Biological Treatment of Fish Processing Saline Wastewater for Reuse as Liquid Fertilizer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fish Processing Wastewater Preparation
2.2. Experimental Setup and Operating Condition
2.3. Analytical Methods
2.3.1. Measurement of Physicochemical Parameter
pH Value
Temperature
Total Suspended Solids (TSS)
- A = Mass of nonfilterable residue on Whatman GF/C filter after evaporation at 105 °C (mg),
- B = Mass of the filter paper prior to sample filtration (mg),
- C = Volume of the sample used for filtration (mL).
Volatile Suspended Solids (VSS)
- A = Mass of nonfilterable residue on Whatman GF/C filter after evaporation at 550 °C (mg),
- B = Mass of nonfilterable residue on Whatman GF/C filter afterignition at 150 °C (mg),
- C = Sample size that have used for filtration (mL).
Biomass Concentration
2.3.2. Measurement of Chemical Parameter: The Organic Content
5-Day Biochemical Oxygen Demand (BOD5)
- DS0 = initial dissolved oxygen in the diluted sample,
- DS5 =final dissolved oxygen in the diluted sample after 5 days of incubation,
- DW0 = initial dissolved oxygen in the dilution water,
- DW5 = final dissolved oxygen in the dilution water after 5 days of incubation,
- SC0 = initial DO in diluted seed solution (seed control),
- SC5 = final DO in diluted seed solution (seed control) after 5 days of incubation,
- F = dilution factor [total volume after dilution (mL)/volume of undiluted sample (mL)],
- f = ratio of seed in diluted sample to seed in seed control,
- [% seed in diluted sample/% seed in diluted seed solution (seed control)].
Chemical Oxygen Demand (COD)
- A = volume of FAS titrant used to titrate the blank (mL),
- B = volume of FAS titrant used to titrate the sample (mL),
- M = Molarity (mol/L) of FAS titrant; 0.01 M was used,
- 8000 = milliequivalent weight of oxygen × 1000 mL/L.
2.3.3. Measurement of Chemical Parameter: The Inorganic Content/Nutrients
Ammoniacal Nitrogen (NH3-N)
Nitrate-Nitrogen (NO3−-N)
Phosphorus (P)/Orthophosphate (PO43−)
3. Results
3.1. Fish Processing Wastewater Characteristics
3.2. Parameter Analysis Results
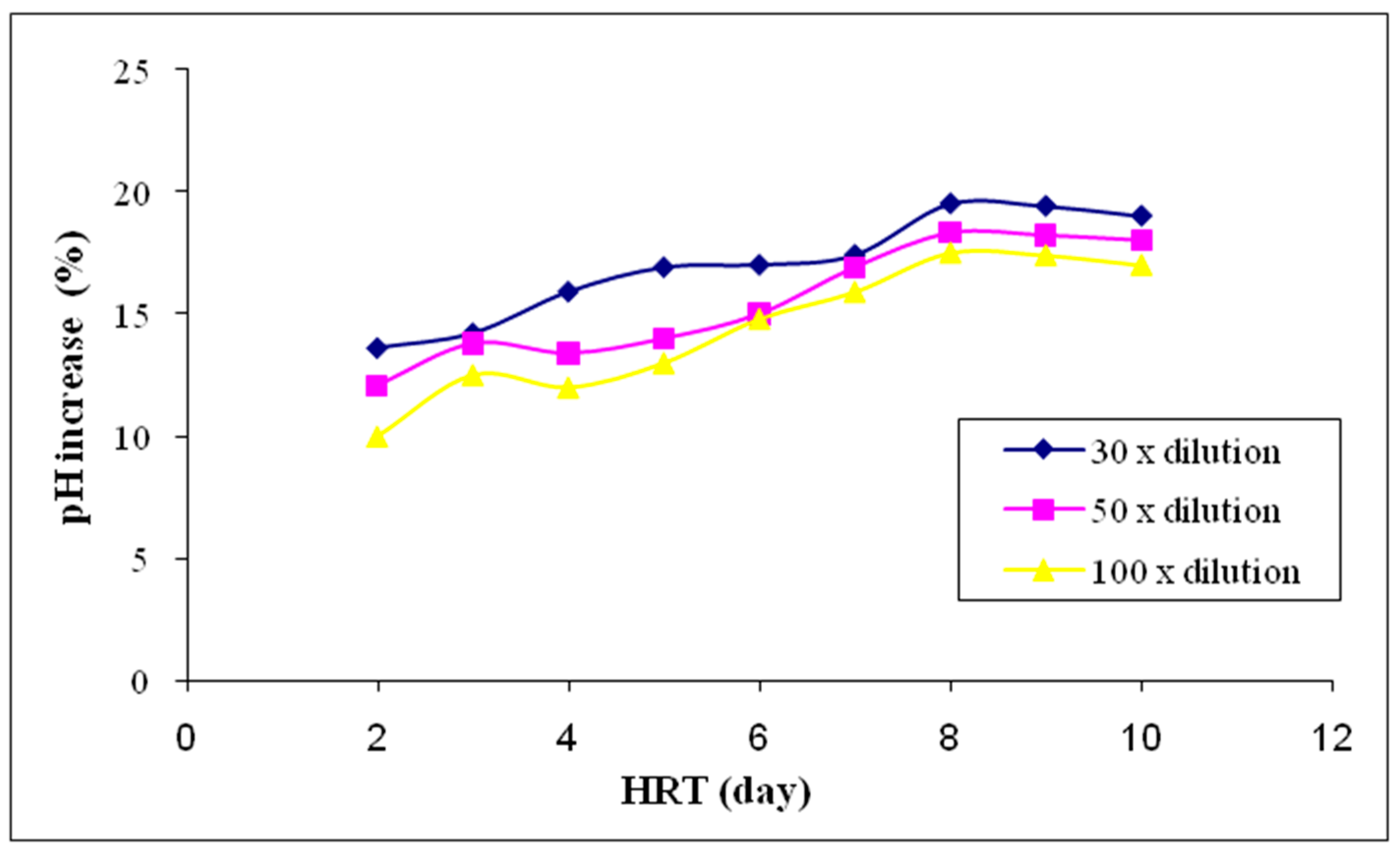
3.2.1. pH Value
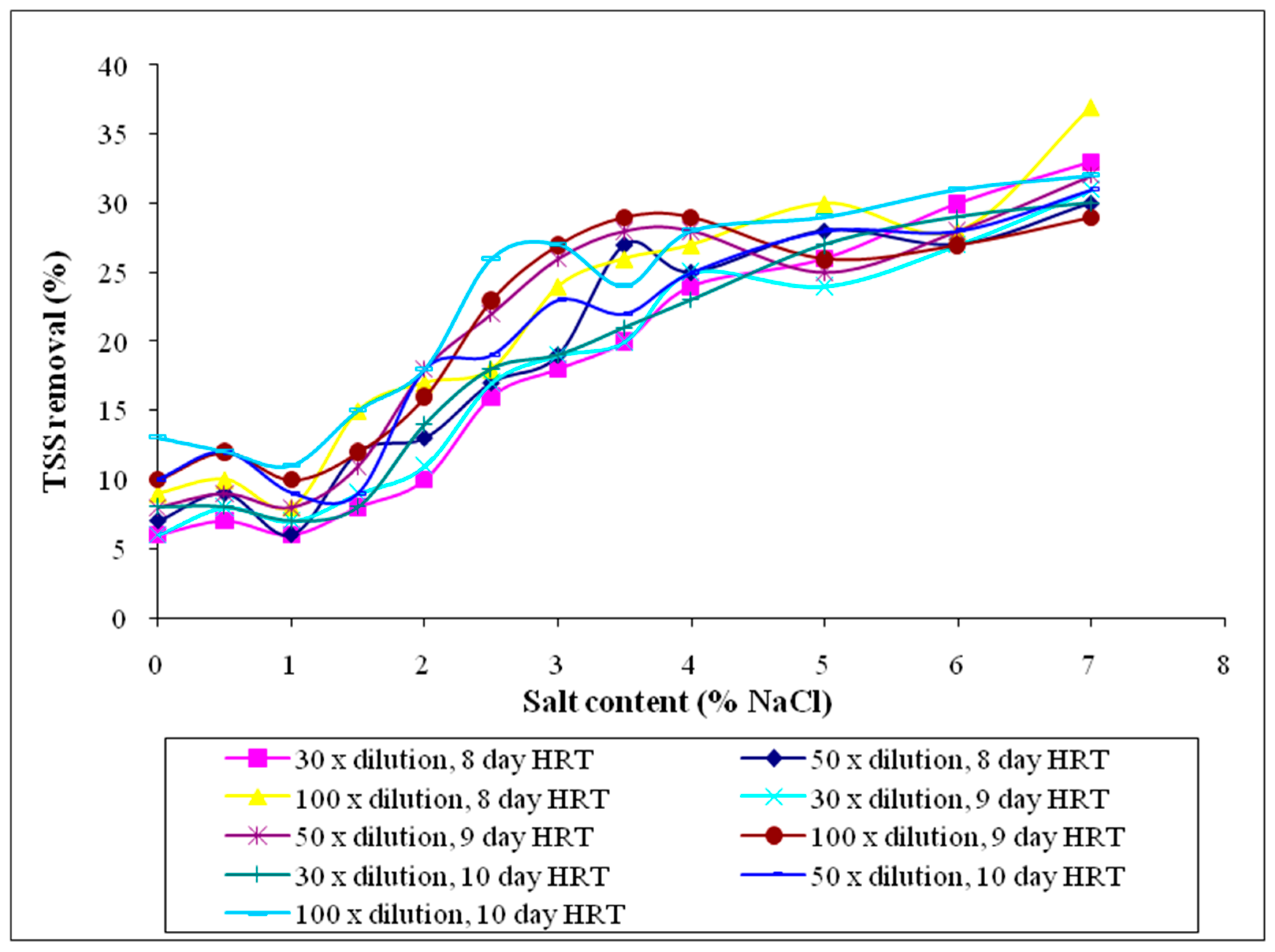
3.2.2. Total Suspended Solids (TSS)
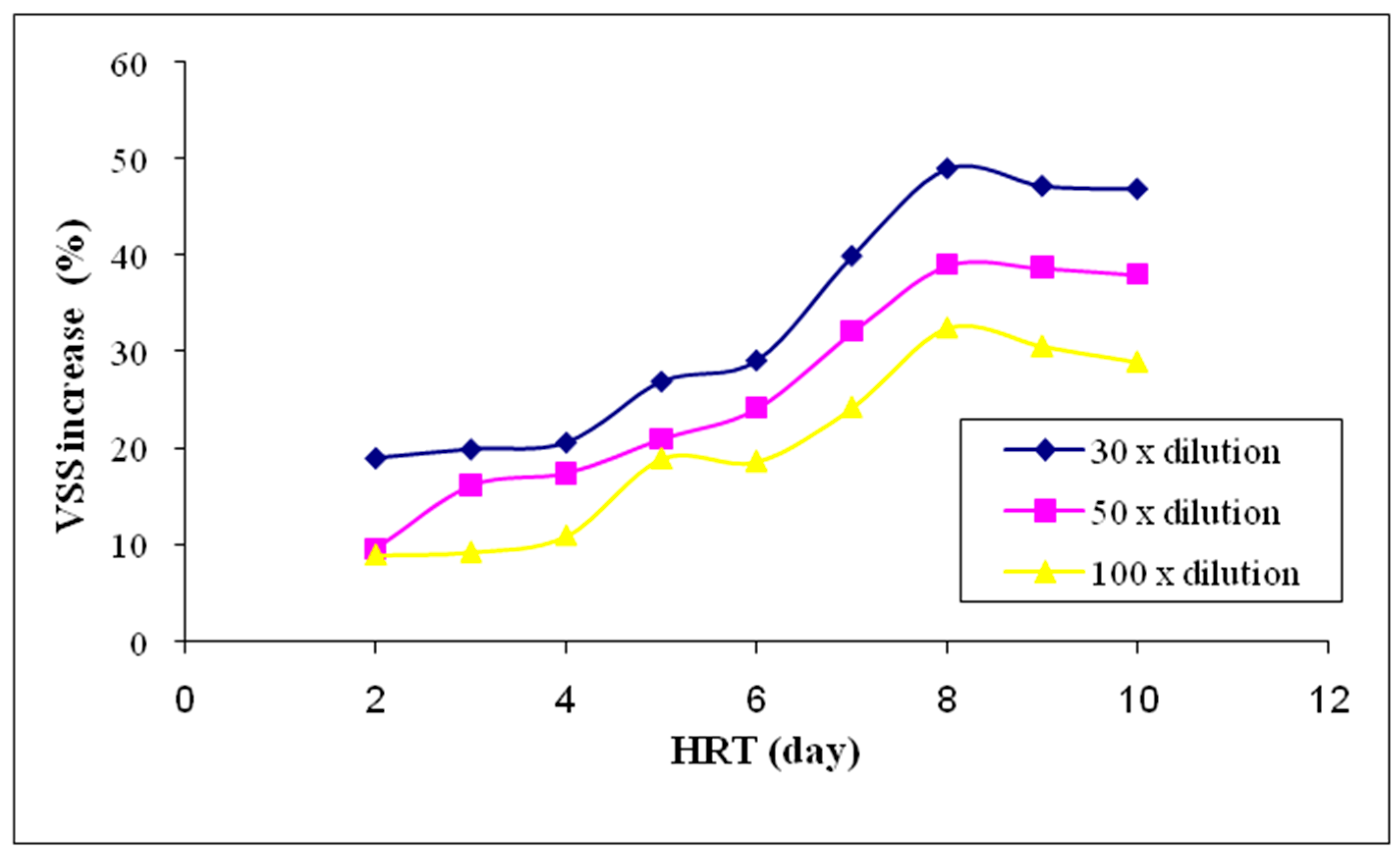
3.2.3. Volatile Suspended Solids (VSS)
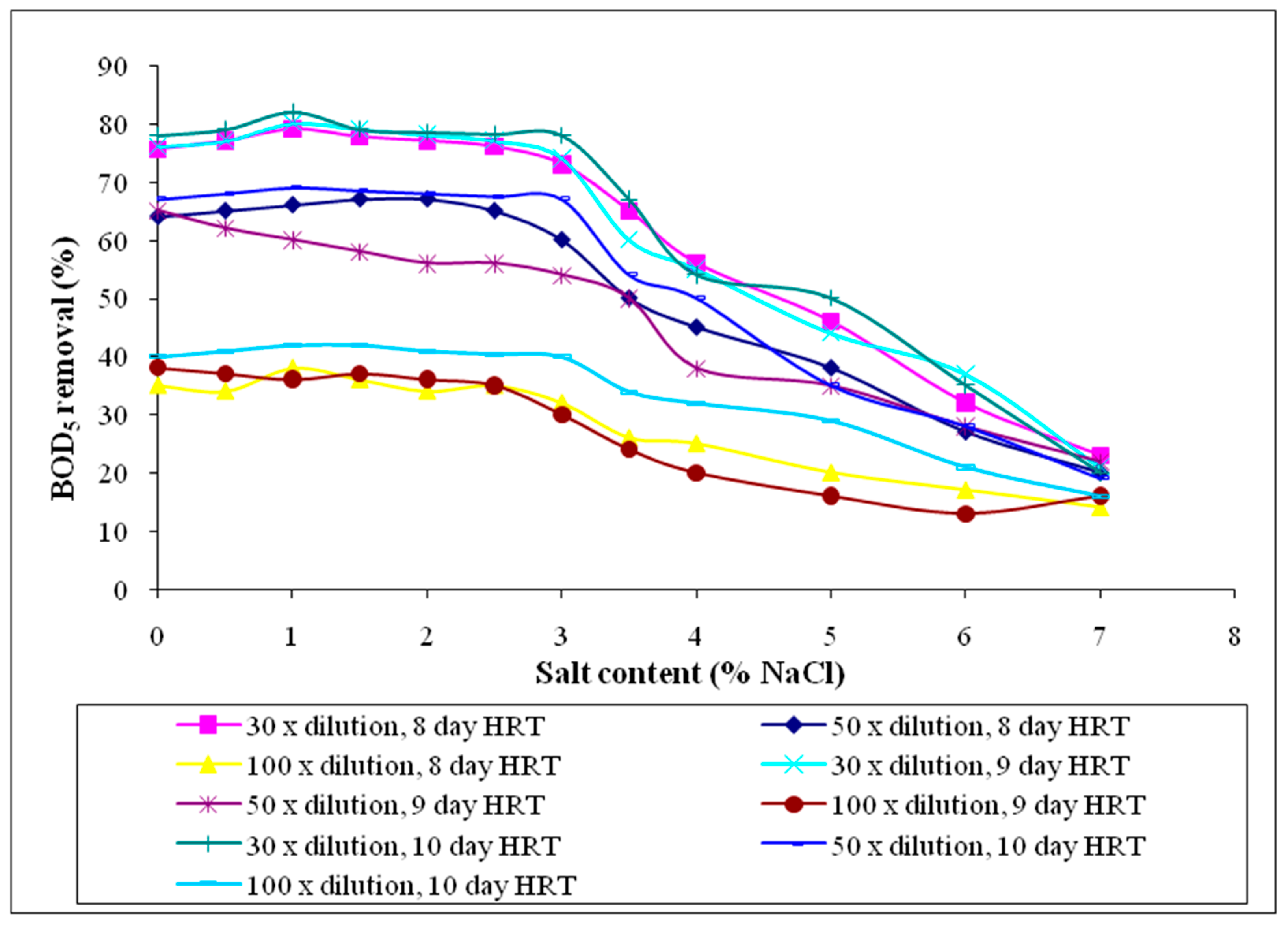
3.2.4. Five-Day Biochemical Oxygen Demand (BOD5)
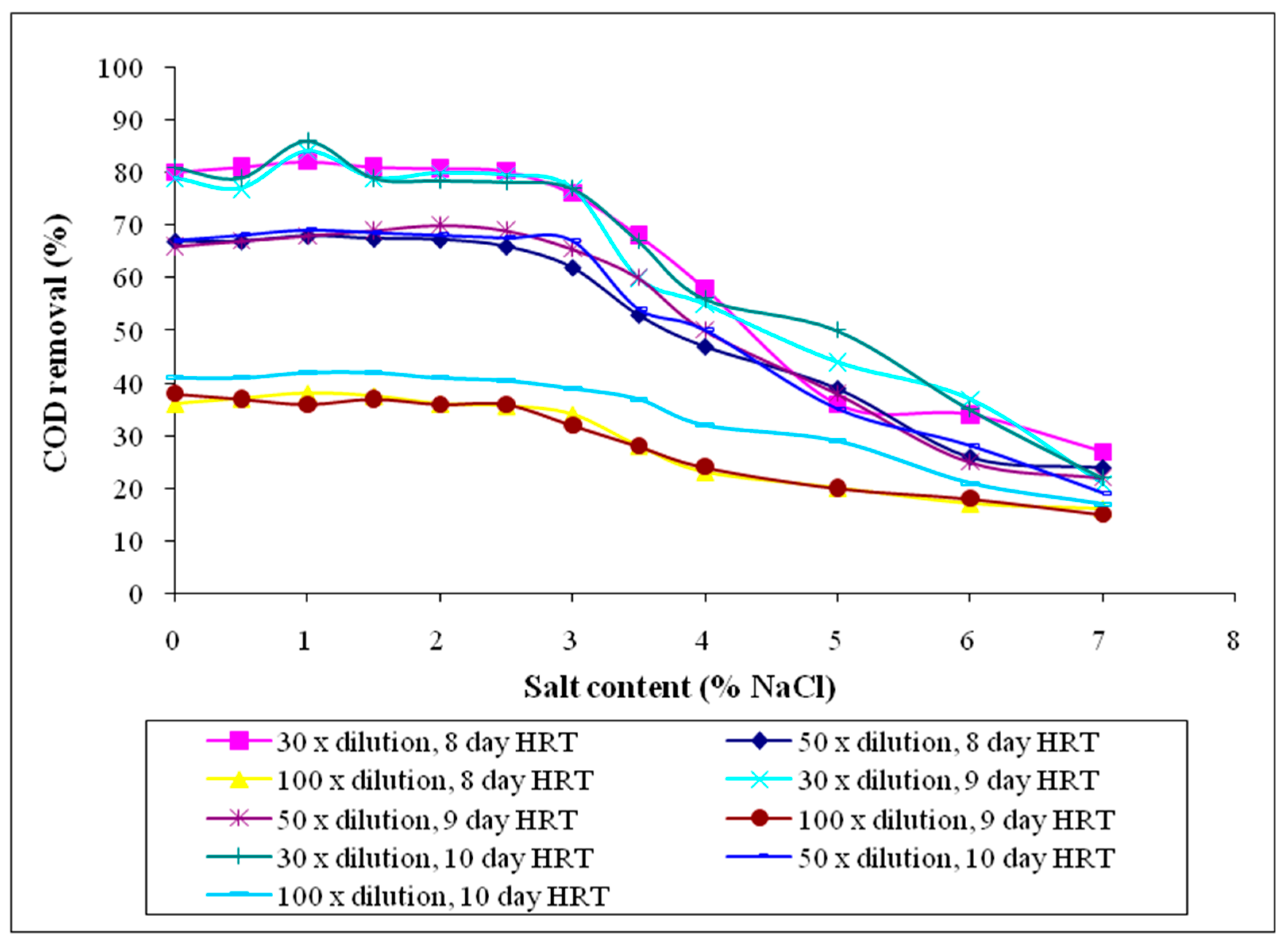
3.2.5. Chemical Oxygen Demand (COD)
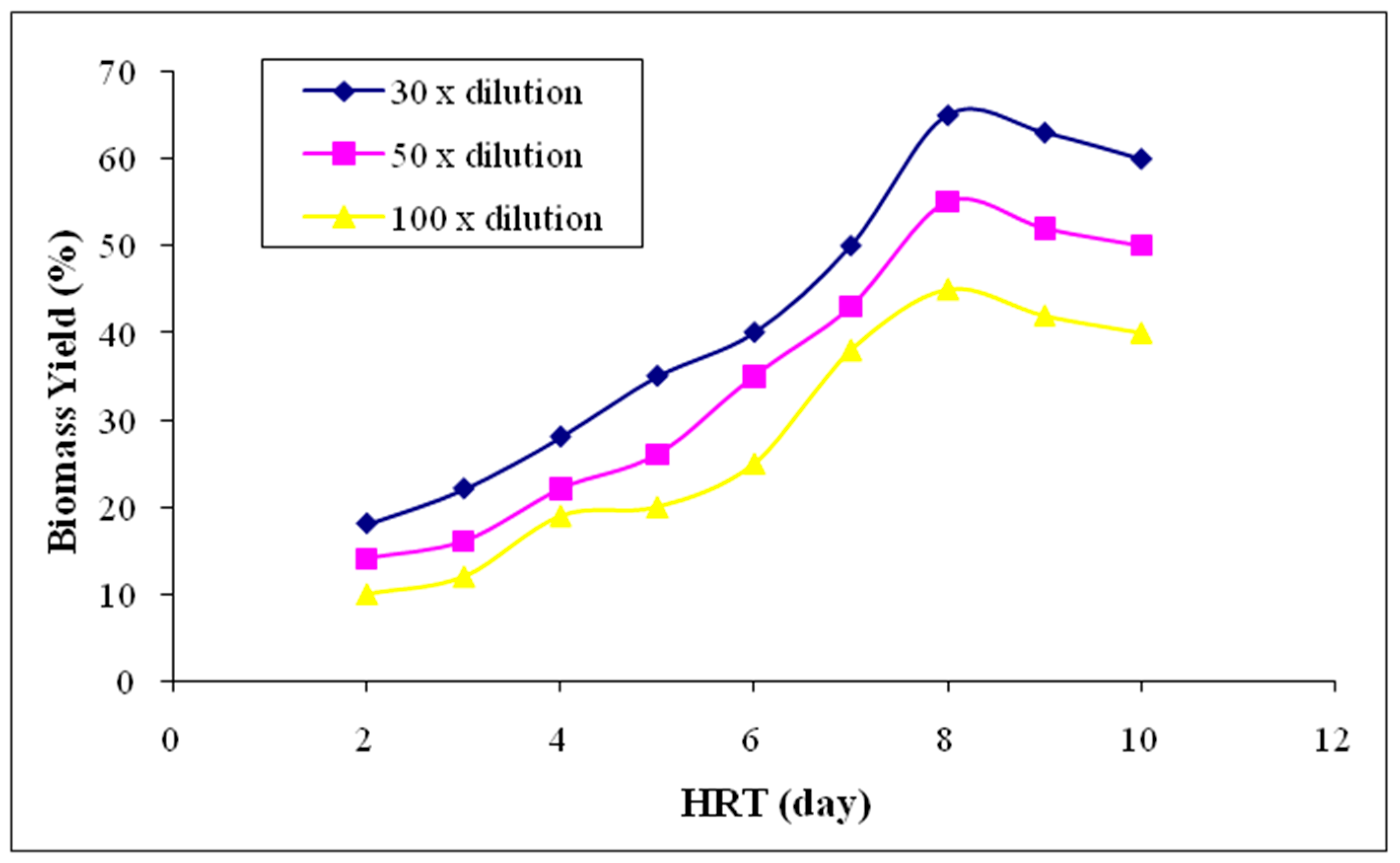
3.2.6. Biomass Yield
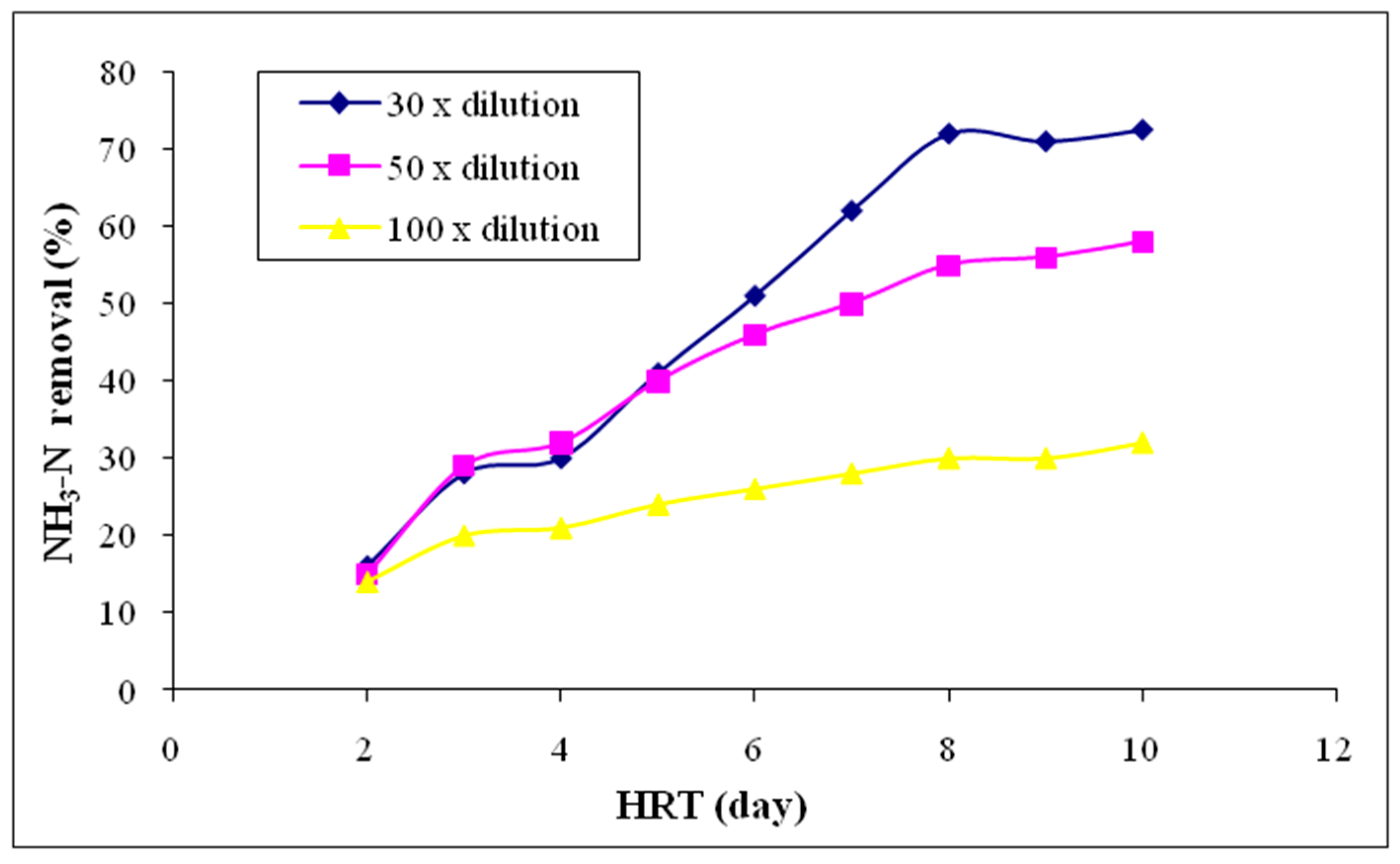
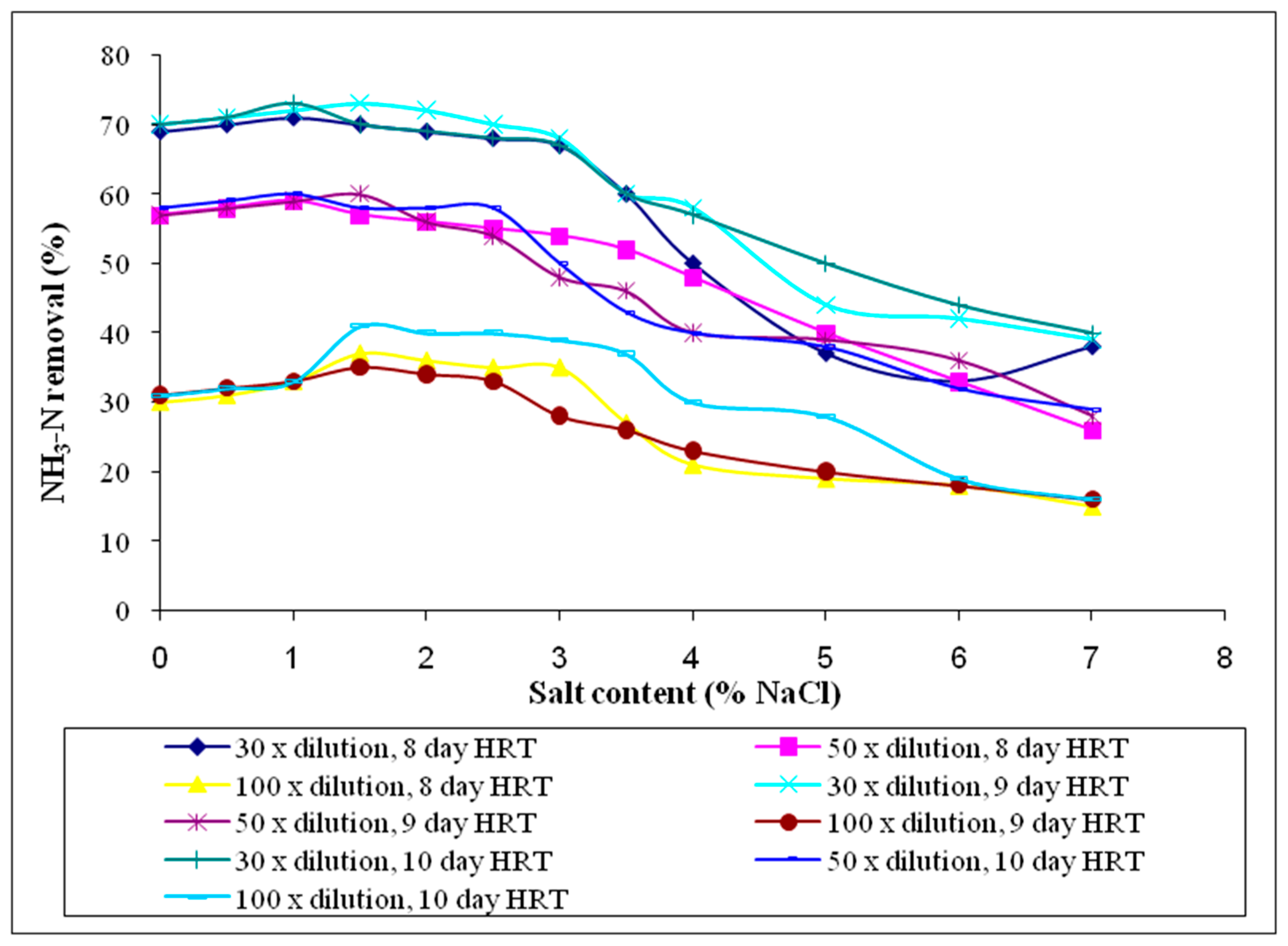
3.2.7. Ammoniacal Nitrogen (NH3-N)
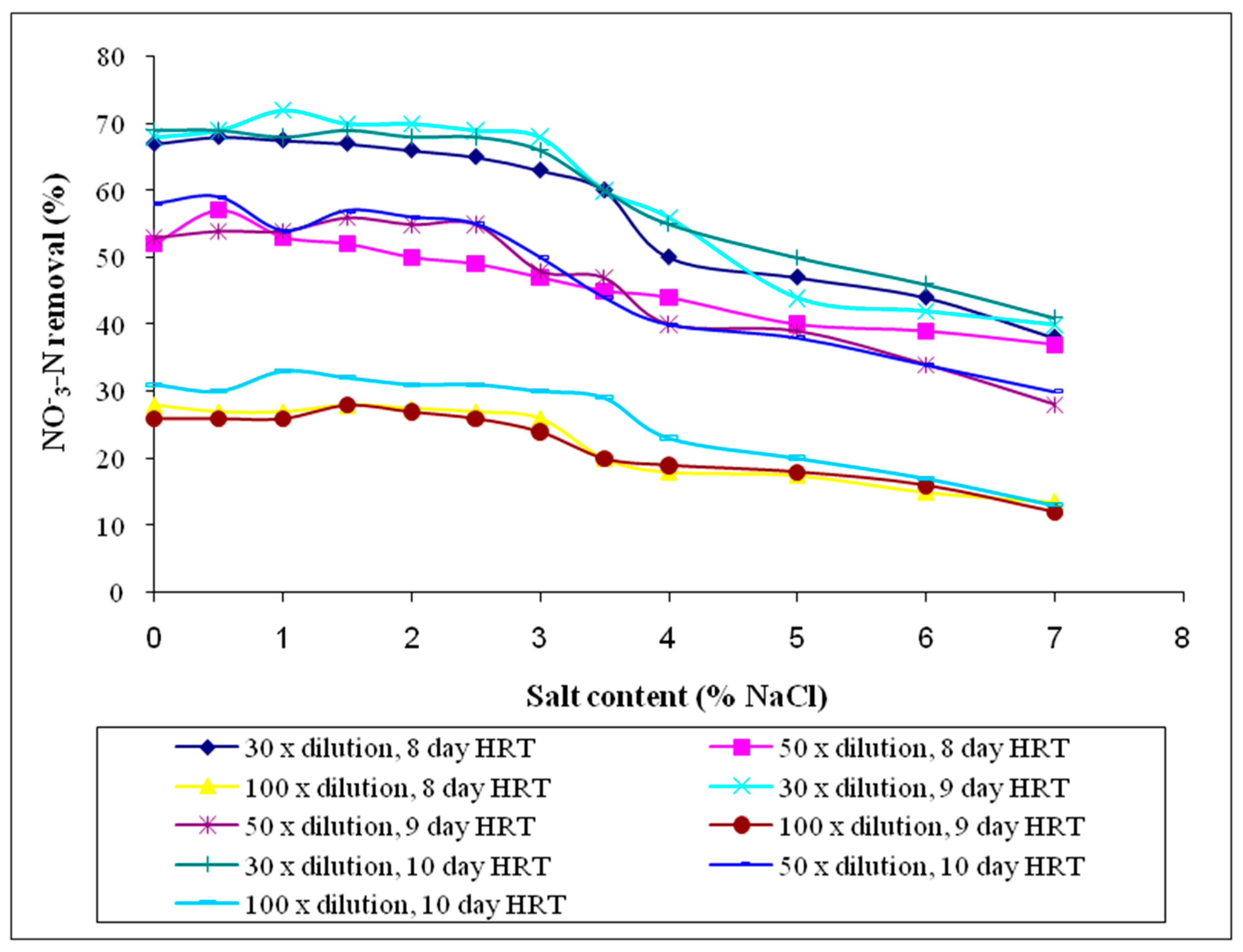
3.2.8. Nitrate-Nitrogen (NO3−-N)
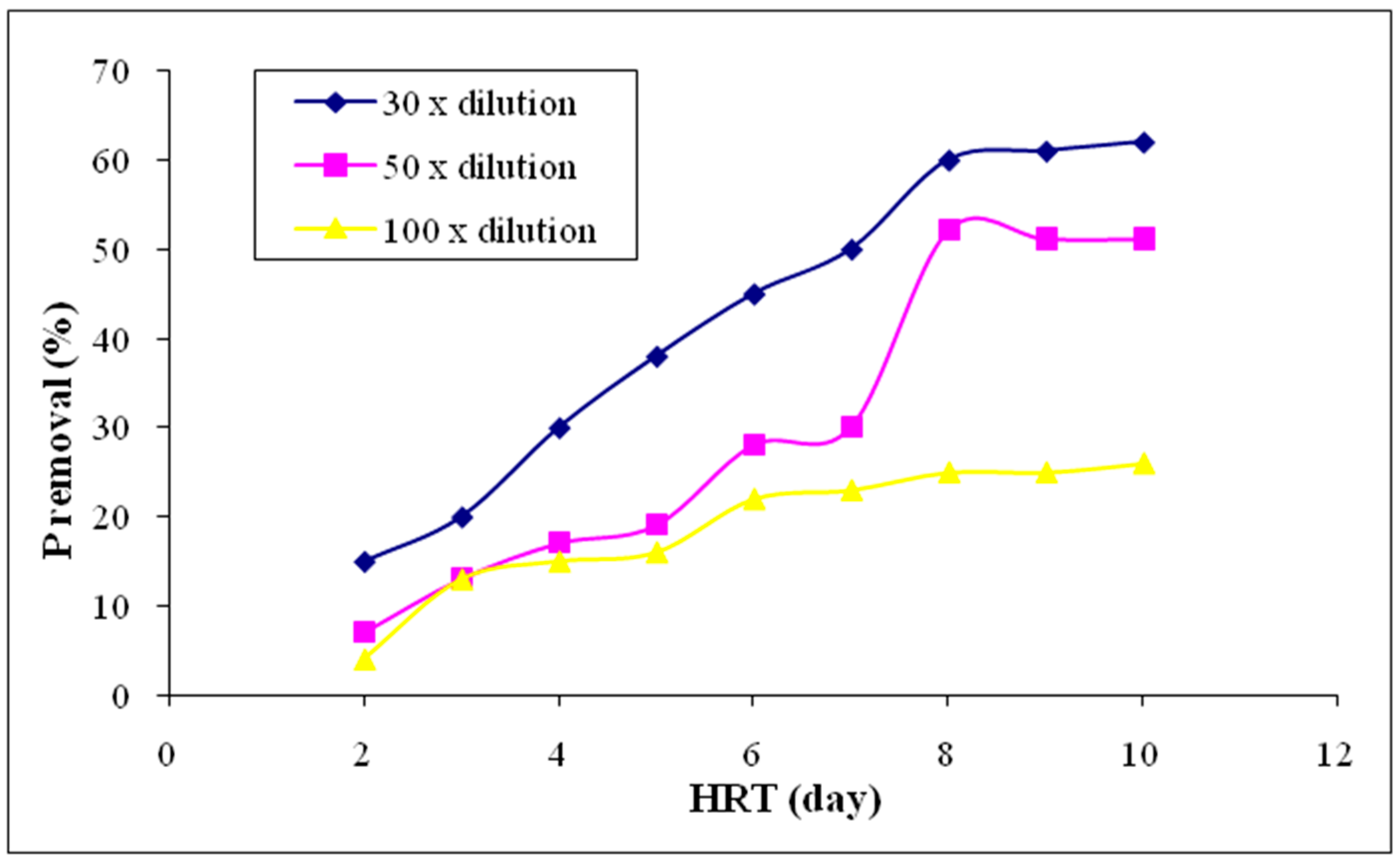
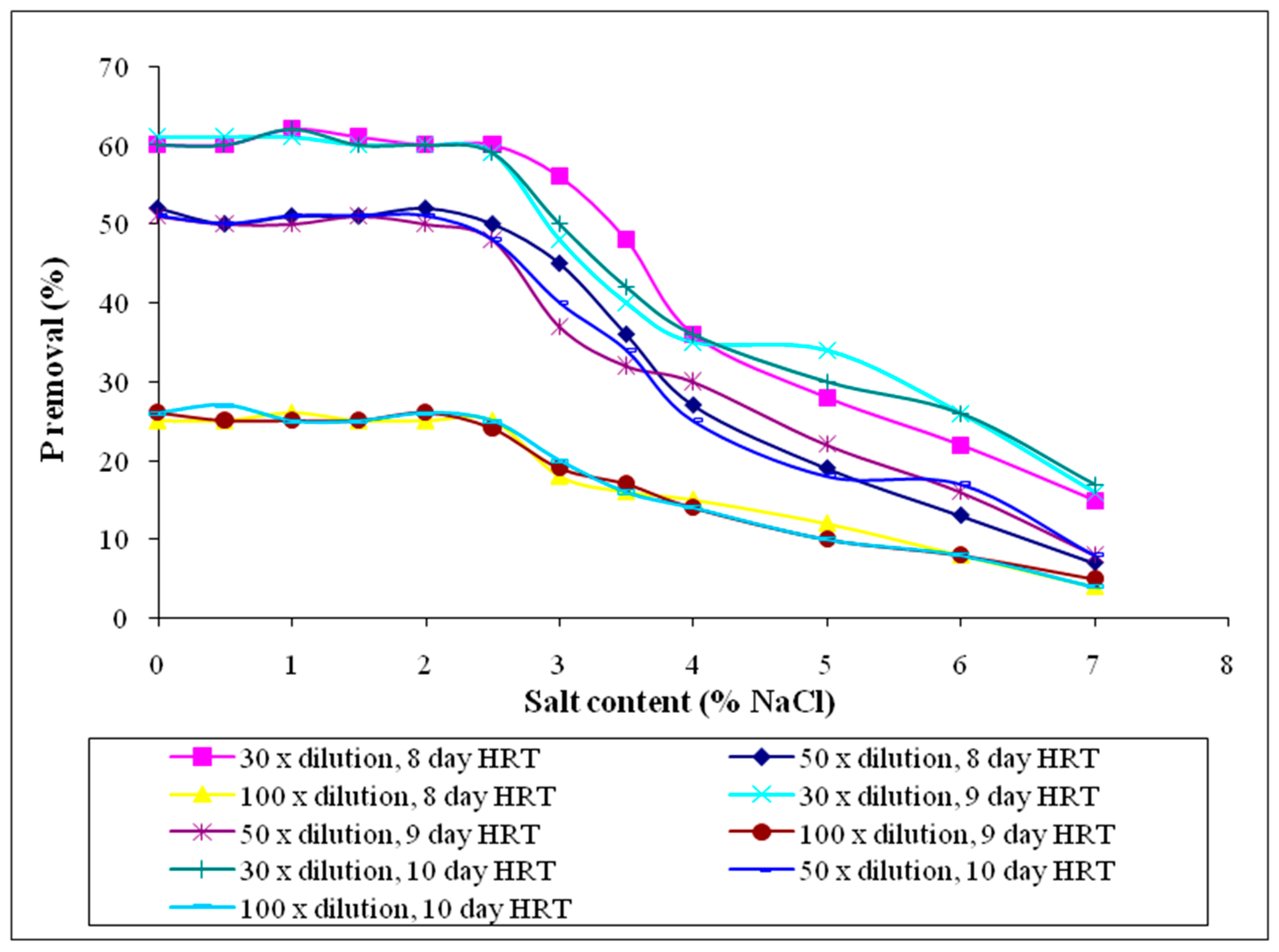
3.2.9. Phosphorus (P)
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Amir, H.M.; Vali, A.; Leila, R. Atmospheric moisture condensation to water recovery by home air conditioners. Am. J. Appl. Sci. 2013, 10, 917–923. [Google Scholar] [CrossRef]
- Abbasi, M.; Dehghani, M.; Moussavi, G.; Azhdarpoor, A. Degradation of organic matter of municipal sewage sludge using ultrasound treatment in Shiraz wastewater treatment plant. Health Scope 2015, 4, e23507. [Google Scholar] [CrossRef]
- Igbinosa, E.O.; Okoh, A.I. Impact of discharge wastewater effluents on the physico-chemical qualities of a receiving watershed in a typical rural community. Int. J. Environ. Sci. Technol. 2009, 6, 175–182. [Google Scholar] [CrossRef]
- Andreas, N.A.; Shane, A.S. Wastewater Treatment and Reuse: Past, Present, and Future. Water 2015, 7, 4887–4895. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. The State of World Fisheries and Aquaculture 2016: Contributing to Food Security and Nutrition for All; FAO: Rome, Italy, 2016; pp. 1–79. [Google Scholar]
- Food and Agriculture Organization of the United Nations. The State of World Fisheries and Aquaculture 2014: Opportunities and Challenges; FAO: Rome, Italy, 2014; pp. 3–69. [Google Scholar]
- Malaysia Investment Development Authority. Food Industry in Malaysia—Ideal Prospects, Immense Opportunities; Food Technology and Sustainable Resources Industries Division: Kuala Lumpur, Malaysia, 2017.
- Department of Statistics Malaysia. Selected Agricultural Indicators, Malaysia, 2016: Production of Agriculture Sector Increased in 2015; The Office of Chief Statistician Malaysia: Putrajaya, Malaysia, 2017.
- Department of Fisheries Malaysia. Status of the Fisheries Sector in Malaysia 2016; Fisheries Information Management Division, Department of Fisheries Malaysia: Putrajaya, Malaysia, 2017.
- Eric, S.L.; Ronald, A.R. Continuous bioprocessing and perfusion: Wider adoption coming as bioprocessing matures. BioProcess. J. 2014, 13, 50–55. [Google Scholar]
- Tay, J.H.; Show, K.Y.; Hung, Y.T. Seafood processing wastewater treatment. In Waste Treatment in the Food Processing Industry; Wang, L.K., Hung, Y.T., Lo, H.H., Yapijakis, C., Eds.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2006; pp. 29–66. [Google Scholar]
- Len, R.G.; Michael, D.P.; Pomeroy, R.S. Fisheries in Southeast Asia: Challenges and opportunities. In Transnational Trends: Middle Eastern and Asian Views; Pandya, A., Laipson, E., Eds.; The Henry L. Stimson Center: Washington, DC, USA, 2008; pp. 171–322. [Google Scholar]
- Sirianuntapiboon, S.; Nimnu, N. Management of water consumption and wastewater of seafood processing industries in Thailand. J. Sci. Technol. 1999, 6, 158–167. [Google Scholar]
- Mitchell, R. Introduction to Environmental Microbiology; Prentice-Hall, Inc.: Englewood Cliffs, NJ, USA, 1974. [Google Scholar]
- Mojiri, A.; Aziz, H.A.; Zaman, N.Q.; Aziz, S.Q.; Zahed, M.A. Powdered ZELIAC augmented sequencing batch reactors (SBR) process for co-treatment of landfill leachate and domestic wastewater. J. Environ. Manag. 2014, 139, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Tay, J.H.; Show, K.Y.; Hung, Y.T. Seafood processing wastewater treatment. In Handbook of Industrial and Hazardous Wastes Treatment, 2nd ed.; Revised and Expanded; Wang, L.K., Hung, Y.T., Lo, H.H., Yapijakis, C., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 2004; pp. 694–737. [Google Scholar]
- Liu, S.X. Food and Agricultural Wastewater Utilization and Treatment, 1st ed.; Blackwell Publishing Professional: Ames, IA, USA, 2007. [Google Scholar]
- Alrumman, S.A.; El-kott, A.F.; Keshk, S.M.A.S. Water Pollution: Source & Treatment. Am. J. Environ. Eng. 2016, 6, 88–98. [Google Scholar]
- Ng, W.J.; Miranda Yap, G.S.; Sivadas, M. Biological treatment of a pharmaceutical wastewater. J. Biol. Waste 1989, 29, 299–311. [Google Scholar] [CrossRef]
- Hall, G.M.; Ahmad, N.H. Surimi and Fish Mince Products. In Fish Processing Technology; Hall, G.M., Ed.; Chapman & Hall: New York, NY, USA, 1992; pp. 72–86. [Google Scholar]
- COWI. Industrial Sector Guide. Cleaner Production Assessment in Fish Processing Industry; UNEP DTIE: Paris, France; Danish Environmental Protection Agency: Copenhagen, Denmark, 1999.
- Mendez, R.; Omil, F.; Soto, M.; Lema, J.M. Pilot plant studies on the anaerobic treatment of different wastewaters from a fish-canning factory. Water Sci. Technol. 1992, 5, 37–44. [Google Scholar]
- Balslev, O.P.; Lynggaard, J.A.; Nickelsen, C. Pilot-scale experiments on anaerobic treatment of wastewater from a fish processing plant. Water Sci. Technol. 1990, 22, 463–474. [Google Scholar]
- Dan, N.P.; Visvanathan, C.; Basu, B. Comparative evaluation of yeast and bacterial treatment of high salinity wastewater based on biokinetic coefficients. Bioresour. Technol. 2003, 87, 51–56. [Google Scholar] [CrossRef]
- Cui, Y.W.; Zhang, H.Y.; Ding, J.R.; Peng, Y.Z. The effects of salinity on nitrification using halophilic nitrifiers in a Sequencing Batch Reactor treating hypersaline wastewater. Sci. Rep. 2016, 6, 24–25. [Google Scholar] [CrossRef] [PubMed]
- Sherly, T.M.V.; Harindranathan, N.; Bright, S.I.S. Physicochemical analysis of seafood processing effluents in Aroor Gramapanchayath, Kerala. IOSR J. Environ. Sci. Toxicol. Food Technol. 2015, 9, 38–44. [Google Scholar]
- Woolard, C.R.; Irvine, R.L. Response of a periodically operated halophilic biofilm reactor to changes in salt content. Water Sci. Technol. 1995, 31, 41–50. [Google Scholar] [CrossRef]
- Stewart, M.J.; Ludwig, H.F.; Kearns, W.H. Effects of varying salinity on extended aeration process. J. Water Pollut. Control Fed. 1962, 37, 1167–1177. [Google Scholar]
- Burnett, W.E. The effect of salinity variations on the activated sludge process. Water Sew. Works 1974, 121, 37–55. [Google Scholar]
- Oren, A.; Gurevich, P.; Malkit, A.; Henis, Y. Microbial degradation of pollutants at high salt concentrations. Biodegradation 1992, 3, 387–398. [Google Scholar] [CrossRef]
- Lefebvre, O.; Moletta, R. Treatment of organic pollution in industrial saline wastewater: A literature review. Water Res. 2006, 40, 3671–3682. [Google Scholar] [CrossRef] [PubMed]
- Hamoda, M.F.; Al-Attar, M.S. Effects of high sodium chloride concentrations on activated sludge treatment. Water Sci. Technol. 1995, 31, 61–72. [Google Scholar] [CrossRef]
- Mostafa, F.H.; Surabhi, S.; Loring, F.N.; James, E.A. Study of Salt Wash Water Toxicity on Wastewater Treatment; Final Report; Purdue University: West Lafayette, IN, USA, 2006. [Google Scholar]
- Joong, K.K.; Jeong, B.K.; Kyoung, S.C.; Yong, K.H. Isolation and identification of microorganisms and their aerobic biodegradation of fish-meal wastewater for liquid-fertilization. Int. Biodeterior. Biodegrad. 2007, 59, 156–165. [Google Scholar]
- Kargi, F.; Uygur, A. Biological treatment of saline wastewater in an aerated percolator unit utilizing halophilic bacteria. Environ. Technol. 1996, 17, 320–325. [Google Scholar] [CrossRef]
- Kargi, F.; Dincer, A.R. Salt inhibition of nitrification and denitrification in saline wastewater. Environ. Technol. 1999, 20, 1147–1153. [Google Scholar]
- Lee, D.; Jean, D. Effects of Salinity on Expression Dewatering of Waste Activated Sludge. J. Colloid Interf. Sci. 1999, 215, 443–445. [Google Scholar]
- Knapp, L.A. Study of Process Control Strategies for Biological Nutrient Removal in an Oxidation Ditch. Master’s Thesis, University of South Florida, Tampa, FL, USA, June 2014. [Google Scholar]
- Moussa, M.S.; Sumanasekera, D.U.; Irahim, S.H.; Lubberding, H.J.; Hooijmans, C.M.; Gijzen, H.J.; Loosdrecht, M.C.M. Long term effects of salt on activity, population structure and floc characteristics in enriched bacterial cultures of nitrifiers. Water Res. 2006, 40, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Bassin, J.P.; Kleerebezem, R.; Muyzer, G.; Rosado, A.S.; Van Loosdrecht, M.C.M.; Dezotti, M. Effect of different salt adaptation strategies on the microbial diversity, activity, and settling of nitrifying sludge in sequencing batch reactors. Appl. Microbiol. Biotechnol. 2012, 93, 1281–1294. [Google Scholar] [CrossRef] [PubMed]
- Panswad, T.; Anan, C. Specific oxygen, ammonia and nitrate uptake rates of a biological nutrient removal process treating elevated salinity wastewater. Bioresour. Technol. 1999, 70, 237–243. [Google Scholar] [CrossRef]
- Metcalf and Eddy. Wastewater Engineering: Treatment, Disposal, and Reuse, 3rd ed.; McGraw-Hill, Inc.: New York, NY, USA, 1991. [Google Scholar]
- Grady, C.P.L.; Daigger, G.T.; Lim, H.C. Biological Wastewater Treatment, 2nd ed.; Marcel Dekker Inc.: New York, NY, USA, 1999. [Google Scholar]
- APHA; AWWA. Standard Methods for Water and Wastewater Examinations, 21st ed.; American Public Health Association (APHA); American Water Works Association (AWWA): Washington, DC, USA, 2005. [Google Scholar]
- APHA; AWWA. Standard Methods for the Examination of Water and Wastewater, 19th ed.; American Public Health Association (APHA); American Water Works Association (AWWA); Water Pollution Control Federation (WPCF): Washington, DC, USA, 1995. [Google Scholar]
- Radojevic, M.; Bashkin, V.N. Practical Environmental Analysis; The Royal Society of Chemistry: Cambridge, UK, 1999. [Google Scholar]
- Mettler Toledo. Delta 320 pH Meter Operation Manual; Mettler-Toledo Instruments (Shanghai) Co. Ltd.: Shanghai, China, 2005. [Google Scholar]
- Middlebrooks, E.J. A nomograph for solution of the BOD equation. Water Sew. Works J. 1965, 112, R230. [Google Scholar]
- HACH. Wastewater and Biosolids Analysis Manual: Digestion and Selected Methods for Determining Metals, Minerals, and Other Related Parameters, 1st ed.; HACH Company: Loveland, CO, USA, 1999. [Google Scholar]
- Ruttanagosrigit, W.; Boyd, C.E.J. Measurement of Chemical Oxygen Demand in Waters of High Chloride Concentration. World Aquac. Soc. 1989, 20, 170–172. [Google Scholar] [CrossRef]
- Wayne, B. The Science of Chemical Oxygen Demand; Technical Information Series, Booklet No. 9; HACH Company: Loveland, CO, USA, 1997. [Google Scholar]
- Cho, K.S.; Cho, K.J.; Park, H.D.; Jeong, S.W.; Nam, S.J.; Lee, T. Characteristics of immobilized PVA beads in nitrate removal. J. Microbiol. Biotechnol. 2006, 16, 414–422. [Google Scholar]
- Kargi, F. Enhanced biological treatment of saline waste-water by using halophilic bacteria. Biotechnol. Lett. 2002, 24, 1569–1572. [Google Scholar] [CrossRef]
- Mueller, D.K.; Spahr, N.E. Water-quality, streamflow, and ancillary data for nutrients in streams and rivers across the nation, 1992–2001. In U.S. Geological Survey Data Series 152; U.S. Geological Survey: Reston, VA, USA, 2005. [Google Scholar]
- HACH. DR/2000 Spectrophotometer Procedures Manual, 2nd ed.; HACH Company: Loveland, CO, USA, 2001. [Google Scholar]
- Campos, J.L.; Mosquera-Corral, A.; Sanchez, M.; Mendez, R.; Lema, J.M. Nitrification in saline wastewater with high ammonia concentration in an activated sludge unit. Water Res. 2002, 36, 2555–2560. [Google Scholar] [CrossRef]
- Rene, E.R.; Kim, S.J.; Park, H.S. Effect of COD/N ratio and salinity on the performance of sequencing batch reactors. Bioresour. Technol. 2008, 99, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.F.; Li, Z.; Wu, P.; Jin, H.Z.; Wang, Z. The performance and phase separated characteristics of an anaerobic baffled reactor treating soybean protein processing wastewater. Bioresour. Technol. 2008, 99, 8027–8033. [Google Scholar] [CrossRef] [PubMed]
- Vallero, M.V.G.; Hulshoff Pol, L.W.; Lettinga, G.; Lens, P.N.L. Effect of NaCl on thermophilic (55 PoPC) methanol degradation in sulfate reducing granular sludge reactors. Water Res. 2003, 37, 2269–2280. [Google Scholar] [CrossRef]
- Woolard, C.R.; Irvine, R.L. Treatment of hypersaline wastewater in the sequencing batch reactor. Water Res. 1995, 29, 1159–1168. [Google Scholar] [CrossRef]
- Panswad, T.; Anan, C. Impact of high chloride wastewater on an anaerobic/anoxic/aerobic process with and without inoculation of chloride acclimated seeds. Water Res. 1999, 33, 1165–1172. [Google Scholar] [CrossRef]
- Sharrer, M.J.; Tal, Y.; Ferrier, D.; Hankins, J.A.; Summerfelt, S.T. Membrane biological reactor treatment of a saline backwash flow from a recirculating aquaculture system. Aquac. Eng. 2007, 36, 159–176. [Google Scholar] [CrossRef]
- Yogalakshmi, K.N.; Joseph, K. Effect of transient sodium chloride shock loads on the performance of submerged membrane bioreactor. Bioresour. Technol. 2010, 101, 7054–7061. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.H.; Wong, M.T.; Okabe, S.; Watanabe, Y. Dynamic response of nitrifying activated sludge batch culture to increased chloride concentration. Water Res. 2003, 37, 3125–3135. [Google Scholar] [CrossRef]
- Münch, E.V.; Lant, P.; Keller, J. Simultaneous nitrification and denitrification in bench scale sequencing batch reactors. Water Res. 1996, 30, 277–284. [Google Scholar] [CrossRef]
- Lyssenko, C.; Wheaton, F. Impact of rapid pulse operating disturbances on ammonia removal by trickling and submerged upflow biofilters for intensive recirculating aquaculture. Aquac. Eng. 2006, 35, 38–50. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, Y.; Liu, S.; Pan, M.; Yang, J.; Chen, S. Denitrification activities and NR2RO production under salt stress with varying COD/N ratios and terminal electron acceptors. Chem. Eng. J. 2013, 215–216, 252–260. [Google Scholar] [CrossRef]
- Uygur, A.; Kargi, F. Salt inhibition on biological nutrient removal from saline wastewater in a sequencing batch reactor. Enzyme Microb. Technol. 2004, 34, 313–318. [Google Scholar] [CrossRef]







| Parameter (mg/L) | Fish Processing Wastewater Concentration | |||
|---|---|---|---|---|
| Simulated Original | 30-Fold Dilution | 50-Fold Dilution | 100-Fold Dilution | |
| TSS | 5530 | 184 | 110 | 55 |
| TDS | 2590 | 86 | 52 | 26 |
| TS | 22,350 | 750 | 450 | 225 |
| VSS | 905 | 30 | 18 | 9 |
| pH | 6.65 | 7.36 | 7.38 | 7.40 |
| BOD | 18,419 | 614 | 368 | 184 |
| COD | 30,000 | 1000 | 600 | 300 |
| NH3-N | 504 | 17 | 10 | 5 |
| NO3−-N | 51 | 1.7 | 1.0 | 0.5 |
| P | 95.5 | 3.2 | 1.91 | 0.96 |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ching, Y.C.; Redzwan, G. Biological Treatment of Fish Processing Saline Wastewater for Reuse as Liquid Fertilizer. Sustainability 2017, 9, 1062. https://doi.org/10.3390/su9071062
Ching YC, Redzwan G. Biological Treatment of Fish Processing Saline Wastewater for Reuse as Liquid Fertilizer. Sustainability. 2017; 9(7):1062. https://doi.org/10.3390/su9071062
Chicago/Turabian StyleChing, Yun Chen, and Ghufran Redzwan. 2017. "Biological Treatment of Fish Processing Saline Wastewater for Reuse as Liquid Fertilizer" Sustainability 9, no. 7: 1062. https://doi.org/10.3390/su9071062




