Estimating Net Photosynthesis of Biological Soil Crusts in the Atacama Using Hyperspectral Remote Sensing
Abstract
:1. Introduction
- To describe for the first time the hyperspectral reflectance signal of BSCs in the Atacama Desert under different water availability conditions.
- To test the suitability of hyperspectral remote sensing data for the estimation of net photosynthesis (NP) of BSCs.
- To test whether a robust transfer function can be established between NP and hyperspectral images acquired under field conditions, which allows mapping NP across larger scales.
2. Materials and Methods
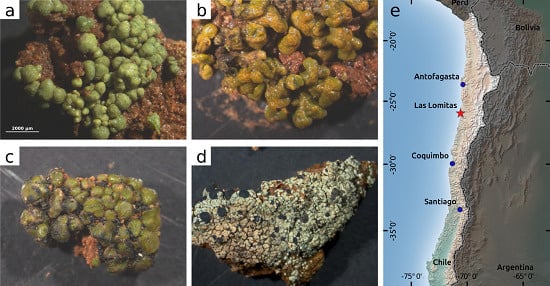
2.1. Area of Investigation
2.2. Sampling of BSCs
2.3. Laboratory Analysis
2.4. Hyperspectral Measurements
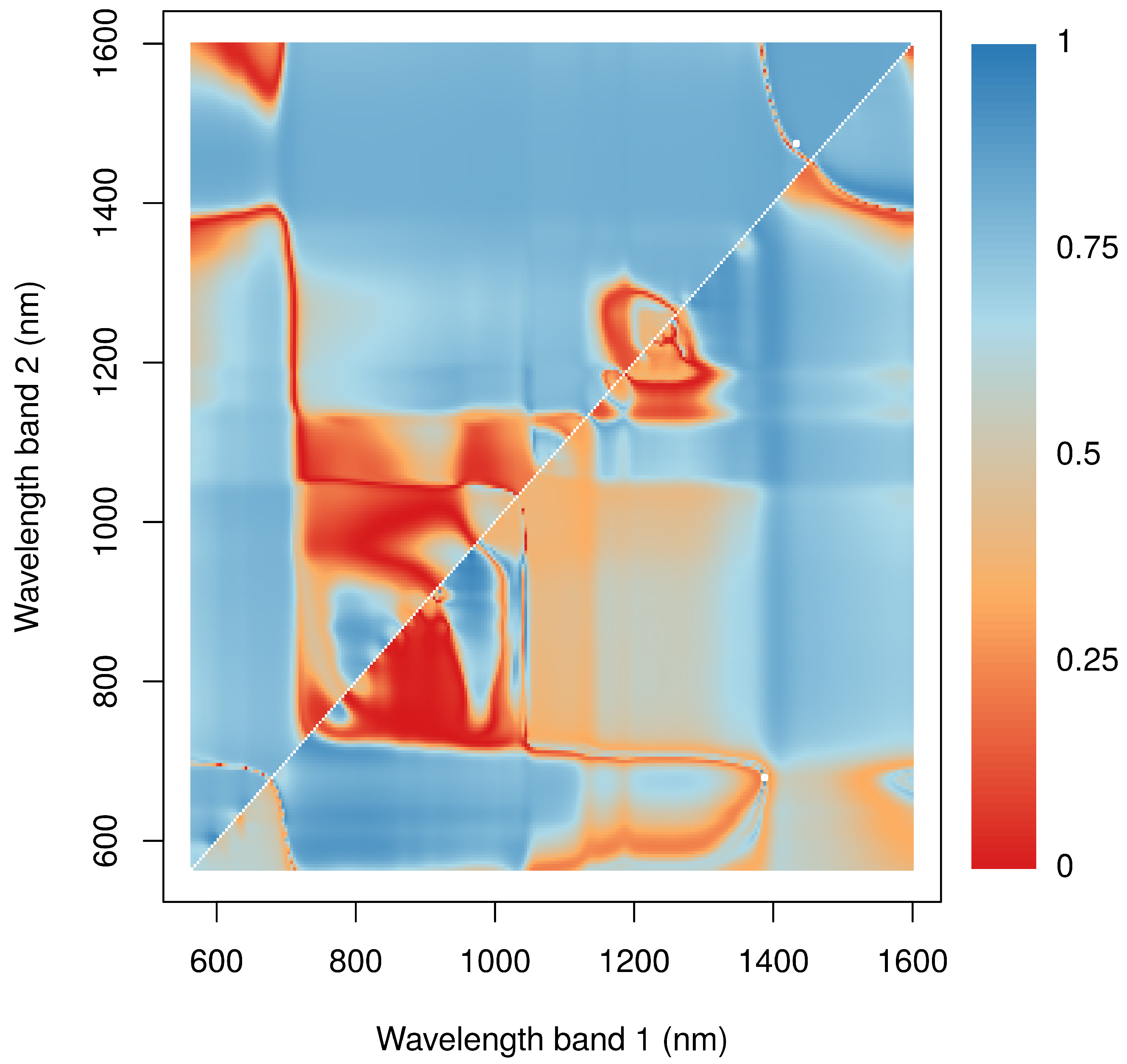
2.5. Hyperspectral Analysis
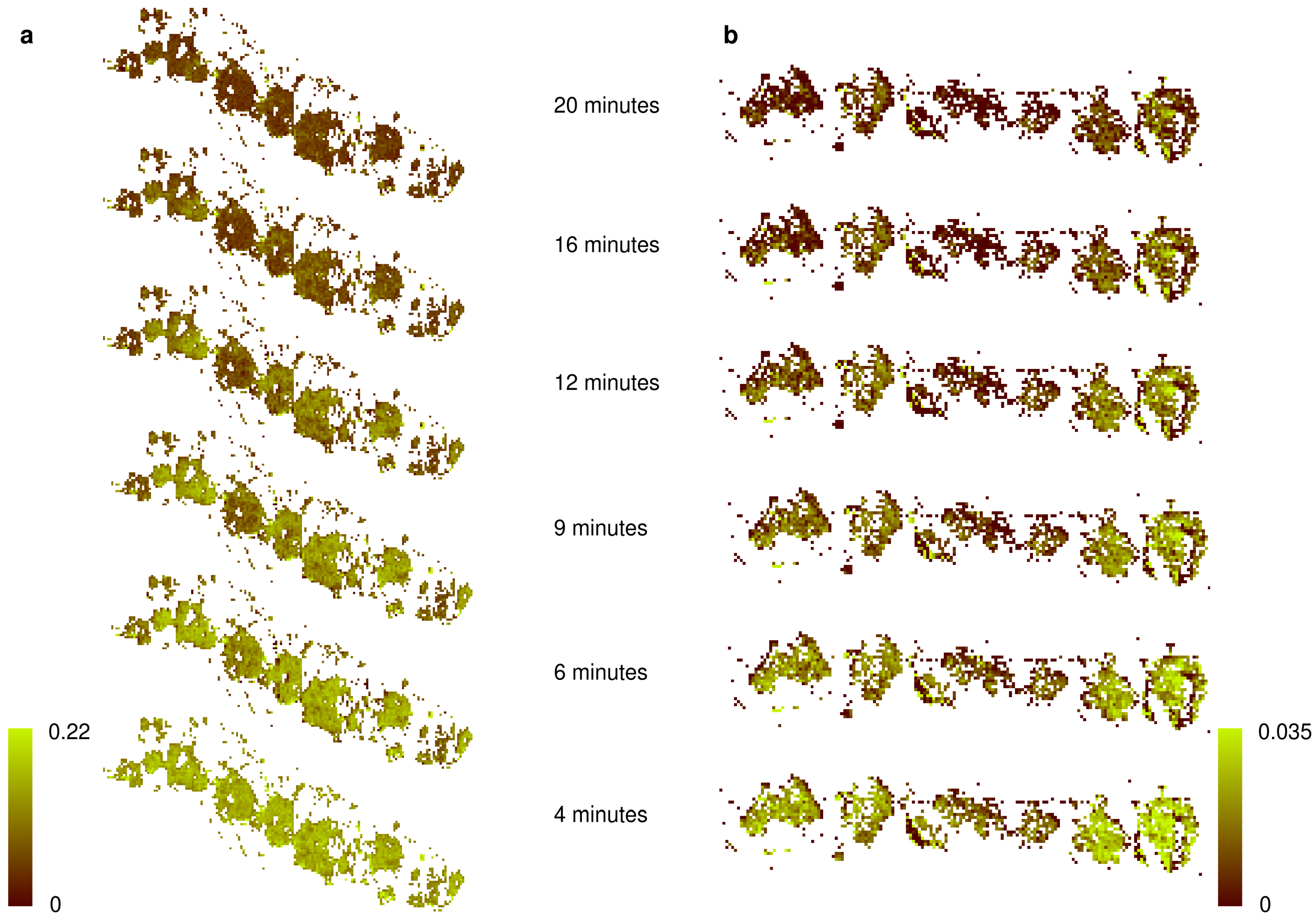
3. Results
4. Discussion
5. Conclusions
- We described for the first time the hyperspectral reflectance signal of BSCs in the Atacama Desert under different water availability conditions. Here, we could demonstrate that hyperspectral reflectance signals among wide-spread species of BSCs in the Atacama Desert differed largely, but water content affected the spectra in a similar manner. Changes in water availability immediately influenced the chlorophyll absorption bands in the visible and the water absorption bands in the near-infrared part of the electromagnetic spectrum.
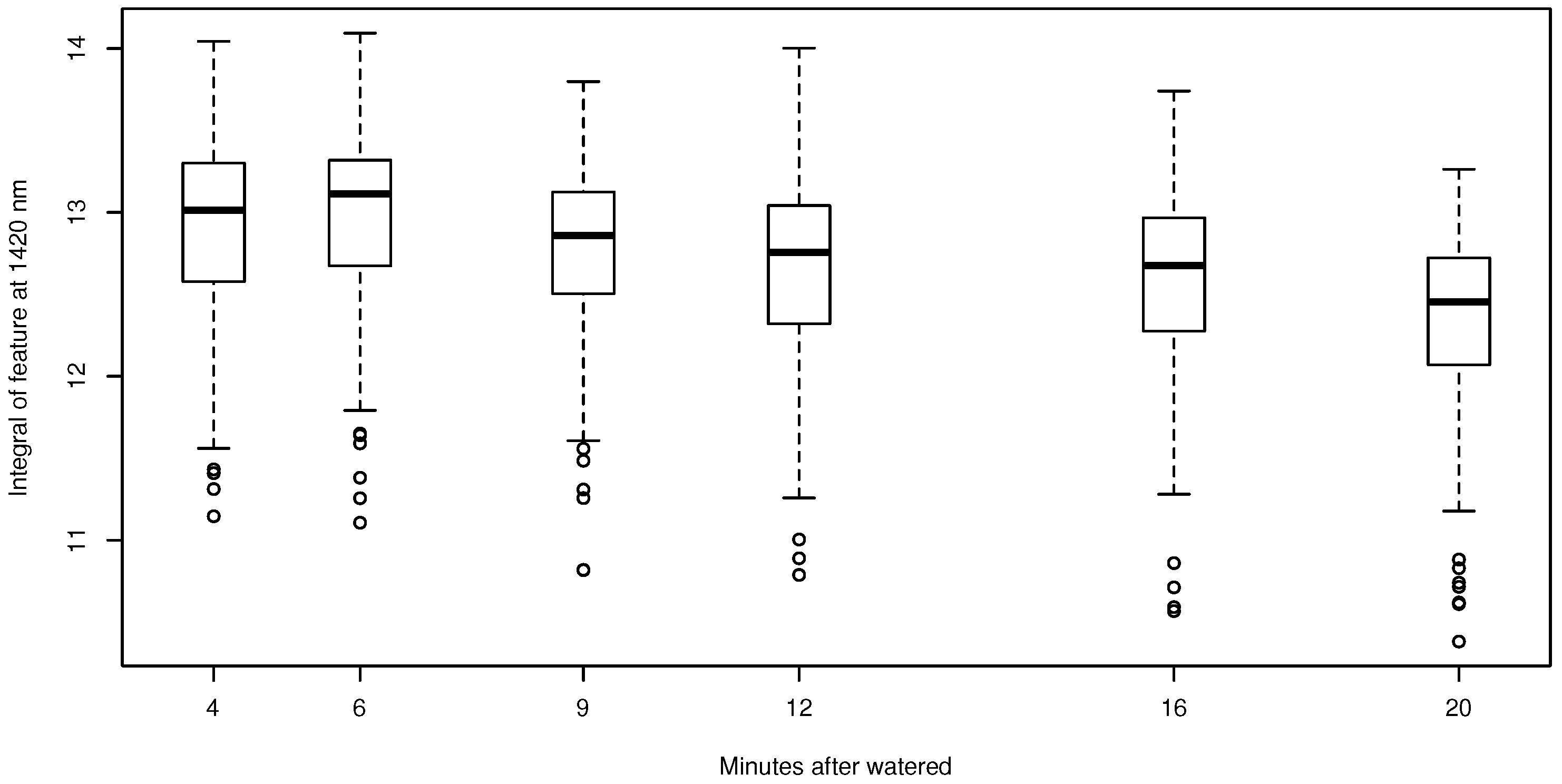
- We tested the suitability of hyperspectral remote sensing data for the NP estimation of BSCs and found that the relationship between water content and NP is highly species dependent, urging the need for species-specific empirical transfer functions between NP and hyperspectral reflectance values. In this respect, the species-dependent transfer functions between the size of the water absorption feature at 1420 nm were better predictors than any variable derived from chlorophyll absorption bands.
- We tested whether the transfer function derived under laboratory conditions can be applied to hyperspectral images acquired in the field, which allows mapping NP across larger scales. Our results were in astonishingly good agreement with the theoretical expectations if the transfer function relied on the water absorption feature at 1420 nm, suggesting that the spectral patterns between laboratory and field measurements were highly comparable and underlining the general possibility for area-wide predictions in the field. However, the use of the water absorption bands limits the usability of space- and air-borne data in future applications because of the strong water absorption in the atmosphere accompanied by the low signal to noise ratio of current sensors. Therefore, we suggest using drones flying at low elevations above the ground to reduce the influence of the atmosphere on the reflectance values measured at the platform. Such kinds of data can be used to provide area-wide NP estimations of BSCs in the southern part of the Atacama Desert in the future, where BSCs are keystone organisms providing key ecosystem functions such as protection against soil erosion, weathering of nitrogen and phosphorus and dust trapping.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Belnap, J.; Lange, O. Biological Soil Crusts: Structure, Function, and Management; Ecological Studies; Springer: Berlin/Heidelberg, Germany, 2001. [Google Scholar]
- Elbert, W.; Weber, B.; Burrows, S.; Steinkamp, J.; Büdel, B.; Andreae, M.O.; Pöschl, U. Contribution of cryptogamic covers to the global cycles of carbon and nitrogen. Nat. Geosci. 2012, 5, 459–462. [Google Scholar] [CrossRef]
- Castenholz, R.W.; Garcia-Pichel, F. Cyanobacterial Responses to UV Radiation. In Ecology of Cyanobacteria II; Springer: Dordrecht, The Netherlands, 2012; pp. 481–499. [Google Scholar]
- Harel, Y.; Ohad, I.; Kaplan, A. Activation of Photosynthesis and Resistance to Photoinhibition in Cyanobacteria within Biological Desert Crust. Plant Phys. 2004, 136, 3070–3079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Caballero, E.; Belnap, J.; Büdel, B.; Crutzen, P.J.; Andreae, M.O.; Pöschl, U.; Weber, B. Dryland photoautotrophic soil surface communities endangered by global change. Nat. Geosci. 2018, 11, 185. [Google Scholar] [CrossRef]
- Warren, S.D. Synopsis: Influence of Biological Soil Crusts on Arid Land Hydrology and Soil Stability; Ecological Studies; Springer: Berlin/Heidelberg, Germany, 2001; pp. 349–360. [Google Scholar]
- Belnap, J. The world at your feet: Desert biological soil crusts. Front. Ecol. Environ. 2003, 1, 181–189. [Google Scholar] [CrossRef]
- Reynolds, R.; Belnap, J.; Reheis, M.; Lamothe, P.; Luiszer, F. Aeolian dust in Colorado Plateau soils: Nutrient inputs and recent change in source. Proc. Nat. Acad. Sci. USA 2001, 98, 7123–7127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castillo-Monroy, A.P.; Maestre, F.T.; Delgado-Baquerizo, M.; Gallardo, A. Biological soil crusts modulate nitrogen availability in semi-arid ecosystems: Insights from a Mediterranean grassland. Plant Soil 2010, 333, 21–34. [Google Scholar] [CrossRef]
- Pointing, S.B.; Belnap, J. Microbial colonization and controls in dryland systems. Nat. Rev. Microbiol. 2012, 10, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Bowker, M.A.; Belnap, J.; Miller, M.E. Spatial modeling of biological soil crusts to support rangeland assessment and monitoring. Rangel. Ecol. Manag. 2006, 59, 519–529. [Google Scholar] [CrossRef]
- Abed, R.M.M.; Polerecky, L.; Al-Habsi, A.; Oetjen, J.; Strous, M.; de Beer, D. Rapid Recovery of Cyanobacterial Pigments in Desiccated Biological Soil Crusts following Addition of Water. PLoS ONE 2014, 9, e112372. [Google Scholar] [CrossRef] [PubMed]
- Karnieli, A.; Tsoar, H. Spectral reflectance of biogenic crust developed on desert dune sand along the Israel-Egypt border. Int. J. Remote Sens. 1995, 16, 369–374. [Google Scholar] [CrossRef]
- Ustin, S.L.; Valko, P.G.; Kefauver, S.C.; Santos, M.J.; Zimpfer, J.F.; Smith, S.D. Remote sensing of biological soil crust under simulated climate change manipulations in the Mojave Desert. Remote Sens. Environ. 2009, 113, 317–328. [Google Scholar] [CrossRef]
- Weber, B.; Olehowski, C.; Knerr, T.; Hill, J.; Deutschewitz, K.; Wessels, D.; Eitel, B.; Büdel, B. A new approach for mapping of Biological Soil Crusts in semidesert areas with hyperspectral imagery. Remote Sens. Environ. 2008, 112, 2187–2201. [Google Scholar] [CrossRef]
- Rodríguez-Caballero, E.; Paul, M.; Tamm, A.; Caesar, J.; Büdel, B.; Escribano, P.; Hill, J.; Weber, B. Biomass assessment of microbial surface communities by means of hyperspectral remote sensing data. Sci. Total Environ. 2017, 586, 1287–1297. [Google Scholar] [CrossRef] [PubMed]
- Chamizo, S.; Stevens, A.; Canton, Y.; Miralles, I.; Domingo, F.; Van Wesemael, B. Discriminating soil crust type, development stage and degree of disturbance in semiarid environments from their spectral characteristics. Eur. J. Soil Sci. 2012, 63, 42–53. [Google Scholar] [CrossRef]
- Gypser, S.; Herppich, W.B.; Fischer, T.; Lange, P.; Veste, M. Photosynthetic characteristics and their spatial variance on biological soil crusts covering initial soils of post-mining sites in Lower Lusatia, NE Germany. Flora 2016, 220, 103–116. [Google Scholar] [CrossRef]
- Rodríguez-Caballero, E.; Escribano, P.; Olehowski, C.; Chamizo, S.; Hill, J.; Canton, Y.; Weber, B. Transferability of multi- and hyperspectral optical biocrust indices. ISPRS J. Photogramm. Remote Sens. 2017, 126, 94–107. [Google Scholar] [CrossRef]
- Escribano, P.; Palacios-Orueta, A.; Oyonarte, C.; Chabrillat, S. Spectral properties and sources of variability of ecosystem components in a Mediterranean semiarid environment. J. Arid Environ. 2010, 74, 1041–1051. [Google Scholar] [CrossRef]
- Rodríguez-Caballero, E.; Escribano, P.; Cantón, Y. Advanced image processing methods as a tool to map and quantify different types of biological soil crust. ISPRS J. Photogramm. Remote Sens. 2014, 90, 59–67. [Google Scholar] [CrossRef]
- Rodríguez-Caballero, E.; Knerr, T.; Weber, B. Importance of biocrusts in dryland monitoring using spectral indices. Remote Sens. Environ. 2015, 170, 32–39. [Google Scholar] [CrossRef]
- Hinchcliffe, G.; Bollard-Breen, B.; Cowan, D.A.; Doshi, A.; Gillman, L.N.; Maggs-Kolling, G.; de Los Rios, A.; Pointing, S.B. Advanced Photogrammetry to Assess Lichen Colonization in the Hyper-Arid Namib Desert. Front. Microbiol. 2017, 8, 2083. [Google Scholar] [CrossRef] [PubMed]
- Rozenstein, O.; Karnieli, A. Identification and characterization of Biological Soil Crusts in a sand dune desert environment across Israel Egypt border using LWIR emittance spectroscopy. J. Arid Environ. 2015, 112, 75–86. [Google Scholar] [CrossRef]
- Raggio, J.; Pintado, A.; Vivas, M.; Sancho, L.G.; Büdel, B.; Colesie, C.; Weber, B.; Schroeter, B.; Lázaro, R.; Green, T.G.A. Continuous chlorophyll fluorescence, gas exchange and microclimate monitoring in a natural soil crust habitat in Tabernas badlands, Almería, Spain: Progressing towards a model to understand productivity. Biodivers. Conserv. 2014, 23, 1809–1826. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J.; Wang, L.; Wang, X.; Gu, Z. The spatial distribution patterns of biological soil crusts in the Gurbantunggut Desert, Northern Xinjiang, China. J. Arid Environ. 2007, 68, 599–610. [Google Scholar] [CrossRef]
- Rundel, P.W. Ecological Relationships of Desert Fog Zone Lichens. Bryologist 1978, 81, 277–293. [Google Scholar] [CrossRef]
- Rundel, P.W.; Dillon, M.O.; Palma, B. Flora and Vegetation of Pan de Azúcar National Park in the Atacama Desert of Northern Chile. Gayana Bot. 1996, 53, 295–315. [Google Scholar]
- Jordan, T.; Riquelme, R.; González, G.; Herrera, C.; Godfrey, L.; Colucci, S.; Gironás-León, J.; Gamboa, C.; Urrutia, J.; Tapia, L.; et al. Hydrological and geomorphological consequences of the extreme precipitation event of 24–26 March 2015, Chile. In Proceedings of the XIV Congreso Geologico Chileno, La Serena, Chile, 4–8 October 2015. [Google Scholar]
- Cereceda, P.; Larrain, H.; Osses, P.; Farías, A.; Egaña, I. The spatial and temporal variability of fog and its relation to fog oases in the Atacama Desert, Chile. Atmos. Res. 2008, 87, 312–323. [Google Scholar] [CrossRef]
- Cereceda, P.; Schemenauer, R.S. The Occurrence of Fog in Chile. J. Appl. Meteorol. 1991, 30, 1097–1105. [Google Scholar] [CrossRef] [Green Version]
- Lehnert, L.W.; Thies, B.; Trachte, K.; Achilles, S.; Osses, P.; Baumann, K.; Schmidt, J.; Samolov, E.; Jung, P.; Leinweber, P.; et al. A Case Study on Fog/Low Stratus Occurrence at Las Lomitas, Atacama Desert (Chile) as a Water Source for Biological Soil Crusts. Aerosol Air Q. Res. 2018, 18, 254–269. [Google Scholar] [CrossRef]
- Thompson, M.V.; Palma, B.; Knowles, J.T.; Holbrook, N.M. Multi-annual climate in Parque Nacional Pan de Azúcar, Atacama Desert, Chile. Rev. Chil. Hist. Nat. 2003, 76, 235–254. [Google Scholar] [CrossRef]
- Gaya, E.; Fernández-Brime, S.; Vargas, R.; Lachlan, R.F.; Gueidan, C.; Ramírez-Mejía, M.; Lutzoni, F. The adaptive radiation of lichen-forming Teloschistaceae is associated with sunscreening pigments and a bark-to-rock substrate shift. Proc. Nat. Acad. Sci. USA 2015, 112, 11600–11605. [Google Scholar] [CrossRef] [PubMed]
- Colesie, C.; Gommeaux, M.; Green, T.A.; Büdel, B. Biological soil crusts in continental Antarctica: Garwood Valley, southern Victoria Land, and Diamond Hill, Darwin Mountains region. Antarc. Sci. 2014, 26, 115–123. [Google Scholar] [CrossRef]
- Ronen, R.; Galun, M. Pigment extraction from lichens with dimethyl sulfoxide (DMSO) and estimation of chlorophyll degradation. Environ. Exp. Bot. 1984, 24, 239–245. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Hijmans, R.J. Raster: Geographic Data Analysis and Modeling. R Package Version 2.5-8. 2016. Available online: https://cran.r-project.org/web/packages/raster/index.html (accessed on 6 June 2018).
- Lehnert, L.W.; Meyer, H.; Obermeier, W.A.; Silva, B.; Regeling, B.; Thies, B.; Bendix, J. Hyperspectral Data Analysis in R: The hsdar-Package. arXiv, 2018; arXiv:1805.05090. [Google Scholar]
- Sims, D.; Gamon, J. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Mutanga, O.; Skidmore, A. Hyperspectral band depth analysis for a better estimation of grass biomass (Cenchrus ciliaris) measured under controlled laboratory conditions. Int. J. Appl. Earth Obs. Geoinform. 2004, 5, 87–96. [Google Scholar] [CrossRef]
- Lange, O.L.; Meyer, A.; Zellner, H.; Heber, U. Photosynthesis and Water Relations of Lichen Soil Crusts: Field Measurements in the Coastal Fog Zone of the Namib Desert. Funct. Ecol. 1994, 8, 253–264. [Google Scholar] [CrossRef]
- Evans, R.D.; Johansen, J.R. Microbiotic Crusts and Ecosystem Processes. Crit. Rev. Plant Sci. 1999, 18, 183–225. [Google Scholar] [CrossRef]
- O’Neill, A.L. Reflectance spectra of microphytic soil crusts in semi-arid Australia. Int. J. Remote Sens. 1994, 15, 675–681. [Google Scholar] [CrossRef]
- Adams, J.B. Interpretation of Visible and Near-Infrared Diffuse Reflectance Spectra of Pyroxenes and Other Rock-Forming Minerals; Academic Press: New York, NY, USA, 1975; pp. 91–116. [Google Scholar]
- Büdel, B.; Vivas, M.; Lange, O.L. Lichen species dominance and the resulting photosynthetic behavior of Sonoran Desert soil crust types (Baja California, Mexico). Ecol. Proc. 2013, 2, 6. [Google Scholar] [CrossRef] [Green Version]
- Gao, B.C.; Goetz, A.F.H.; Wiscombe, W.J. Cirrus cloud detection from Airborne Imaging Spectrometer data using the 1.38 μm water vapor band. Geophys. Res. Lett. 1993, 20, 301–304. [Google Scholar] [CrossRef]
- Pengra, B.W.; Johnston, C.A.; Loveland, T.R. Mapping an invasive plant, Phragmites australis, in coastal wetlands using the EO-1 Hyperion hyperspectral sensor. Remote Sens. Environ. 2007, 108, 74–81. [Google Scholar] [CrossRef]





| Species | NRI | Polynomial Regression | ||||
|---|---|---|---|---|---|---|
| Acarospora cf. gypsi-deserti | 1388 | 679 | −43.0170 | 76.1124 | −3.3 × 10 | 0.95 |
| 1388 | 675 | −43.4680 | 76.8389 | −3.4 × 10 | 0.95 | |
| 970 | 929 | −0.8014 | 2.9343 | −2.1 × 10 | 0.94 | |
| 966 | 925 | −0.8759 | 3.0839 | −2.2 × 10 | 0.93 | |
| 974 | 933 | −0.8830 | 3.1640 | −2.3 × 10 | 0.93 | |
| 970 | 925 | −1.0468 | 3.5271 | −2.5 × 10 | 0.93 | |
| 966 | 921 | −1.2512 | 3.9865 | −2.7 × 10 | 0.93 | |
| 970 | 921 | −1.4212 | 4.4215 | −3.0 × 10 | 0.93 | |
| 1599 | 1404 | −0.2212 | 0.1737 | −1.8 × 10 | 0.93 | |
| 979 | 937 | −1.0409 | 3.5662 | −2.6 × 10 | 0.93 | |
| Caloplaca santessoniana | 1475 | 1433 | −0.0172 | 0.0247 | −9.7 × 10 | 0.88 |
| 1599 | 1392 | 0.0254 | −0.0183 | −3.3 × 10 | 0.88 | |
| 1595 | 1392 | 0.0225 | −0.0151 | −3.3 × 10 | 0.88 | |
| 1106 | 1057 | 0.0809 | −0.1292 | 5.2 × 10 | 0.87 | |
| 1579 | 1396 | 0.0028 | 0.0050 | −4.1 × 10 | 0.87 | |
| 1554 | 1392 | 0.0540 | −0.0747 | 3.2 ×10 | 0.87 | |
| 1479 | 1429 | −0.0128 | 0.0206 | −7.3 × 10 | 0.87 | |
| 1574 | 1396 | 0.0021 | 0.0060 | −6.2 × 10 | 0.87 | |
| 1583 | 1396 | 0.0034 | 0.0041 | −2.8 × 10 | 0.87 | |
| 1591 | 1392 | 0.0201 | −0.0126 | −3.1 × 10 | 0.87 | |
| Species | Variable | Absorption Feature | Polynomial Regression | |||
|---|---|---|---|---|---|---|
| a | b | c | ||||
| Acarospora cf. gypsi-deserti | Integral | 0.040 | 0.1226 | −0.0412 | 0.150 | |
| −0.121 | 0.9825 | −0.8627 | 0.561 | |||
| 1.061 | −1.8794 | 0.8985 | 0.246 | |||
| −0.254 | 0.3645 | −0.0687 | 0.880 | |||
| Width | 0.302 | −0.0478 | −0.1503 | 0.444 | ||
| −0.311 | 1.3344 | −0.9299 | 0.361 | |||
| −0.026 | 0.2143 | −0.0741 | 0.041 | |||
| −43.513 | 83.7620 | −40.1740 | 0.333 | |||
| Caloplaca santessoniana | Integral | 0.030 | 0.0499 | −0.0347 | 0.127 | |
| 0.053 | −0.0024 | −0.0047 | 0.197 | |||
| −0.267 | 0.2835 | −0.0184 | 0.743 | |||
| −0.072 | 0.0816 | −0.0126 | 0.817 | |||
| Width | 0.166 | −0.2188 | 0.0727 | 0.719 | ||
| 0.158 | −0.2657 | 0.1168 | 0.396 | |||
| −0.179 | 0.3029 | −0.0979 | 0.626 | |||
| −3.354 | 5.9404 | −2.5865 | 0.799 | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lehnert, L.W.; Jung, P.; Obermeier, W.A.; Büdel, B.; Bendix, J. Estimating Net Photosynthesis of Biological Soil Crusts in the Atacama Using Hyperspectral Remote Sensing. Remote Sens. 2018, 10, 891. https://doi.org/10.3390/rs10060891
Lehnert LW, Jung P, Obermeier WA, Büdel B, Bendix J. Estimating Net Photosynthesis of Biological Soil Crusts in the Atacama Using Hyperspectral Remote Sensing. Remote Sensing. 2018; 10(6):891. https://doi.org/10.3390/rs10060891
Chicago/Turabian StyleLehnert, Lukas W., Patrick Jung, Wolfgang A. Obermeier, Burkhard Büdel, and Jörg Bendix. 2018. "Estimating Net Photosynthesis of Biological Soil Crusts in the Atacama Using Hyperspectral Remote Sensing" Remote Sensing 10, no. 6: 891. https://doi.org/10.3390/rs10060891