 2012, 118, 116–126. [Google Scholar] [CrossRef] [Green Version]
- Olmanson, L.G.; Bauer, M.E.; Brezonik, P.L. A 20-year Landsat water clarity census of Minnesota’s 10,000 lakes. Remote Sens. Environ. 2008, 112, 4086–4097. [Google Scholar] [CrossRef]
- Van der Woerd, H.J.; Wernand, M.R. True Colour Classification of Natural Waters with Medium-Spectral Resolution Satellites: SeaWiFS, MODIS, MERIS and OLCI. Sensors 2015, 15, 25663–25680. [Google Scholar] [CrossRef] [Green Version]
- Van der Woerd, J.H.; Wernand, R.M. Hue-Angle Product for Low to Medium Spatial Resolution Optical Satellite Sensors. Remote Sens. 2018, 10, 180. [Google Scholar] [CrossRef]
- Lowe, D.J.; Green, J.D. The basis for lake diversity: Origins and development of lakes. In Inland Waters of New Zealand; Viner, A.B., Ed.; DSIR Science Publishing Centre: Wellington, New Zealand, 1987; p. 494. [Google Scholar]
- Vant, W.N.; Davies-Colley, R.J. Factors affecting clarity of New-Zealand lakes. New Zeal. J. Mar. Fresh. 1984, 18, 367–377. [Google Scholar] [CrossRef]
- Davies-Colley, R.J.; Vant, W.N. Absorption of light by yellow substance in fresh-water lakes. Limnol. Oceanogr. 1987, 32, 416–425. [Google Scholar] [CrossRef]
- Gallegos, C.L.; Davies-Colley, R.J.; Gall, M. Optical closure in lakes with contrasting extremes of reflectance. Limnol. Oceanogr. 2008, 53, 2021–2034. [Google Scholar] [CrossRef] [Green Version]
- Leathwick, J.R.; West, D.; Gerbeaux, P.; Kelly, D.; Robertson, H.; Bronwn, D.; Chadderton, W.L.; Ausseil, A.G. Freshwater Ecosystems of New Zealand (FENZ) Geodatabase Version One—User Guide; NIWA: Hamilton, New Zealand, 2010. [Google Scholar]
- Snelder, T. Definition of a Multivariate Classification of New Zealand Lakes; National Institute of Water and Atmospheric Research: Christchurch, New Zealand, 2006. [Google Scholar]
- Larned, S.; Snelder, T.; Unwin, M.; McBride, G.B.; Verburg, P.; McMillan, H. Analysis of Water Quality in New Zealand Lakes and Rivers; National Institute of Water and Atmospheric Research: Christchurch, New Zealand, 2015. [Google Scholar]
- Burns, N.M.; Rutherford, J.C.; Clayton, J.S. A Monitoring and Classification System for New Zealand Lakes and Reservoirs. Lake Reserv. Manag. 1999, 15, 255–271. [Google Scholar] [CrossRef]
- Verburg, P.; Hamill, K.; Unwin, M.; Abell, J. Lake Water Quality in New Zealand 2010: Status and Trends; National Institute of Water and Atmospheric Research: Christchurch, New Zealand, 2010. [Google Scholar]
- LINZ Land Information New Zealand—Aerial Imagery. Sourced from the LINZ Data Service and Licensed by the Copyright Holder for Re-Use under the Creative Commons Attribution 3.0 New Zealand. Available online: https://data.linz.govt.nz/ (accessed on 13 August 2018).
- Roy, D.P.; Wulder, M.A.; Loveland, T.R.; Woodcock, C.E.; Allen, R.G.; Anderson, M.C.; Helder, D.; Irons, J.R.; Johnson, D.M.; Kennedy, R.; et al. Landsat-8: Science and product vision for terrestrial global change research. Remote Sens. Environ. 2014, 145, 154–172. [Google Scholar] [CrossRef]
- USGS. Product Guide—Landsat 8 Surface Reflectance Code (LaSRC) Product, Version 4.3 2018. Available online: https://landsat.usgs.gov/sites/default/files/documents/lasrc_product_guide.pdf (accessed on 13 August 2018).
- Vermote, E.; Justice, C.; Claverie, M.; Franch, B. Preliminary analysis of the performance of the Landsat 8/OLI land surface reflectance product. Remote Sens. Environ. 2016, 185, 46–56. [Google Scholar] [CrossRef]
- Kay, S.; Hedley, D.J.; Lavender, S. Sun Glint Correction of High and Low Spatial Resolution Images of Aquatic Scenes: A Review of Methods for Visible and Near-Infrared Wavelengths. Remote Sens. 2009, 1, 697–730. [Google Scholar] [CrossRef]
- Nicolas, J.M.; Deschamps, P.Y.; Frouin, R. Spectral reflectance of oceanic whitecaps in the visible and near infrared: Aircraft measurements over open ocean. Geophys Res. Lett. 2001, 28, 4445–4448. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.; Wang, S.; Woodcock, C.E. Improvement and expansion of the Fmask algorithm: Cloud, cloud shadow, and snow detection for Landsats 4–7, 8, and Sentinel 2 images. Remote Sens. Environ. 2015, 159, 269–277. [Google Scholar] [CrossRef]
- Foga, S.; Scaramuzza, P.L.; Guo, S.; Zhu, Z.; Dilley, R.D.; Beckmann, T.; Schmidt, G.L.; Dwyer, J.L.; Joseph Hughes, M.; Laue, B. Cloud detection algorithm comparison and validation for operational Landsat data products. Remote Sens. Environ. 2017, 194, 379–390. [Google Scholar] [CrossRef] [Green Version]
- McFeeters, S.K. The use of the Normalized Difference Water Index (NDWI) in the delineation of open water features. Int. J. Remote Sens. 1996, 17, 1425–1432. [Google Scholar] [CrossRef]
- Barsi, J.; Lee, K.; Kvaran, G.; Markham, B.; Pedelty, J. The Spectral Response of the Landsat-8 Operational Land Imager. Remote Sens. 2014, 6, 10232–10251. [Google Scholar] [CrossRef] [Green Version]
- Pahlevan, N.; Lee, Z.P.; Wei, J.W.; Schaaf, C.B.; Schott, J.R.; Berk, A. On-orbit radiometric characterization of OLI (Landsat-8) for applications in aquatic remote sensing. Remote Sens. Environ. 2014, 154, 272–284. [Google Scholar] [CrossRef]
- Alföldi, T.T.; Munday, J.C. Water quality analysis by digital chromaticity mapping of Landsat data. Can. J. Remote Sens. 1978, 4, 108–126. [Google Scholar] [CrossRef]
- Munday, J.C. Chromaticity of path radiance and atmospheric correction of Landsat data. Remote Sens. Environ. 1983, 13, 525–538. [Google Scholar] [CrossRef]
- Mobley, C.D. Hydrolight Users’ Guide. 2013. Available online: https://www.sequoiasci.com/wp-content/uploads/2013/07/HE52UsersGuide.pdf (accessed on 10 August 2018).
- IOCCG Synthesized Dataset from IOCCG Report 5. 2015. Available online: http://ioccg.org/what-we-do/ioccg-publications/ioccg-reports/synthesized-dataset-from-ioccg-report-5/ (accessed on 10 August 2018).
- USGS. USGS Spectral Viewer. 2018. Available online: https://landsat.usgs.gov/using-usgs-spectral-viewer (accessed on 10 August 2018).
- Landcare. Land Cover Database Version 4.1, Mainland New Zealand. Landcare Research: 2015. Available online: https://lris.scinfo.org.nz/layer/48423-lcdb-v41-land-cover-database-version-41-mainland-new-zealand/ (accessed on 10 August 2018).
- Tait, A.; Liley, B. Interpolation of daily solar radiation for New Zealand using a satellite data-derived cloud cover surface. Weather Clim. 2009, 29, 70–88. [Google Scholar]
- NIWA. Sunshine Hours Annual Average 1972–2013. Available online: https://statisticsnz.shinyapps.io/sunshine_hours/ (accessed on 10 August 2018).
- Dean-Speirs, T.; Neilson, K. Waikato Region Shallow Lakes Management Plan. 2014. Available online: https://www.waikatoregion.govt.nz/services/publications/technical-reports/tr/tr201458 (accessed on 10 August 2018).
- Von Westernhagen, N.; Hamilton, D.P.; Pilditch, C.A. Temporal and spatial variations in phytoplankton productivity in surface waters of a warm-temperate, monomictic lake in New Zealand. Hydrobiologia 2010, 652, 57–70. [Google Scholar] [CrossRef] [Green Version]
- Wood, S.A.; Paul, W.J.; Hamilton, D.P. Cyanobacterial Biovolumes for the Rotorua Lakes. 2008. Available online: https://www.boprc.govt.nz/media/32233/Cawthron-090803-CyanobacterialbiovolumesforRotorualakes.pdf (accessed on 10 August 2018).
- USGS. Path/Row Shapefiles. 2018. Available online: https://landsat.usgs.gov/pathrow-shapefiles (accessed on 10 August 2018).
- Ministry for the Environment. A Guide to the National Policy Statement for Freshwater Management 2014; MfE: Wellington, New Zealand, 2015.
- Ministry for the Environment. National Policy Statement for Freshwater Management 2014; MfE: Wellington, New Zealand, 2014.
- Drusch, M.; Del Bello, U.; Carlier, S.; Colin, O.; Fernandez, V.; Gascon, F.; Hoersch, B.; Isola, C.; Laberinti, P.; Martimort, P.; et al. Sentinel-2: ESA’s Optical High-Resolution Mission for GMES Operational Services. Remote Sens. Environ. 2012, 120, 25–36. [Google Scholar] [CrossRef]
- Moore, T.S.; Dowell, M.D.; Bradt, S.; Ruiz Verdu, A. An optical water type framework for selecting and blending retrievals from bio-optical algorithms in lakes and coastal waters. Remote Sens. Environ. 2014, 143, 97–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spyrakos, E.; O’Donnell, R.; Hunter, P.D.; Miller, C.; Scott, M.; Simis, S.G.H.; Neil, C.; Barbosa, C.C.F.; Binding, C.E.; Bradt, S.; et al. Optical types of inland and coastal waters. Limnol. Oceanogr. 2017, 63, 846–870. [Google Scholar] [CrossRef] [Green Version]
- Eleveld, A.M.; Ruescas, B.A.; Hommersom, A.; Moore, S.T.; Peters, W.S.; Brockmann, C. An Optical Classification Tool for Global Lake Waters. Remote Sens. 2017, 9, 420. [Google Scholar] [CrossRef]
- Karlsson, J.; Byström, P.; Ask, J.; Ask, P.; Persson, L.; Jansson, M. Light limitation of nutrient-poor lake ecosystems. Nature 2009, 460, 506. [Google Scholar] [CrossRef] [PubMed]
- Busch, J.A.; Price, I.; Jeansou, E.; Zielinski, O.; van der Woerd, H.J. Citizens and satellites: Assessment of phytoplankton dynamics in a NW Mediterranean aquaculture zone. Int. J. Appl. Earth Obs. 2016, 47, 40–49. [Google Scholar] [CrossRef]
- Brauman, K.A.; Daily, G.C.; Duarte, T.K.e.; Mooney, H.A. The Nature and Value of Ecosystem Services: An Overview Highlighting Hydrologic Services. Annu. Rev. Environ. Resour. 2007, 32, 67–98. [Google Scholar] [CrossRef]
- MfE. National Environmental Monitoring Standards—Water Quality, Part 3 (Draft). Ministry for Business, Innovation and Employment, 2017. Available online: http://nems.org.nz/documents/water-quality-part-3-lakes/ (accessed on 10 August 2018).
- Davies-Colley, R.J.; Smith, D.G.; Speed, D.J.; Nagels, J.W. Matching natural water colors to Munsell standards. J. Am. Water Resour. Assoc. 1997, 33, 1351–1361. [Google Scholar] [CrossRef]
- Hayward, S.; Meredith, A.; Stevenson, M. Review of Proposed NRRP Water Quality Objectives and Standards for Rivers and Lakes in the Canterbury Region; Environment Canterbury: Christchurch, New Zealand, 2009.
- Schallenberg, M.; Sorrell, B. Regime shifts between clear and turbid water in New Zealand lakes: Environmental correlates and implications for management and restoration. New Zeal. J. Mar. Fresh. 2009, 43, 701–712. [Google Scholar] [CrossRef] [Green Version]
- Foote, K.; Joy, M.; Death, R. New Zealand Dairy Farming: Milking Our Environment for All Its Worth. Environ. Manag. 2015, 56, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Snelder, T.H.; Larned, S.T.; McDowell, R.W. Anthropogenic increases of catchment nitrogen and phosphorus loads in New Zealand. New Zeal. J. Mar. Fresh. 2017. [Google Scholar] [CrossRef]
- McDowell, R.W.; Wilcock, R.J. Water quality and the effects of different pastoral animals. New Zeal. Vet. J. 2008, 56, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Allan, M.G.; Hamilton, D.P.; Trolle, D.; Muraoka, K.; McBride, C. Spatial heterogeneity in geothermally-influenced lakes derived from atmospherically corrected Landsat thermal imagery and three-dimensional hydrodynamic modelling. Int. J. Appl. Earth Obs. 2016, 50, 106–116. [Google Scholar] [CrossRef]
- Hicks, B.J.; Stichbury, G.A.; Brabyn, L.K.; Allan, M.G.; Ashraf, S. Hindcasting water clarity from Landsat satellite images of unmonitored shallow lakes in the Waikato region, New Zealand. Environ. Monit. Assess. 2013, 185, 7245–7261. [Google Scholar] [CrossRef] [PubMed]
- Allan, M.G.; Hamilton, D.P.; Hicks, B.J.; Brabyn, L. Landsat remote sensing of chlorophyll a concentrations in central North Island lakes of New Zealand. Int. J. Remote Sens. 2011, 32, 2037–2055. [Google Scholar] [CrossRef]
- Allan, M.G.; Hamilton, D.P.; Hicks, B.; Brabyn, L. Empirical and semi-analytical chlorophyll a algorithms for multi-temporal monitoring of New Zealand lakes using Landsat. Environ. Monit. Assess. 2015, 187. [Google Scholar] [CrossRef] [PubMed]
- Lymburner, L.; Botha, E.; Hestir, E.; Anstee, J.; Sagar, S.; Dekker, A.; Malthus, T. Landsat 8: Providing continuity and increased precision for measuring multi-decadal time series of total suspended matter. Remote Sens. Environ. 2016, 185, 108–118. [Google Scholar] [CrossRef]
- Pahlevan, N.; Schott, J.R.; Franz, B.A.; Zibordi, G.; Markham, B.; Bailey, S.; Schaaf, C.B.; Ondrusek, M.; Greb, S.; Strait, C.M. Landsat 8 remote sensing reflectance (Rrs) products: Evaluations, intercomparisons, and enhancements. Remote Sens. Environ. 2017, 190, 289–301. [Google Scholar] [CrossRef]
- Bernardo, N.; Watanabe, F.; Rodrigues, T.; Alcântara, E. Atmospheric correction issues for retrieving total suspended matter concentrations in inland waters using OLI/Landsat-8 image. Adv. Sp. Res. 2017, 59, 2335–2348. [Google Scholar] [CrossRef]
- Vanhellemont, Q.; Ruddick, K. Advantages of high quality SWIR bands for ocean colour processing: Examples from Landsat-8. Remote Sens. Environ. 2015, 161, 89–106. [Google Scholar] [CrossRef]
- Doxani, G.; Vermote, E.; Roger, J.C.; Gascon, F.; Adriaensen, S.; Frantz, D.; Hagolle, O.; Hollstein, A.; Kirches, G.; Li, F.; et al. Atmospheric Correction Inter-Comparison Exercise. Remote Sens. 2018, 10, 352. [Google Scholar] [CrossRef]
- Franz, B.A.; Bailey, S.W.; Kuring, N.; Werdell, P.J. Ocean Color Measurements with the Operational Land Imager on Landsat-8: Implementation and Evaluation in SeaDAS. Available online: https://www.spiedigitallibrary.org/journals/Journal-of-Applied-Remote-Sensing/volume-9/issue-01/096070/Ocean-color-measurements-with-the-Operational-Land-Imager-on Landsat/10.1117/1.JRS.9.096070.full?SSO=1 (accessed on 10 August 2018).
- Wulder, M.A.; White, J.C.; Loveland, T.R.; Woodcock, C.E.; Belward, A.S.; Cohen, W.B.; Fosnight, E.A.; Shaw, J.; Masek, J.G.; Roy, D.P. The global Landsat archive: Status, consolidation, and direction. Remote Sens. Environ. 2016, 185, 271–283. [Google Scholar] [CrossRef]

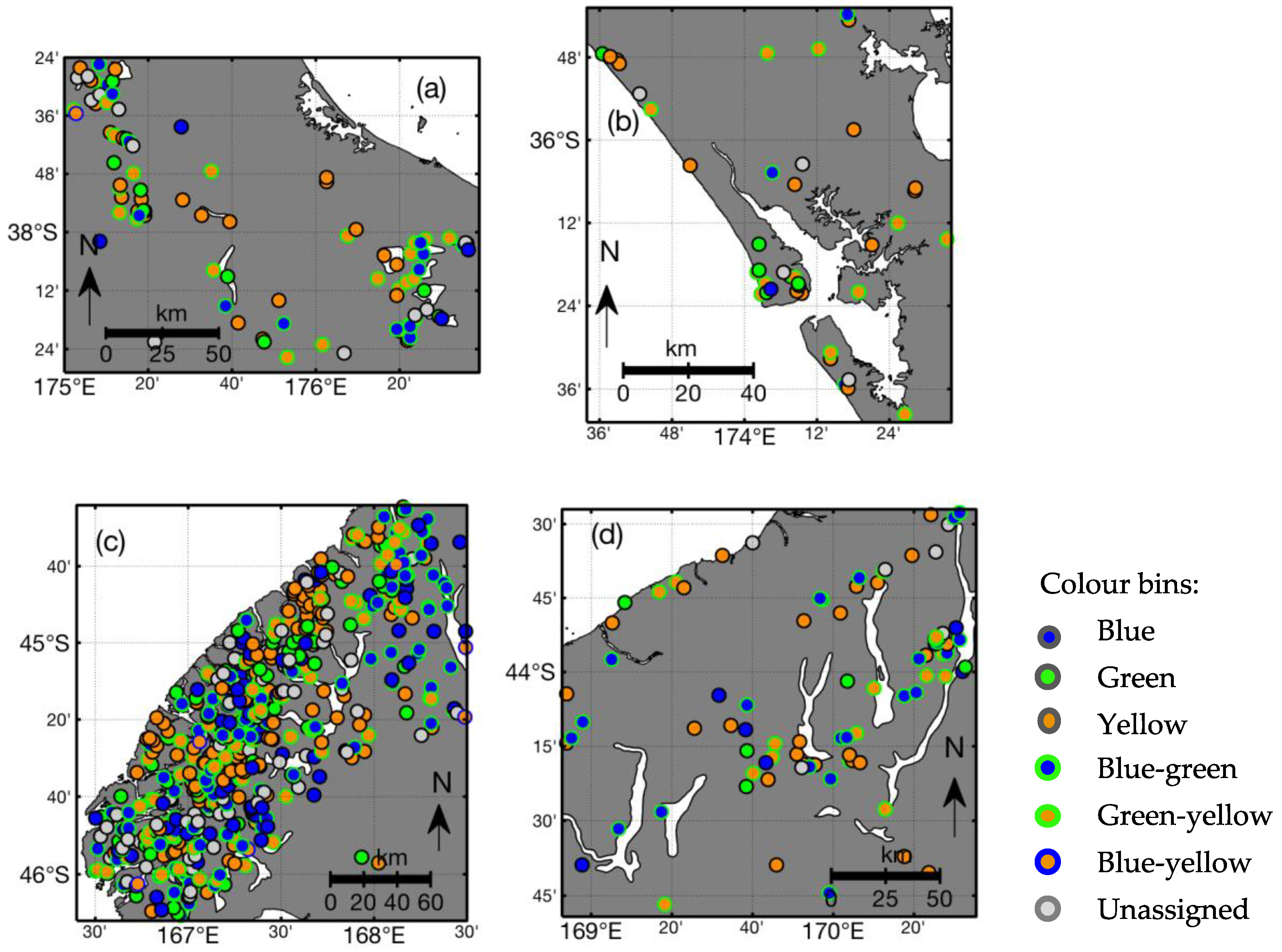
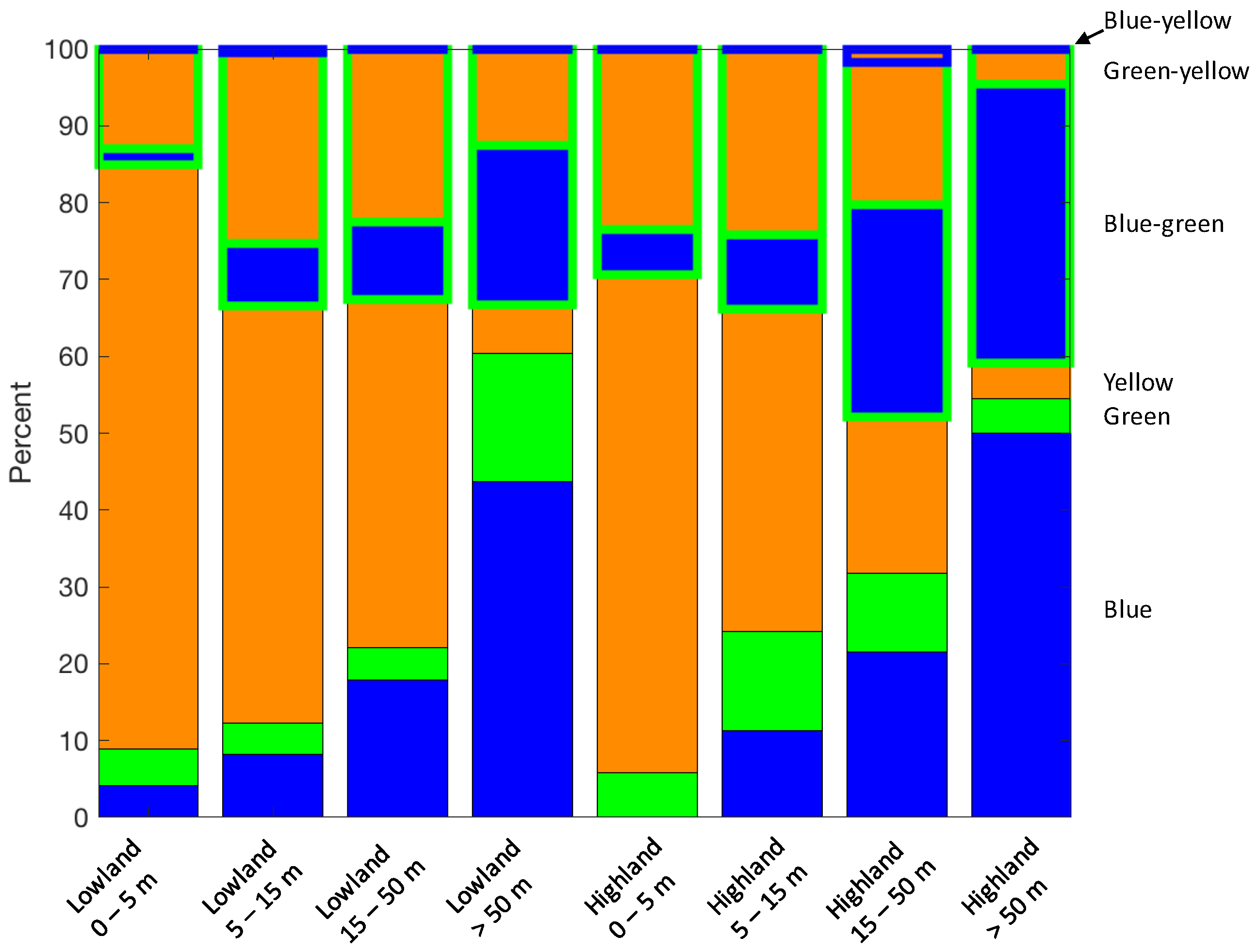
| Colour Bin 1 | Criterion | Number of Lakes 2 |
|---|---|---|
| Blue | ≥60% blue | 277 (18.6%) |
| Green | ≥60% green | 124 (8.3%) |
| Yellow | ≥60% yellow | 547 (36.8%) |
| Blue-green | ≥40% blue ≥20% green | 287 (19.3%) |
| Green-yellow | ≥40% yellow ≥20% green | 302 (20.3%) |
| Blue-yellow | ≥40% blue ≥40% yellow | 15 (1.0%) |
| Unassigned | None of the above | 195 (13.1%) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lehmann, M.K.; Nguyen, U.; Allan, M.; Van der Woerd, H.J. Colour Classification of 1486 Lakes across a Wide Range of Optical Water Types. Remote Sens. 2018, 10, 1273. https://doi.org/10.3390/rs10081273
Lehmann MK, Nguyen U, Allan M, Van der Woerd HJ. Colour Classification of 1486 Lakes across a Wide Range of Optical Water Types. Remote Sensing. 2018; 10(8):1273. https://doi.org/10.3390/rs10081273
Chicago/Turabian StyleLehmann, Moritz K., Uyen Nguyen, Mathew Allan, and Hendrik Jan Van der Woerd. 2018. "Colour Classification of 1486 Lakes across a Wide Range of Optical Water Types" Remote Sensing 10, no. 8: 1273. https://doi.org/10.3390/rs10081273