5 Key Challenges and Solutions for Governing Complex Adaptive (Food) Systems
Abstract
:1. Introduction
2. Characteristics of Complex Adaptive (Food) Systems
2.1. Multi-Causality: There Is No Smoking Gun and No Silver Bullet
2.2. Cumulative Impacts: Death by a Thousand Cuts
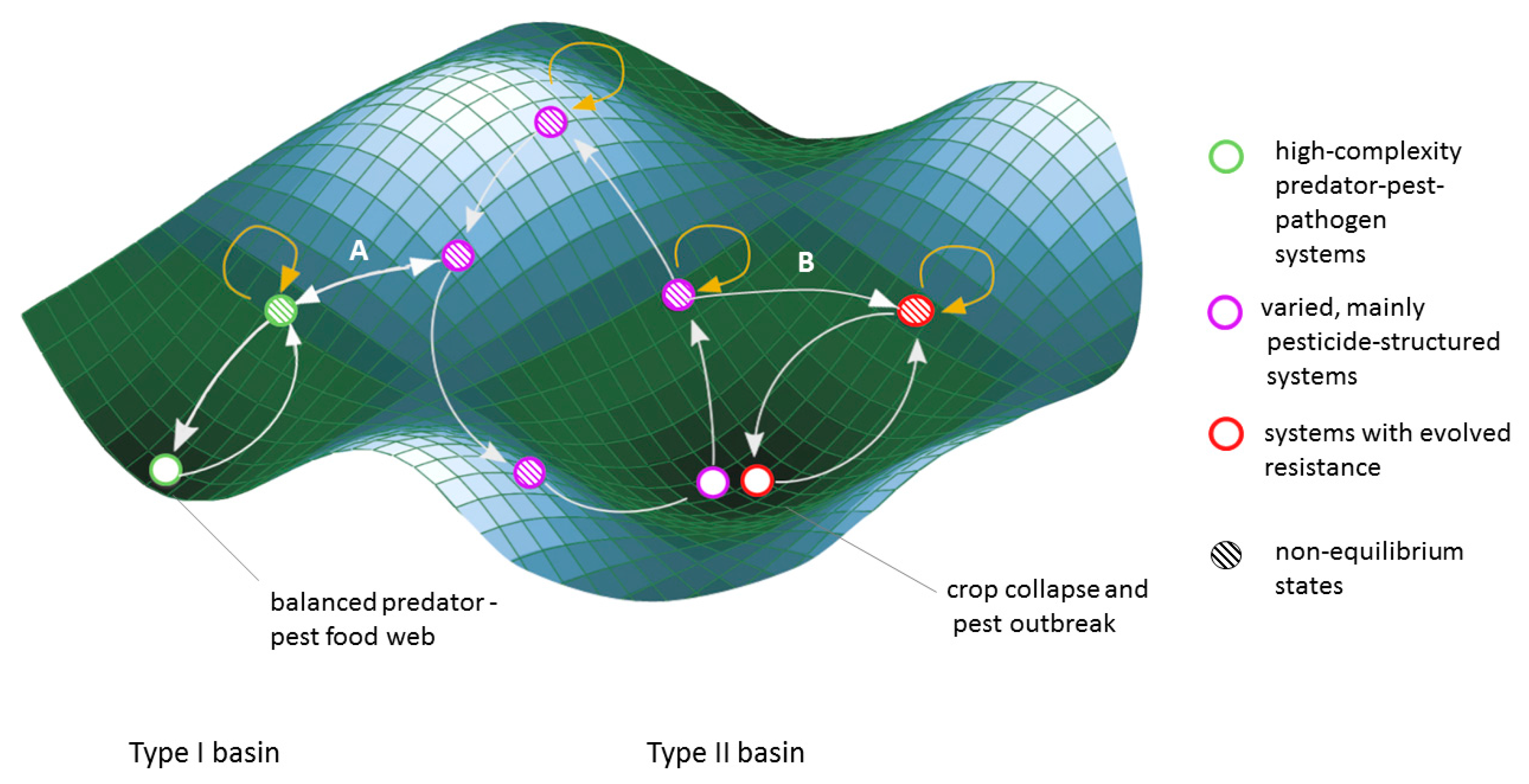
2.3. Regime Shifts: Systems Change in Fits and Spurts, and Can Shift Unexpectedly
2.4. Teleconnections: ”Transporting” Impacts across Time and Space
2.5. Multi-Scalarity: Drivers and Impacts Cross Scales
3. Case Studies
3.1. Searching for a Smoking Gun for Pollinator Declines
3.2. Paying for a Thousand Band-Aids? Rethinking PES to Integrate Complexity in Solving Distant Problems
3.3. Regime Shifts and Pests: Pesticide Resistance and Pest Control
3.4. Sourcing Stifling Sediment: Teleconnections between Oyster Beds and Farms via Nitrogen Run-Off in Tasman Bay, New Zealand
3.5. Conflicting Scales in Governance of Puget Sound Riparian Restoration
4. Discussion: Rethinking Agriculture
4.1. Governance Scale Shift: Farming Is Not Only Agricultural Production, but Also Land Management
4.2. Production Scale Shift: From Prescriptive to Place-Based Farming Practices
4.3. People Scale Shift: Seeking Systems that Support Participation of All People as Both Citizens and Consumers
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Robertson, G.P.; Swinton, S.M. Reconciling agricultural productivity and environmental integrity: A grand challenge for agriculture. Front. Ecol. Environ. 2005, 3, 38–46. [Google Scholar] [CrossRef]
- Foley, J.A.; Ramankutty, N.; Brauman, K.A.; Cassidy, E.S.; Gerber, J.S.; Johnston, M.; Mueller, N.D.; O’Connell, C.; Ray, D.K.; West, P.C.; et al. Solutions for a cultivated planet. Nature 2011, 478, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Green, R.E.; Cornell, S.J.; Scharlemann, J.P.W.; Balmford, A. Farming and the fate of wild nature. Science 2005, 307, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Mendenhall, C.D.; Karp, D.S.; Meyer, C.F.J.; Hadly, E.A.; Daily, G.C. Predicting biodiversity change and averting collapse in agricultural landscapes. Nature 2014, 509, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D.; Balzar, C.; Hill, J.; Befort, B.L. Global food demand and the sustainable intensification of agriculture. Proc. Natl. Acad. Sci. USA 2011, 108, 20260–20264. [Google Scholar] [CrossRef] [PubMed]
- Vitousek, P.M.; Aber, J.D.; Howarth, R.W.; Likens, G.E.; Matson, P.A.; Schindler, D.W.; Schlesinger, W.H.; Tilman, D.G. Human alteration of the global nitrogen cycle: Sources and consequences. Ecol. Appl. 1997, 7, 737–750. [Google Scholar] [CrossRef]
- Donner, S.D.; Kucharik, C.J. Corn-based ethanol production compromises goal of reducing nitrogen export by the Mississippi River. Proc. Natl. Acad. Sci. USA 2008, 105, 4513–4518. [Google Scholar] [CrossRef] [PubMed]
- Rohr, J.R.; McCoy, K.A. A qualitative meta-analysis reveals consistent effects of atrazine on freshwater fish and amphibians. Environ. Health Persp. 2010, 118, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Van Engelsdorp, D.; Evans, J.D.; Saegerman, C.; Mullin, C.; Haubruge, E.; Nguyen, B.K.; Frazier, M.; Frazier, J.; Cox-Foster, D.; Chen, Y.; et al. Colony collapse disorder: A descriptive study. PLoS ONE 2009, 4, e6481. [Google Scholar]
- Garibaldi, L.A.; Aizen, M.A.; Klein, A.M.; Cunningham, S.A.; Harder, L.D. Global growth and stability of agricultural yield decrease with pollinator dependence. Proc. Natl. Acad. Sci. USA 2011, 108, 5909–5914. [Google Scholar] [CrossRef] [PubMed]
- Arthur, B.W.; Durlauf, S.N.; Lane, D.A. The Economy as an Evolving Complex System II; Addison-Wesley: Reading, MA, USA, 1997; Volume 27, pp. 1–6. [Google Scholar]
- Holland, J.H. Hidden Order; Addison-Wesley: Reading, MA, USA, 1995. [Google Scholar]
- Bullock, J.M.; Dhanjal Adams, K.L.; Milne, A.; Oliver, T.H.; Todman, L.C.; Whitmore, A.P.; Pywell, R.F. Resilience and food security: Rethinking an ecological concept. J. Ecol. 2017, 105, 880–884. [Google Scholar] [CrossRef]
- Wittman, H.; Chappell, M.J.; Abson, D.J.; Kerr, R.B.; Blesh, J.; Hanspach, J.; Perfecto, I.; Fischer, J. A social-ecological perspective on harmonizing food security and biodiversity conservation. Reg. Environ. Chang. 2016, 17, 1291–1301. [Google Scholar] [CrossRef]
- Wittman, H. Reframing agrarian citizenship: Land, life and power in Brazil. J. Rural Stud. 2009, 25, 120–130. [Google Scholar] [CrossRef]
- Darnhofer, I. Resilience and why it matters for farm management. Eur. Rev. Agric. Econ. 2014, 41, 461–484. [Google Scholar] [CrossRef]
- Vandermeer, J.H.; Perfecto, I. Syndromes of production in agriculture: Prospects for social-ecological regime change. Ecol. Soc. 2012, 17, 388–395. [Google Scholar] [CrossRef]
- Bennett, E.M.; Carpenter, S.; Gordon, L.; Ramankutty, N. Toward a more resilient agriculture. Solutions 2014, 5, 65–75. [Google Scholar]
- Rammel, C.; Stagl, S.; Wilfing, H. Managing complex adaptive systems—A co-evolutionary perspective on natural resource management. Ecol. Econ. 2007, 63, 9–21. [Google Scholar] [CrossRef]
- Mahon, R.; McConney, P.; Roy, R.N. Governing fisheries as complex adaptive systems. Mar. Policy 2008, 32, 104–112. [Google Scholar] [CrossRef]
- Levin, P.S.; Mollmann, C. Marine ecosystem regime shifts: Challenges and opportunities for ecosystem-based management. Philos. Trans. R. Soc. B 2015, 370, 1659. [Google Scholar] [CrossRef]
- Ban, N.C.; Mills, M.; Tam, J.; Hicks, C.C.; Klain, S.C.; Stoeckl, N.; Bottrill, M.C.; Levine, J.; Pressey, R.L.; Satterfield, T.; et al. A social-ecological approach to conservation planning: Embedding social considerations. Front. Ecol. Environ. 2013, 11, 194–202. [Google Scholar] [CrossRef] [Green Version]
- Schianetz, K.; Kavanagh, L. Sustainability indicators for tourism destinations: A complex adaptive systems approach using systemic indicator systems. J. Sustain. Tour. 2008, 16, 601–628. [Google Scholar] [CrossRef]
- Gross, J.E.; McAllister, R.R.J.; Abel, N.; Smith, D.M.S.; Maru, Y. Australian rangelands as complex adaptive systems: A conceptual model and preliminary results. Environ. Modell. Softw. 2006, 21, 1264–1272. [Google Scholar] [CrossRef]
- Klerkx, L.; Aarts, N.; Leeuwis, C. Adaptive management in agricultural innovation systems: The interactions between innovation networks and their environment. Agric. Syst. 2010, 103, 390–400. [Google Scholar] [CrossRef]
- Levin, S.A. The problem of pattern and scale in ecology: The Robert H. MacArthur Award lecture. Ecology 1992, 73, 1943–1967. [Google Scholar] [CrossRef]
- Darling, E.S.; Côté, I.M. Quantifying the evidence for ecological synergies. Ecol. Lett. 2008, 11, 1278–1286. [Google Scholar] [CrossRef] [PubMed]
- Holling, C.S. Resilience and stability of ecological systems. Annu. Rev. Ecol. Syst. 1973, 4, 1–23. [Google Scholar] [CrossRef]
- Beisner, B.E.; Haydon, D.T.; Cuddington, K. Alternative stable states in ecology. Front. Ecol. Environ. 2003, 1, 376–382. [Google Scholar] [CrossRef]
- Folke, C.; Carpenter, S.; Walker, B.; Scheffer, M.; Elmqvist, T.; Gunderson, L.; Holling, C.S. Regime shifts, resilience, and biodiversity in ecosystem management. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 557–581. [Google Scholar] [CrossRef]
- Liu, J.; Dietz, T.; Carpenter, S.R.; Alberti, M.; Folke, C.; Moran, E.; Pell, A.N.; Deadman, P.; Kratz, T.; Lubchenco, J. Complexity of coupled human and natural systems. Science 2007, 317, 1513–1516. [Google Scholar] [CrossRef] [PubMed]
- Cash, D.; Adger, W.N.; Berkes, F.; Garden, P. Scale and cross-scale dynamics: Governance and information in a multilevel world. Ecol. Soc. 2006, 11, 3213–3217. [Google Scholar] [CrossRef]
- Levin, S.A. Multiple scales and the maintenance of biodiversity. Ecosystems 2000, 3, 498–506. [Google Scholar] [CrossRef]
- Singh, G.G.; Sinner, J.; Ellis, J.; Kandlikar, M.; Halpern, B.S.; Satterfield, T.; Chan, K.M.A. Mechanisms and risk of cumulative impacts to coastal ecosystem services: An expert elicitation approach. J. Environ. Manag. 2017, 199, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Crain, C.M.; Kroeker, K.; Halpern, B.S. Interactive and cumulative effects of multiple human stressors in marine systems. Ecol. Lett. 2008, 11, 1304–1315. [Google Scholar] [CrossRef] [PubMed]
- Halpern, B.S.; Mcleod, K.L.; Rosenberg, A.A.; Crowder, L.B. Managing for cumulative impacts in ecosystem-based management through ocean zoning. Ocean Coast. Manag. 2008, 51, 203–211. [Google Scholar] [CrossRef]
- O’Brien, K.; Leichenko, R.; Kelkar, U.; Venema, H.; Aandahl, G.; Tompkins, H.; Javed, A.; Bhadwal, S.; Barg, S.; Nygaard, L.; et al. Mapping vulnerability to multiple stressors: climate change and globalization in India. Glob. Environ. Chang. 2004, 14, 303–313. [Google Scholar] [CrossRef]
- Folke, C. Resilience: The emergence of a perspective for social–ecological systems analyses. Glob. Environ. Chang. 2006, 16, 253–267. [Google Scholar] [CrossRef]
- Otto, S.P.; Day, T. A Biologist’s Guide to Mathematical Modeling in Ecology and Evolution; Princeton University Press: Princeton, NJ, USA, 2007. [Google Scholar]
- Lewontin, R.C. The meaning of stability. Brookhaven Symp. Biol. 1969, 22, 13–24. [Google Scholar] [PubMed]
- Seto, K.C.; Reenberg, A.; Boone, C.G.; Fragkias, M.; Haase, D.; Langanke, T.; Marcotullio, P.; Munroe, D.K.; Olah, B.; Simon, D. Urban land teleconnections and sustainability. Proc. Natl. Acad. Sci. USA 2012, 109, 7687–7692. [Google Scholar] [CrossRef] [PubMed]
- Adger, W.N.; Eakin, H.; Winkels, A. Nested and teleconnected vulnerabilities to environmental change. Front. Ecol. Environ. 2008, 7, 150–157. [Google Scholar] [CrossRef]
- Eakin, H.; Winkels, A.; Sendzimir, J. Nested vulnerability: Exploring cross-scale linkages and vulnerability teleconnections in Mexican and Vietnamese coffee systems. Environ. Sci. Policy 2009, 12, 398–412. [Google Scholar] [CrossRef]
- Collins, S.L.; Carpenter, S.R.; Swinton, S.M.; Orenstein, D.E.; Childers, D.L.; Gragson, T.L.; Grimm, N.B.; Grove, J.M.; Harlan, S.L.; Kaye, J.P.; et al. An integrated conceptual framework for long-term social–ecological research. Front. Ecol. Environ. 2011, 9, 351–357. [Google Scholar] [CrossRef]
- Eisler, M.C.; Lee, M.R.; Tarlton, J.F.; Martin, G.B.; Beddington, J.; Dungait, J.A.; Greathead, H.; Liu, J.; Mathew, S.; Miller, H. Agriculture: Steps to sustainable livestock. Nature 2014, 507, 32–34. [Google Scholar] [CrossRef] [PubMed]
- Guswa, A.J.; Brauman, K.A.; Brown, C.; Hamel, P.; Keeler, B.L.; Sayre, S.S. Ecosystem services: Challenges and opportunities for hydrologic modeling to support decision making. Water Resour. Res. 2014, 50, 4535–4544. [Google Scholar] [CrossRef]
- Brown, C.J.; Saunders, M.I.; Possingham, H.P.; Richardson, A.J. Managing for interactions between local and global stressors of ecosystems. PLoS ONE 2013, 8, e65765. [Google Scholar] [CrossRef] [PubMed]
- Christensen, M.R.; Graham, M.D.; Vinebrooke, R.D.; Findlay, D.L.; Paterson, M.J.; Turner, M.A. Multiple anthropogenic stressors cause ecological surprises in boreal lakes. Glob. Chang. Biol. 2006, 12, 2316–2322. [Google Scholar] [CrossRef]
- Jackson, J.B.; Kirby, M.X.; Berger, W.H.; Bjorndal, K.A.; Botsford, L.W.; Bourque, B.J.; Bradbury, R.H.; Cooke, R.; Erlandson, J.; Estes, J.A. Historical overfishing and the recent collapse of coastal ecosystems. Science 2001, 293, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, W.Z.; Zhang, X.C.; Zheng, F.L. Impacts of land use change and climate variability on hydrology in an agricultural catchment on the Loess Plateau of China. J. Hydrol. 2009, 377, 35–42. [Google Scholar] [CrossRef]
- Piao, S.; Ciais, P.; Huang, Y.; Shen, Z.; Peng, S.; Li, J.; Zhou, L.; Liu, H.; Ma, Y.; Ding, Y.; et al. The impacts of climate change on water resources and agriculture in China. Nature 2010, 467, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Walker, B.; Meyers, J. Thresholds in ecological and social–ecological systems: A developing database. Ecol. Soc. 2004, 9, 3438–3447. [Google Scholar] [CrossRef]
- Scheffer, M.; Carpenter, S.R. Catastrophic regime shifts in ecosystems: Linking theory to observation. Trends Ecol. Evol. 2003, 18, 648–656. [Google Scholar] [CrossRef]
- Meadows, D.H. Thinking in Systems; Chelsea Green Publishing: Windsor County, VT, USA, 2008. [Google Scholar]
- Scheffer, M.; Hosper, S.H.; Meijer, M.L.; Moss, B.; Jeppesen, E. Alternative equilibria in shallow lakes. Trends Ecol. Evol. 1993, 8, 275–279. [Google Scholar] [CrossRef]
- Cardille, J.A.; Bennett, E.M. Ecology: Tropical teleconnections. Nat. Geosci. 2010, 3, 154–155. [Google Scholar] [CrossRef]
- Güneralp, B.; Seto, K.C.; Ramachandran, M. Evidence of urban land teleconnections and impacts on hinterlands. Curr. Opin. Environ. Sustain. 2013, 5, 445–451. [Google Scholar] [CrossRef]
- Liu, J.; Hull, V.; Batistella, M.; DeFries, R.; Dietz, T.; Fu, F.; Hertel, T.W.; Izaurralde, R.C.; Lambin, E.F.; Li, S.; et al. Framing sustainability in a telecoupled world. Ecol. Soc. 2013, 18, 344–365. [Google Scholar] [CrossRef]
- Dias, P.C. Sources and sinks in population biology. Trends Ecol. Evol. 1996, 11, 326–330. [Google Scholar] [CrossRef]
- Kreitzman, M.; Ashander, J.; Driscoll, J.; Bateman, A.W.; Chan, K.M.A.; Lewis, M.A.; Krkosek, M. Wild salmon sustain the effectiveness of parasite control on salmon farms: Conservation implications from an evolutionary ecosystem service. Conserv. Lett. 2017. [Google Scholar] [CrossRef]
- Fischer, J.; Abson, D.J.; Butsic, V.; Chappell, M.J.; Ekroos, J.; Hanspach, J.; Kuemmerle, T.; Smith, H.G.; Wehrden, H. Land sparing versus land sharing: Moving forward. Conserv. Lett. 2014. [Google Scholar] [CrossRef]
- Gosnell, H.; Abrams, J. Amenity migration: Diverse conceptualizations of drivers, socioeconomic dimensions, and emerging challenges. GeoJournal 2011, 76, 303–322. [Google Scholar] [CrossRef]
- Durand, F. What’s the Deal with Interstellar’s Idea that Corn and Okra Are the Only Crops Left in the Future? Let’s Ask a Scientist. Available online: http://www.thekitchn.com/could-a-crop-blight-really-wipe-out-everything-but-corn-and-okra-food-on-film-212647 (accessed on 9 June 2015).
- Tilman, D.; Reich, P.B.; Isbell, F. Biodiversity impacts ecosystem productivity as much as resources, disturbance, or herbivory. Proc. Natl. Acad. Sci. USA 2012, 109, 10394–10397. [Google Scholar] [CrossRef] [PubMed]
- Potts, S.G.; Imperatriz-Fonseca, V.; Ngo, H.T.; Aizen, M.A.; Biesmeijer, J.C.; Breeze, T.D.; Dicks, L.V.; Garibaldi, L.A.; Hill, R.; Settele, J.; et al. Safeguarding pollinators and their values to human well-being. Nature 2016, 540, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.M.; Vaissiere, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B 2007, 274, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.R.; Singh, G.M.; Mozaffarian, D.; Myers, S.S. Effects of decreases of animal pollinators on human nutrition and global health: A modelling analysis. Lancet 2015, 386, 1964–1972. [Google Scholar] [CrossRef]
- Gordon, R.; Bresolin-Schott, N.; East, I.J. Nomadic beekeeper movements create the potential for widespread disease in the honeybee industry. Aust. Vet. J. 2014, 92, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Committee on the Status of Pollinators in North America; Board on Life Sciences; Resources, Board on Agriculture and Natural Resources; Division on Earth and Life Studies; National Research Council of the National Academies. Status of Pollinators in North America; National Academies Press: Washington, DC, USA, 2007. [Google Scholar]
- Garibaldi, L.A.; Steffan-Dewenter, I.; Winfree, R.; Aizen, M.A.; Bommarco, R.; Cunningham, S.A.; Kremen, C.; Carvalheiro, L.G.; Harder, L.D.; Afik, O.; et al. Wild pollinators enhance fruit set of crops regardless of honey bee abundance. Science 2013, 339, 1608–1611. [Google Scholar] [CrossRef] [PubMed]
- Winfree, R.; Williams, N.M.; Dushoff, J.; Kremen, C. Native bees provide insurance against ongoing honey bee losses. Ecol. Lett. 2007, 10, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- Stanley, D.A.; Garratt, M.P.D.; Wickens, J.B.; Wickens, V.J.; Potts, S.G.; Raine, N.E. Neonicotinoid pesticide exposure impairs crop pollination services provided by bumblebees. Nature 2015, 528, 548–550. [Google Scholar] [CrossRef] [PubMed]
- Whitehorn, P.R.; O’Connor, S.; Wackers, F.L.; Goulson, D. Neonicotinoid pesticide reduces bumble bee colony growth and queen production. Science 2012, 336, 351–352. [Google Scholar] [CrossRef] [PubMed]
- Cutler, G.C.; Scott-Dupree, C.D.; Drexler, D.M. Honey bees, neonicotinoids and bee incident reports: The Canadian situation. Pest Manag. Sci. 2013, 70, 779–783. [Google Scholar] [CrossRef] [PubMed]
- Godfray, H.C.J.; Blacquière, T.; Field, L.M.; Hails, R.S.; Potts, S.G.; Raine, N.E.; Vanbergen, A.J.; McLean, A.R. A restatement of recent advances in the natural science evidence base concerning neonicotinoid insecticides and insect pollinators. Proc. Roy. Soc. B Biol. Sci. 2015, 282, 1818. [Google Scholar] [CrossRef] [PubMed]
- Henry, M.; Béguin, M.; Requier, F.; Rollin, O.; Odoux, J.F.; Aupinel, P.; Aptel, J.; Tchamitchian, S.; Decourtye, A. A common pesticide decreases foraging success and survival in honey bees. Science 2012, 336, 348–350. [Google Scholar] [CrossRef] [PubMed]
- Di Prisco, G.; Cavaliere, V.; Annoscia, D.; Varricchio, P.; Caprio, E.; Nazzi, F.; Gargiulo, G.; Pennacchio, F. Neonicotinoid clothianidin adversely affects insect immunity and promotes replication of a viral pathogen in honey bees. Proc. Natl. Acad. Sci. USA 2013, 110, 18466–18471. [Google Scholar] [CrossRef] [PubMed]
- Mogren, C.L.; Lundgren, J.G. Neonicotinoid-contaminated pollinator strips adjacent to cropland reduce honey bee nutritional status. Sci. Rep. 2016, 6, 29608. [Google Scholar] [CrossRef] [PubMed]
- Rundlöf, M.; Andersson, G.K.S.; Bommarco, R.; Fries, I.; Hederström, V.; Herbertsson, L.; Jonsson, O.; Klatt, B.K.; Pedersen, T.R.; Yourstone, J.; et al. Seed coating with a neonicotinoid insecticide negatively affects wild bees. Nature 2015, 521, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Lundin, O.; Rundlöf, M.; Smith, H.G.; Fries, I.; Bommarco, R. Neonicotinoid insecticides and their impacts on bees: A systematic review of research approaches and identification of knowledge gaps. PLoS ONE 2015, 10, e0136928. [Google Scholar] [CrossRef] [PubMed]
- Gill, R.J.; Baldock, K.C.R.; Brown, M.J.F.; Cresswell, J.E.; Dicks, L.V.; Fountain, M.T.; Garratt, M.P.D.; Gough, L.A.; Heard, M.S.; Holland, J.M.; et al. Protecting an Ecosystem Service: Approaches to Understanding and Mitigating Threats to Wild Insect Pollinators, 1st ed.; Elsevier: Toronto, ON, Canada, 2016; Volume 54, pp. 135–206. [Google Scholar]
- Vanbergen, A.J.; Initiative, T.I.P. Threats to an ecosystem service: Pressures on pollinators. Front. Ecol. Environ. 2013, 11, 251–259. [Google Scholar] [CrossRef] [Green Version]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Kremen, C.; Williams, N.M.; Thorp, R.W. Crop pollination from native bees at risk from agricultural intensification. Proc. Natl. Acad. Sci. USA 2002, 99, 16812–16816. [Google Scholar] [CrossRef] [PubMed]
- Kovács-Hostyánszki, A.; Espíndola, A.; Vanbergen, A.J.; Settele, J.; Kremen, C.; Dicks, L.V. Ecological intensification to mitigate impacts of conventional intensive land use on pollinators and pollination. Ecol. Lett. 2017, 20, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Senapathi, D.; Biesmeijer, J.C.; Breeze, T.D.; Kleijn, D.; Potts, S.G.; Carvalheiro, L.G. Pollinator conservation—The difference between managing for pollination services and preserving pollinator diversity. Curr. Opin. Insect Sci. 2015, 12, 93–101. [Google Scholar] [CrossRef]
- Aguilar, R.; Ashworth, L.; Galetto, L.; Aizen, M.A. Plant reproductive susceptibility to habitat fragmentation: Review and synthesis through a meta-analysis. Ecol. Lett. 2006, 9, 968–980. [Google Scholar] [CrossRef] [PubMed]
- Di Pasquale, G.; Salignon, M.; Le Conte, Y.; Belzunces, L.P.; Decourtye, A.; Kretzschmar, A.; Suchail, S.; Brunet, J.L.; Alaux, C. Influence of pollen nutrition on honey bee health: Do pollen quality and diversity matter? PLoS ONE 2013, 8, e72016. [Google Scholar] [CrossRef] [PubMed]
- Runckel, C.; Flenniken, M.L.; Engel, J.C.; Ruby, J.G.; Ganem, D.; Andino, R.; DeRisi, J.L. Temporal analysis of the honey bee microbiome reveals four novel viruses and seasonal prevalence of known viruses, nosema, and crithidia. PLoS ONE 2011, 6, e20656. [Google Scholar] [CrossRef] [PubMed]
- Fürst, M.A.; McMahon, D.P.; Osborne, J.L.; Paxton, R.J.; Brown, M.J.F. Disease associations between honeybees and bumblebees as a threat to wild pollinators. Nature 2014, 506, 364–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Memmott, J.; Craze, P.G.; Waser, N.M.; Price, M.V. Global warming and the disruption of plant-pollinator interactions. Ecol. Lett. 2007, 10, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Hegland, S.J.; Nielsen, A.; Lázaro, A.; Bjerknes, A.-L.; Totland, Ø. How does climate warming affect plant-pollinator interactions? Ecol. Lett. 2009, 12, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Goulson, D.; Nicholls, E. The canary in the coalmine; bee declines as an indicator of environmental health. Sci. Prog. 2016, 99, 312–326. [Google Scholar] [CrossRef] [PubMed]
- González-Varo, J.P.; Biesmeijer, J.C.; Bommarco, R.; Potts, S.G.; Schweiger, O.; Smith, H.G.; Steffan Dewenter, I.; Szentgyörgyi, H.; Woyciechowski, M.; Vilà, M. Combined effects of global change pressures on animal-mediated pollination. Trends Ecol. Evol. 2013, 28, 524–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, K.M.A.; Anderson, E.; Chapman, M.; Jespersen, K.; Olmsted, P. Payments for ecosystem services: Rife with problems and potential—for transformation towards sustainability. Ecol. Econ. 2017, 140, 110–122. [Google Scholar] [CrossRef]
- Wunder, S. When payments for environmental services will work for conservation. Conserv. Lett. 2013, 6, 230–237. [Google Scholar] [CrossRef]
- Owens, P.N.; Batalla, R.J.; Collins, A.J.; Gomez, B.; Hicks, D.M.; Horowitz, A.J.; Kondolf, G.M.; Marden, M.; Page, M.J.; Peacock, D.H.; et al. Fine-grained sediment in river systems: Environmental significance and management issues. River Res. Appl. 2005, 21, 693–717. [Google Scholar] [CrossRef]
- Carpenter, S.R.; Caraco, N.F.; Correll, D.L.; Howarth, R.W.; Sharpley, A.N.; Smith, V.H. Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecol. Appl. 1998, 8, 559–568. [Google Scholar] [CrossRef]
- Mumby, P.J.; Hastings, A.; Edwards, H.J. Thresholds and the resilience of Caribbean coral reefs. Nature 2007, 450, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Groffman, P.M.; Baron, J.S.; Blett, T.; Gold, A.J.; Goodman, I.; Gunderson, L.H.; Levinson, B.M.; Palmer, M.A.; Paerl, H.W.; Peterson, G.D.; et al. Ecological thresholds: The key to successful environmental management or an important concept with no practical application? Ecosystems 2006, 9, 1–13. [Google Scholar] [CrossRef]
- Oreskes, N. Science and public policy: What’s proof got to do with it? Environ. Sci. Policy 2004, 7, 369–383. [Google Scholar] [CrossRef]
- Naeem, S.; Ingram, J.C.; Varga, A.; Agardy, T.; Barten, P.; Bennett, G.; Bloomgarden, E.; Bremer, L.L.; Burkill, P.; Cattau, M.; Ching, C.; et al. Get the science right when paying for nature’s services. Science 2015, 347, 1206–1207. [Google Scholar] [CrossRef] [PubMed]
- Pascual, U.; Phelps, J.; Garmendia, E.; Brown, K.; Corbera, E.; Martin, A.; Gómez-Baggethun, E.; Muradian, R. Social equity matters in payments for ecosystem services. BioScience 2014, 64, 1027–1036. [Google Scholar] [CrossRef]
- Vatn, A. An institutional analysis of payments for environmental services. Ecol. Econ. 2010, 69, 1245–1252. [Google Scholar] [CrossRef]
- Frey, B.S.; Jegen, R. Motivation crowding theory. J. Econ. Surv. 2001, 15, 589–611. [Google Scholar] [CrossRef]
- Fehr, E.; Falk, A. Psychological foundations of incentives. Eur. Econ. Rev. 2002, 46, 687–724. [Google Scholar] [CrossRef]
- Meadows, D.H. Leverage points. Solu. J. 2010, 1, 41–49. [Google Scholar]
- Levine, J.; Chan, K.M.A.; Satterfield, T. Ecological economics. Ecol. Econ. 2015, 114, 22–32. [Google Scholar] [CrossRef]
- Roopnarine, P.D.; Angielczyk, K.D. Community stability and selective extinction during the Permian-Triassic mass extinction. Science 2015, 350, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Bommarco, R.; Miranda, F.; Bylund, H.; Björkman, C. Insecticides suppress natural enemies and increase pest damage in cabbage. J. Econ. Entomol. 2011, 104, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Angelo, M.J. The Law and Ecology of Pesticides and Pest Management; Taylor & Francis Group: Abingdon, UK, 2013; pp. 1–330. [Google Scholar]
- Dutcher, J.D. A review of resurgence and replacement causing pest outbreaks in IPM. In General Concepts in Integrated Pest and Disease Management; Springer: Dordrecht, The Netherlands, 2007; pp. 27–43. [Google Scholar]
- Rand, T.A.; Tylianakis, J.M.; Tscharntke, T. Spillover edge effects: The dispersal of agriculturally subsidized insect natural enemies into adjacent natural habitats. Ecol. Lett. 2006, 9, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Arbuckle, J.G. Farmer perspectives on pesticide resistance. Exten. Community Econ. Dev. Publ. 2014, 25, 1–8. [Google Scholar]
- Comins, H.N. The development of insecticide resistance in the presence of migration. J. Theor. Biol. 1977, 64, 177–197. [Google Scholar] [CrossRef]
- May, R.M.; Dobson, A.P. Population Dynamics and the Rate of Evolution of Pesticide Resistance; National Academy Press: Washington, DC, USA, 1986; pp. 170–193. [Google Scholar]
- Tabashnik, B.E.; Brévault, T.; Carrière, Y. Insect resistance to Bt crops: Lessons from the first billion acres. Nat. Biotechnol. 2013, 31, 510–521. [Google Scholar] [CrossRef] [PubMed]
- Whalon, M.E.; Mota-Sanchez, D.; Hollingworth, R.M. Analysis of Global Pesticide Resistance in Arthropods; CABI: Wallingford, UK, 2008; pp. 5–31. [Google Scholar]
- Uyenoyama, M.K. Pleiotropy and the evolution of genetic systems conferring resistance to pesticides. In Pesticide Resistance: Strategies and Tactics for Management; National Academies Press: Washington, DC, USA, 1986; pp. 207–221. [Google Scholar]
- Gould, F.; Kennedy, G.G.; Johnson, M.T. Effects of natural enemies on the rate of herbivore adaptation to resistant host plants. Entomol. Exp. Appl. 2011, 58, 1–14. [Google Scholar] [CrossRef]
- Liu, X.; Chen, M.; Collins, H.L.; Onstad, D.W.; Roush, R.T.; Zhang, Q.; Earle, E.D.; Shelton, A.M. Natural enemies delay insect resistance to Bt crops. PLoS ONE 2014, 9, e90366. [Google Scholar] [CrossRef] [PubMed]
- Gassmann, A.J.; Hannon, E.R.; Sisterson, M.S.; Stock, S.P.; Carrière, Y.; Tabashnik, B.E. Effects of entomopathogenic nematodes on evolution of pink bollworm resistance to Bacillus thuringiensis toxin Cry1Ac. J. Econ. Entomol. 2012, 105, 994–1005. [Google Scholar] [CrossRef] [PubMed]
- Berticat, C.; Duron, O.; Heyse, D.; Raymond, M. Insecticide resistance genes confer a predation cost on mosquitoes, Culex pipiens. Genet. Res. 2004, 83, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Letourneau, D.K.; Bothwell, S.G. Comparison of organic and conventional farms: Challenging ecologists to make biodiversity functional. Front. Ecol. Environ. 2008, 6, 430–438. [Google Scholar] [CrossRef]
- Macfadyen, S.; Gibson, R.; Polaszek, A.; Morris, R.J.; Craze, P.G.; Planqué, R.; Symondson, W.O.C.; Memmott, J. Do differences in food web structure between organic and conventional farms affect the ecosystem service of pest control? Ecol. Lett. 2009, 12, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Crowder, D.W.; Northfield, T.D.; Strand, M.R.; Snyder, W.E. Organic agriculture promotes evenness and natural pest control. Nature 2010, 466, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Vandermeer, J.; Perfecto, I.; Philpott, S. Ecological complexity and pest control in organic coffee production: Uncovering an autonomous ecosystem service. BioScience 2010, 60, 527–537. [Google Scholar] [CrossRef]
- Letourneau, D.K.; Goldstein, B. Pest damage and arthropod community structure in organic vs. conventional tomato production in California. J. Appl. Ecol. 2001, 38, 557–570. [Google Scholar] [CrossRef]
- Handley, S. An Analysis of Historical Impacts and Composition of the Benthic Environment of Tasman and Golden Bays; National Institute of Water and Atmospheric Research: Nelson, New Zealand, 2006. [Google Scholar]
- Gillespie, P.A.; Forrest, R.W.; Peake, B.M.; Basher, L.R.; Clement, D.M.; Dunmore, R.A.; Hicks, D.M. Spatial delineation of the depositional footprint of the Motueka River outwelling plume in Tasman Bay, New Zealand. N. Z. J. Mar. Freshw. Res. 2011, 45, 455–475. [Google Scholar] [CrossRef]
- Gibbs, M.M. Sedimentation, suspension, and resuspension in Tasman Bay and Beatrix Bay, New Zealand, two contrasting coastal environments which thermally stratify in summer. N. Z. J. Mar. Freshw. Res. 2001, 35, 951–970. [Google Scholar] [CrossRef]
- Cornelisen, C.D.; Gillespie, P.A.; Kirs, M.; Young, R.G.; Forrest, R.W.; Barter, P.J.; Knight, B.R.; Harwood, V.J. Motueka River plume facilitates transport of ruminant faecal contaminants into shellfish growing waters, Tasman Bay. N. Z. J. Mar. Freshw. Res. 2011, 45, 477–495. [Google Scholar] [CrossRef]
- Brown, S.N. Ecology and Enhancement of the Flat Oyster Ostrea chilensis (Philippi, 1845) in Central New Zealand. Ph.D. Thesis, University of Canterbury, Christchurch, New Zealand, 2011. [Google Scholar]
- Thrush, S.F.; Hewitt, J.E.; Cummings, V.J.; Ellis, J.I.; Hatton, C.; Lohrer, A.; Norkko, A. Muddy waters: Elevating sediment input to coastal and estuarine habitats. Front. Ecol. Environ. 2004, 2, 299–306. [Google Scholar] [CrossRef]
- Knick, S.T.; Rotenberry, J.T. Ghosts of habitats past: Contribution of landscape change to current habitats used by shrubland birds. Ecology 2000, 81, 220–227. [Google Scholar] [CrossRef]
- Biggs, R.; Carpenter, S.R.; Brock, W.A. Turning back from the brink: Detecting an impending regime shift in time to avert it. Proc. Natl. Acad. Sci. USA 2009, 106, 826–831. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.; Cummings, V.; Hewitt, J.; Thrush, S.; Norkko, A. Determining effects of suspended sediment on condition of a suspension feeding bivalve (Atrina zelandica): Results of a survey, a laboratory experiment and a field transplant experiment. J. Exp. Mar. Biol. Ecol. 2002, 267, 147–174. [Google Scholar] [CrossRef]
- MacKenzie, L.; Adamson, J. Water column stratification and the spatial and temporal distribution of phytoplankton biomass in Tasman Bay, New Zealand: Implications for aquaculture. N. Z. J. Mar. Freshw. Res. 2004, 38, 705–728. [Google Scholar] [CrossRef]
- Schreiber, S.; Rudolf, V.H.W. Crossing habitat boundaries: Coupling dynamics of ecosystems through complex life cycles. Ecol. Lett. 2008, 11, 576–587. [Google Scholar] [CrossRef] [PubMed]
- Tuckey, B.J.; Gibbs, M.T.; Knight, B.R.; Gillespie, P.A. Tidal circulation in Tasman and Golden Bays: Implications for river plume behaviour. N. Z. J. Mar. Freshw. Res. 2006, 40, 305–324. [Google Scholar] [CrossRef]
- Kelsey, J. The New Zealand Experiment: A World Model for Structural Adjustment? Bridget Williams Books: Wellington, New Zealand, 2015. [Google Scholar]
- Gibbs, M.T. Interactions between bivalve shellfish farms and fishery resources. Aquaculture 2004, 240, 267–296. [Google Scholar] [CrossRef]
- Brown, C.J.; Trebilco, R. Unintended cultivation, shifting baselines, and conflict between objectives for fisheries and conservation. Conserv. Biol. 2014, 28, 677–688. [Google Scholar] [CrossRef] [PubMed]
- McGuire, J.; Morton, L.W.; Cast, A.D. Reconstructing the good farmer identity: Shifts in farmer identities and farm management practices to improve water quality. Agric. Hum. Values 2012, 30, 57–69. [Google Scholar] [CrossRef]
- McLeod, K.; Leslie, H. Ecosystem-Based Management for the Oceans; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Ehler, C.; Douvere, F. Navigating the Future of Marine World Heritage: Results from the First World Heritage Marine Site Managers Meeting Honolulu, Hawaii, 1–3 December 2010. Available online: http://www.unesco.org/library/PDF/193111e.pdf (accessed on 6 September 2017).
- Lindenmayer, D.; Hobbs, R.J.; Montague-Drake, R.; Alexandra, J.; Bennett, A.; Burgman, M.; Cale, P.; Calhoun, A.; Cramer, V.; Cullen, P.; et al. A checklist for ecological management of landscapes for conservation. Ecol. Lett. 2008, 11, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Opdam, P.; Wascher, D. Climate change meets habitat fragmentation: Linking landscape and biogeographical scale levels in research and conservation. Biol. Conserv. 2004, 117, 285–297. [Google Scholar] [CrossRef]
- Rosenberg, A.A.; McLeod, K. Implementing ecosystem-based approaches to management for the conservation of ecosystem services: Politics and socio-economics of ecosystem-based management of marine resources. Mar. Ecol. Prog. Ser. 2005, 300, 271–274. [Google Scholar] [CrossRef]
- Gregory, R.; Failing, L.; Harstone, M.; Long, G.; McDaniels, T. Structured Decision Making: A Practical Guide to Environmental Management Choices; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Guerry, A.D.; Ruckelshaus, M.H.; Arkema, K.K.; Bernhardt, J.R.; Guannel, G.; Kim, C.K.; Marsik, M.; Papenfus, M.; Toft, J.E.; Verutes, G.; et al. Modeling benefits from nature: Using ecosystem services to inform coastal and marine spatial planning. Int. J. Biodivers. Sci. Ecosyst. Serv. Manag. 2012, 8, 107–121. [Google Scholar] [CrossRef]
- Nelson, E.; Mendoza, G.; Regetz, J.; Polasky, S.; Tallis, H.; Cameron, D.R.; Chan, K.M.A.; Daily, G.C.; Goldstein, J.; Kareiva, P.M.; et al. Modeling multiple ecosystem services, biodiversity conservation, commodity production, and tradeoffs at landscape scales. Front. Ecol. Environ. 2009, 7, 4–11. [Google Scholar] [CrossRef]
- Tietje, O. Identification of a small reliable and efficient set of consistent scenarios. Eur. J. Oper. Res. 2005, 162, 418–432. [Google Scholar] [CrossRef]
- Robinson, J.; Carmichael, J.; Van Wynsberghe, R.; Journeay, M.; Rogers, L. Sustainability as a problem of design: Interactive science in the Georgia Basin. Integr. Asses. 2006, 6, 165–192. [Google Scholar]
- Schneider, R.R.; Stelfox, J.B.; Boutin, S.; Wasel, S. Managing the cumulative impacts of land uses in the western canadian sedimentary basin: A modeling approach. Ecol. Soc. 2003, 7, 928–930. [Google Scholar] [CrossRef]
- Metcalf, S.S.; Wheeler, E.; Bendor, T.K.; Lubinski, K.S.; Hannon, B.M. Sharing the floodplain: Mediated modeling for environmental management. Environ. Model. Softw. 2010, 25, 1282–1290. [Google Scholar] [CrossRef]
- Steinitz, C.; Arias, H.; Bassett, S.; Flaxman, M.; Goode, T.; Maddock, T., III; Mouat, D.; Peiser, R.; Shearer, A. Alternative Futures for Changing Landscapes; Island Press: Washington, DC, USA, 2003. [Google Scholar]
- Porras, I.; Barton, D.N.; Miranda, M. Learning from 20 Years of Payments for Ecosystem Services in Costa Rica; Shaping Sustainable Markets Papers: London, UK, 2013. [Google Scholar]
- Ruhl, J.B. Farms, Their Environmental Harms, and Environmental Law: Taking the Great Leap from Anti-Law to Positive Law in Farm Policy. Available online: http://scholarship.law.berkeley.edu/cgi/viewcontent.cgi?article=1623&context=elq (accessed on 7 September 2017).
- Tanentzap, A.J.; Lamb, A.; Walker, S.; Farmer, A. Resolving conflicts between agriculture and the natural environment. PLoS Biol. 2015, 13, e1002242. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.N. Can cows and conservation mix? BioScience 2001, 51, 85–90. [Google Scholar] [CrossRef]
- Curtin, C.G.; Sayre, N.F.; Lane, B.D. Transformations of the Chihuahuan Borderlands: Grazing, fragmentation, and biodiversity conservation in desert grasslands. Environ. Sci. Policy 2002, 5, 55–68. [Google Scholar] [CrossRef]
- Austin, M.A.; Buffett, D.A.; Nicolson, D.J.; Scudder, G.G.E.; Stevens, V. (Eds.) Taking Nature’s Pulse: The Status of Biodiversity in British Columbia; Biodiversity BC: Victoria, BC, Canada, 2008; p. 268. [Google Scholar]
- Wilcove, D.S.; Lee, J. Using economic and regulatory incentives to restore endangered species: Lessons learned from three new programs. Conserv. Biol. 2004, 18, 639–645. [Google Scholar] [CrossRef]
- Wunder, S. Are direct payments for environmental services spelling doom for sustainable forest management in the tropics? Ecol. Soc. 2006, 11, 473–482. [Google Scholar] [CrossRef]
- Rigby, D.; Cáceres, D. Organic farming and the sustainability of agricultural systems. Agric. Syst. 2001, 68, 21–40. [Google Scholar] [CrossRef]
- Ostrom, E.; Cox, M. Moving beyond panaceas: A multi-tiered diagnostic approach for social-ecological analysis. Environ. Conserv. 2010, 37, 451–463. [Google Scholar] [CrossRef]
- Escobar, A. Culture sits in places: Reflections on globalism and subaltern strategies of localization. Political Geogr. 2001, 20, 139–174. [Google Scholar] [CrossRef]
- Marsden, T. Sustainable place-making for sustainability science: The contested case of agri-food and urban–rural relations. Sustain. Sci. 2013, 8, 213–226. [Google Scholar] [CrossRef]
- Horlings, L.G.; Horlings, L.G. Values in place; a value-oriented approach toward sustainable place-shaping. Reg. Stud. Reg. Sci. 2015, 2, 257–274. [Google Scholar] [CrossRef]
- Klassen, S.E.; Wittman, H. Place-based food systems: “Re-valuing local” and fostering socio-ecological sustainability. In Sustainable Food Futures: Multidisciplinary Solutions; Duncan, J., Bailey, M., Eds.; Routledge: Abingdon, UK, 2017. [Google Scholar]
- Seufert, V.; Ramankutty, N.; Mayerhofer, T. What is this thing called organic?—How organic farming is codified in regulations. Food Policy 2017, 68, 10–20. [Google Scholar] [CrossRef]
- Jacobsen, S.E.; Sørensen, M.; Pedersen, S.M.; Weiner, J. Feeding the world: Genetically modified crops versus agricultural biodiversity. Agron. Sustain. Dev. 2013, 33, 651–662. [Google Scholar] [CrossRef]
- Stubbs, T.L.; Kennedy, A.C. Microbial weed control and microbial herbicides. In Herbicides—Environmental Impact Studies and Management Approaches; Alvarez-Fernandez, R., Ed.; InTech: Rijeka, Croatia, 2012; pp. 135–166. [Google Scholar]
- Kennedy, A.C.; Johnson, B.N.; Stubbs, T.L. Host range of a deleterious rhizobacterium for biological control of downy brome. Weed Sci. 2001, 49, 792–797. [Google Scholar] [CrossRef]
- Seufert, V.; Ramankutty, N.; Foley, J.A. Comparing the yields of organic and conventional agriculture. Nature 2012, 485, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Levin, S.A.; Lubchenco, J. Resilience, robustness, and marine ecosystem-based management. BioScience 2008, 58, 27–32. [Google Scholar] [CrossRef]
- Levin, S.; Xepapadeas, T.; Crépin, A.S.; Norberg, J.; de Zeeuw, A.; Folke, C.; Hughes, T.; Arrow, K.; Barrett, S.; Daily, G.; et al. Social-ecological systems as complex adaptive systems: Modeling and policy implications. Environ. Dev. Econ. 2012, 18, 111–132. [Google Scholar] [CrossRef]
- Leslie, P.; McCabe, J.T. Response diversity and resilience in social-ecological systems. Curr. Anthropol. 2013, 54, 114–143. [Google Scholar] [CrossRef] [PubMed]
- Baumgart-Getz, A.; Prokopy, L.S.; Floress, K. Why farmers adopt best management practice in the USA: A meta-analysis of the adoption literature. J. Environ. Manag. 2012, 96, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.W. Environmental crises and the metabolic rift in world-historical perspective. Org. Environ. 2016, 13, 123–157. [Google Scholar] [CrossRef]
- Clapp, J. Distant agricultural landscapes. Sustain. Sci. 2014, 10, 305–316. [Google Scholar] [CrossRef]
- Wittman, H. Reworking the metabolic rift: La Vía Campesina, agrarian citizenship, and food sovereignty. J. Peasant Stud. 2009, 36, 805–826. [Google Scholar] [CrossRef]
- Levkoe, C.Z. Towards a transformative food politics. Local Environ. 2011, 16, 687–705. [Google Scholar] [CrossRef]
- Altieri, M.A. Agroecology, Small Farms, and Food Sovereignty. Available online: http://monthlyreview.org/2009/07/01/agroecology-small-farms-and-food-sovereignty/ (accessed on 1 March 2015).
- Blesh, J.; Wittman, H. “Brasilience:” assessing resilience in land reform settlements in the Brazilian Cerrado. Hum. Ecol. 2015, 43, 531–546. [Google Scholar] [CrossRef]
- Wittman, H. Food sovereignty: A new rights framework for food and nature? Environ. Soc. Adv. Res. 2011, 2, 87–105. [Google Scholar]
- Loos, J.; Abson, D.J.; Chappell, M.J.; Hanspach, J.; Mikulcak, F.; Tichit, M.; Fischer, J. Putting meaning back into “sustainable intensification”. Front. Ecol. Environ. 2014, 12, 356–361. [Google Scholar] [CrossRef]
- Weber, C.L.; Matthews, H.S. Food-Miles and the Relative Climate Impacts of Food Choices in the United States. Environ. Sci. Technol. 2008, 42, 3508–3513. [Google Scholar] [CrossRef] [PubMed]
- Stokstad, E. Could less meat mean more food? Science 2010, 327, 810–811. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D.; Clark, M. Global diets link environmental sustainability and human health. Nature 2014, 515, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Erb, K.H.; Lauk, C.; Kastner, T.; Mayer, A.; Theurl, M.C.; Haberl, H. Exploring the biophysical option space for feeding the world without deforestation. Nat. Commun. 2016, 7, 11382. [Google Scholar] [CrossRef] [PubMed]
- Kremen, C.; Iles, A.; Bacon, C. Diversified farming systems: An agroecological, systems-based alternative to modern industrial agriculture. Ecol. Soc. 2012, 17, 44. [Google Scholar] [CrossRef]
- Raynolds, L.T.; Murray, D.; Heller, A. Regulating sustainability in the coffee sector: A comparative analysis of third-party environmental and social certification initiatives. Agric. Hum. Values 2007, 24, 147–163. [Google Scholar] [CrossRef]
- Schipanski, M.E.; MacDonald, G.K.; Rosenzweig, S.; Chappell, M.J.; Bennett, E.M.; Kerr, R.B.; Blesh, J.; Crews, T.; Drinkwater, L.; Lundgren, J.G.; et al. Realizing resilient food systems. BioScience 2016, 66, 600–610. [Google Scholar] [CrossRef]
- Kahneman, D. Thinking, Fast and Slow; Farrar, Straus and Giroux: New York, NY, USA, 2011. [Google Scholar]
- Heath, C.; Heath, D. Switch: How to Change Things When Change Is Hard, 1st ed.; Crown Business: Danvers, MA, USA, 2010. [Google Scholar]
- Eckhardt, G.M.; Belk, R.; Devinney, T.M. Why don’t consumers consume ethically? J. Consum. Behav. 2010, 9, 426–436. [Google Scholar] [CrossRef]
- Sagoff, M. Aggregation and deliberation in valuing environmental public goods: A look beyond contingent pricing. Ecol. Econ. 1998, 24, 213–230. [Google Scholar] [CrossRef]
- Kahan, D.M.; Braman, D. Cultural cognition and public policy. Yale Law Policy Rev. 2006, 24, 149–172. [Google Scholar]
| CAS Characteristic | 1. Multi-Causality: There Is No Smoking Gun and No Silver Bullet | 2. Cumulative Impacts: Death by a Thousand Cuts | 3. Regime Shifts: Systems Change in Fits and Spurts, and Can Flip Unexpectedly | 4. Teleconnections: “Transporting” Impacts across Time and Space | 5. Multi-Scalarity: Drivers and Impacts Cross Scales |
|---|---|---|---|---|---|
| Definition | Any given ecological or social pattern is simultaneously the product of many different processes [ 26] | The combined total effect of multiple effects that limit the ability of people to enjoy ecosystem services [ 34] | A system can change non-linearly and non-reversibly between alternative stable states with significantly different components | Links between distant areas that are enabled via larger scale processes | Patterns can emerge at one scale via changes at other scales |
| Associated terms/concepts | Multi-causality | Cumulative impacts | Non-linearity, regime shifts, hysteresis, basins of attraction, attractors, stable states, steady states, bifurcations, oscillations, periodic behavior | Teleconnections, legacy effects, cross-system impacts | Multi-scalarity, shifting baselines, pulse and press disturbances |
| Key Literature | [ 26] | [ 35,36,37] | [ 28,38,39,40] | [ 41,42,43] | [ 26,44] |
| Case Study | 1. Multi-Causality: There Is No Smoking Gun and No Silver Bullet | 2. Cumulative Impacts: Death By a Thousand Cuts | 3. Regime Shifts: Systems Change in Fits and Spurts, and Can Flip Unexpectedly | 4. Teleconnections: “Transporting” Impacts across Time and Space | 5. Multi-Scalarity: Drivers and Impacts Cross Scales |
|---|---|---|---|---|---|
| (1) Pollinators | Neonicotinoid pesticides are considered by many to be the cause of pollinator declines. While there is mounting evidence that demonstrates their toxic and sometimes lethal effects on pollinator populations, and important policy and legislative actions have been taken to reduce neonicotinoid use as a result, this one-dimensional view of pollinator challenges is problematic as it can excuse inaction, or obscure the other contributing causes. | There are several contributing causes to decreased resilience of pollinator populations, including compromised immune systems from pesticide exposure, reduced abundance and appropriateness of food sources, decreased natural and semi-natural habitat as nesting sites, increased exposure to pests and parasites, and increased environmental shocks from climate change. The intensification and extensification of agriculture has contributed to all of these causes. | The sudden collapse of honeybee colonies (coined Colony Collapse Disorder) can be considered a micro example of a systems flip. At larger scales, a sudden systems flip or collapse of pollinator populations is also possible as stressors reach critical thresholds that populations are no longer able to withstand. | Local pollinator populations around the world (both wild and managed) are being significantly impacted via the same global drivers. Land conversion of biodiverse areas such as tropical forests to agricultural land in order to feed our growing population, and the increased intensity of management (reduced biodiversity, increased input use) are all contributing to local pollinator declines. | The dynamics and stressors that are contributing to pollinator declines are happening at multiple scales, from in-field biodiversity, to global market conditions influencing farmer decision-making, to widespread habitat destruction from agricultural extensification. In the global agri-food system, agricultural production is often driven by demand from non-local markets, yet critical agro-ecosystem dynamics are limited to smaller scales (e.g., the foraging range of many pollinators is within the scale of a farm-field). |
| (2) PES | One of the benefits of PES is that, as a voluntary program, it allows policymakers to address agricultural impacts without demonstrating proof (as is often expected before prohibitive legislation). | Agricultural impacts are one set among many, but within agriculture, PES have envisioned the actions of separate farmers as separate impacts to be addressed separately by ‘buying’ behavior change (via an incremental addition of an extrinsic motivation). But such an approach misses the point that there are larger system dynamics at play, and it may be possible to intervene in such a way as to change norms, not just individual behaviours. | PES programs are sometimes looked to as solutions to problems in downstream systems. While this recognition of teleconnections and multi-scalar dynamics are welcome, it’s also the case that those downstream systems shouldn’t be expected to change linearly as a result of altered inputs via the PES. | Agricultural intensification and associated environmental impacts are a result of teleconnections from consumer demand and—in some cases—pressure from integrated value-chain retailers (e.g., Walmart). But perhaps what’s needed is to expand PES so as to directly connect improvements in farm management directly to the concerns of consumers, who often value environmental outcomes and demonstrate a willingness to buy accordingly. | PES are an example of recognizing that on-farm actions can have considerable consequences at other scales, e.g., in downstream aquatic ecosystems (Chesapeake, Golden/Tasman Bays, Gulf of Mexico). |
| (3) Pest control | Pesticide resistance is driven by multiple factors, including pressure from pest predators, availability of habitat fragments without pesticide pressure and farm and landscape scale diversity. | If pesticides have acquired resistance to multiple pests, viable pest-control options become increasingly difficult to find, and the alternative is a more severe collapse in production. | Pest control systems with multiple pest predators and competitors exist in the basin of attraction of a self-regulating, functioning food web. Intensive pesticide use can shift these systems to a different basin of attraction where crop collapse results in the event of pesticide failure. | Once pests evolve genetic pesticide resistance, the trait can travel through metapopulations and spread to areas where the resistance mutation would not have arisen. On the positive side, meta-populations of beneficial insects can help repopulate depleted areas if management changes to support their survival and connectivity. | Changes to the ecological community on a local scale (the absence of pest predators and competitors) and local selective forces (intensive pesticide use) drive the acquisition of permanent genetic changes with large scale impacts (both in time and space). |
| (4) Sediment in Tasman bay | While sedimentation is seen as a major issue in Tasman bay, it has many contributing causes. A further complication is that historical fishing practices have led to the context where sediment is as problematic as it is today. | Future climate change may reinforce current feedbacks as more intense storms may increase sediment runoff to the bay. | Changing the benthic community from a three dimensional, high bivalve biomass floor to a system with a flat silty bottom through historic fishing practices has exposed Tasman bay to sedimentation and sediment resuspension, as there does not exist the density of filter feeders to help sediment settle out. | Demand for New Zealand dairy, meat and other agricultural products in other continents can shape land use practices around Tasman bay, resulting in changes to sediment input into the bay. | Land use around the bays that are sources of sediment are regional in scale. Because New Zealand agriculture is fully subject to global markets, global demand affects land use (global scale). Ships (commercial and fishing) in the bays also resuspend sediment at a local scale. |
| (5) Scales in Puget Sound | While loss, fragmentation and destruction of salmon habitat is considered to be the limiting factor (aka smoking gun) for salmon returns, the many other impacts to salmon, including dams, climate change, and non-point source pollution as well as fisheries related impacts allows different actors to point blame elsewhere. Even if habitat is greatly improved, it is no silver bullet as the other factors could still combine to impede recovery. | Salmon face multiple impacts including from land use, dams, fisheries, and indirect global impacts such as climate change. Impacts from individual farms such as run-off and lack of riparian habitat are individually small, yet cumulatively important. | Potential collapses in salmon runs could have dramatic social, cultural and ecological consequences. | Ocean currents and migration mean that salmon may face impacts generated in distant places, including changes in ocean temperature and acidity driven by distant emissions. | Restoration projects are completed on the parcel scale and in relatively short term contracts yet seek to have large spatial scale impacts on salmon returns. Historical impacts have dramatically altered the baseline expectations of many Puget Sound residents. Yet Treaty Tribes employ a much longer term baseline going back prior to colonization and the resultant widespread landscape changes. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chapman, M.; Klassen, S.; Kreitzman, M.; Semmelink, A.; Sharp, K.; Singh, G.; Chan, K.M.A. 5 Key Challenges and Solutions for Governing Complex Adaptive (Food) Systems. Sustainability 2017, 9, 1594. https://doi.org/10.3390/su9091594
Chapman M, Klassen S, Kreitzman M, Semmelink A, Sharp K, Singh G, Chan KMA. 5 Key Challenges and Solutions for Governing Complex Adaptive (Food) Systems. Sustainability. 2017; 9(9):1594. https://doi.org/10.3390/su9091594
Chicago/Turabian StyleChapman, Mollie, Susanna Klassen, Maayan Kreitzman, Adrian Semmelink, Kelly Sharp, Gerald Singh, and Kai M. A. Chan. 2017. "5 Key Challenges and Solutions for Governing Complex Adaptive (Food) Systems" Sustainability 9, no. 9: 1594. https://doi.org/10.3390/su9091594




