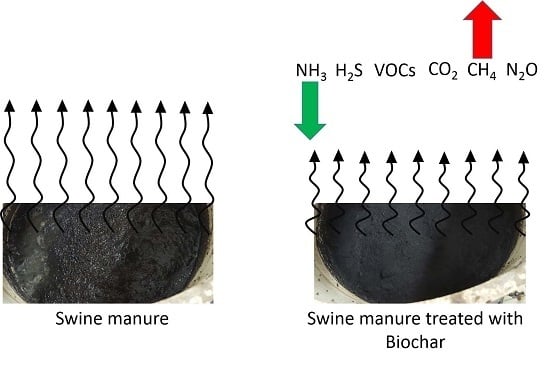
Pilot-Scale Testing of Non-Activated Biochar for Swine Manure Treatment and Mitigation of Ammonia, Hydrogen Sulfide, Odorous Volatile Organic Compounds (VOCs), and Greenhouse Gas Emissions
Abstract
:1. Introduction
- Can biochar float on top of swine manure for at least 1-month period?
- Can a thin layer of biochar be effective at mitigating gaseous emissions?
- Can biochar mitigate emissions of any key gases of concern (odorous VOCs such as sulfur-containing VOCs, volatile fatty acids, phenolics, and indolics), or NH3, H2S and greenhouse gases (CH4, N2O, and CO2)?
- Can non-activated, non-functionalized biochar be an economically-feasible option for controlling gaseous emissions from stored manure?
2. Materials and Methods
2.1. Experimental Design
2.2. Manure Storage Simulators
2.3. Gas Concentration Measurements
2.4. Swine Manure Analysis
2.5. Estimation of Time and Dose Effect on Gaseous Emissions
2.6. Statistical Analysis
3. Results and Discussion
3.1. Visual Observations of the Biochar Surface Layer Over Time
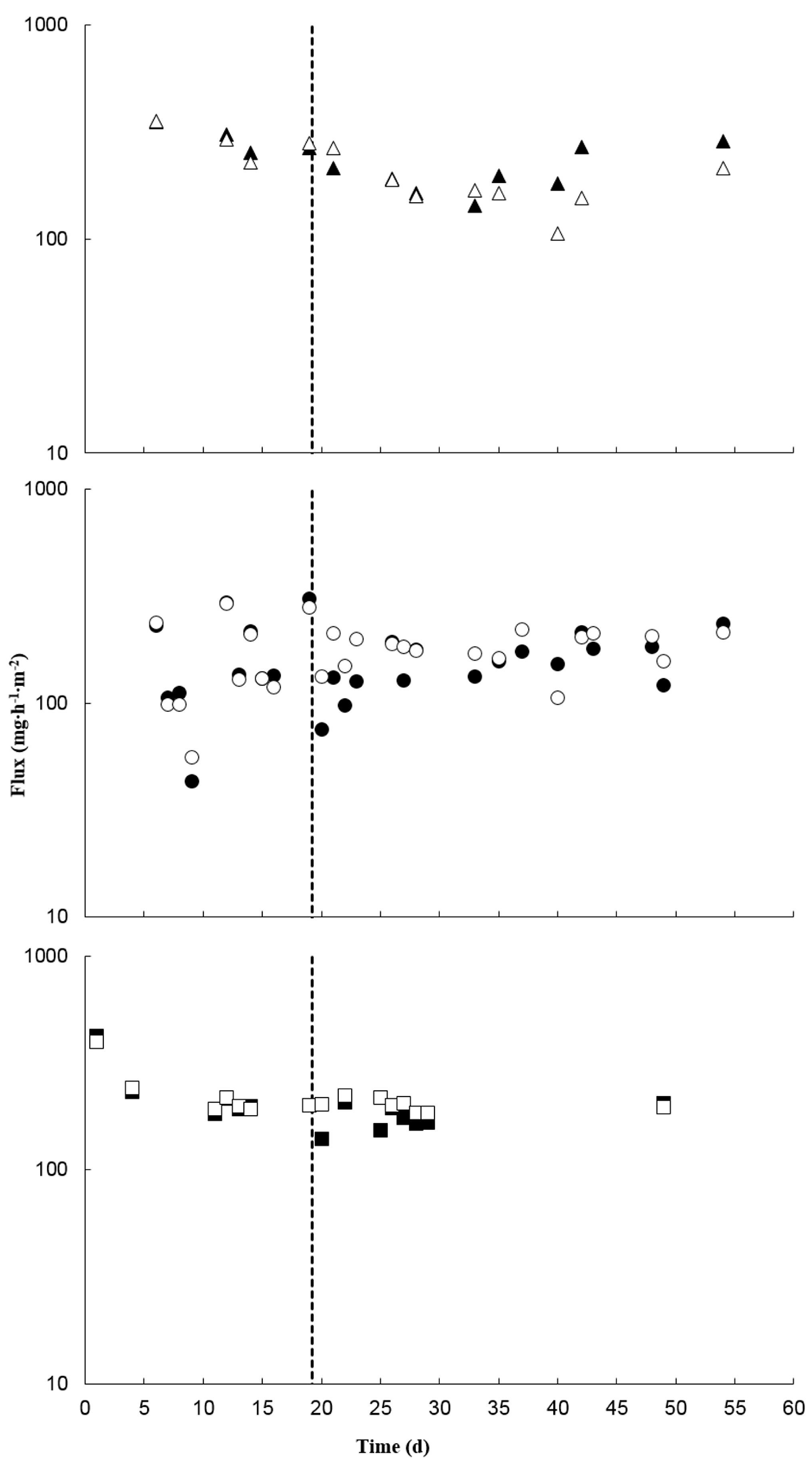
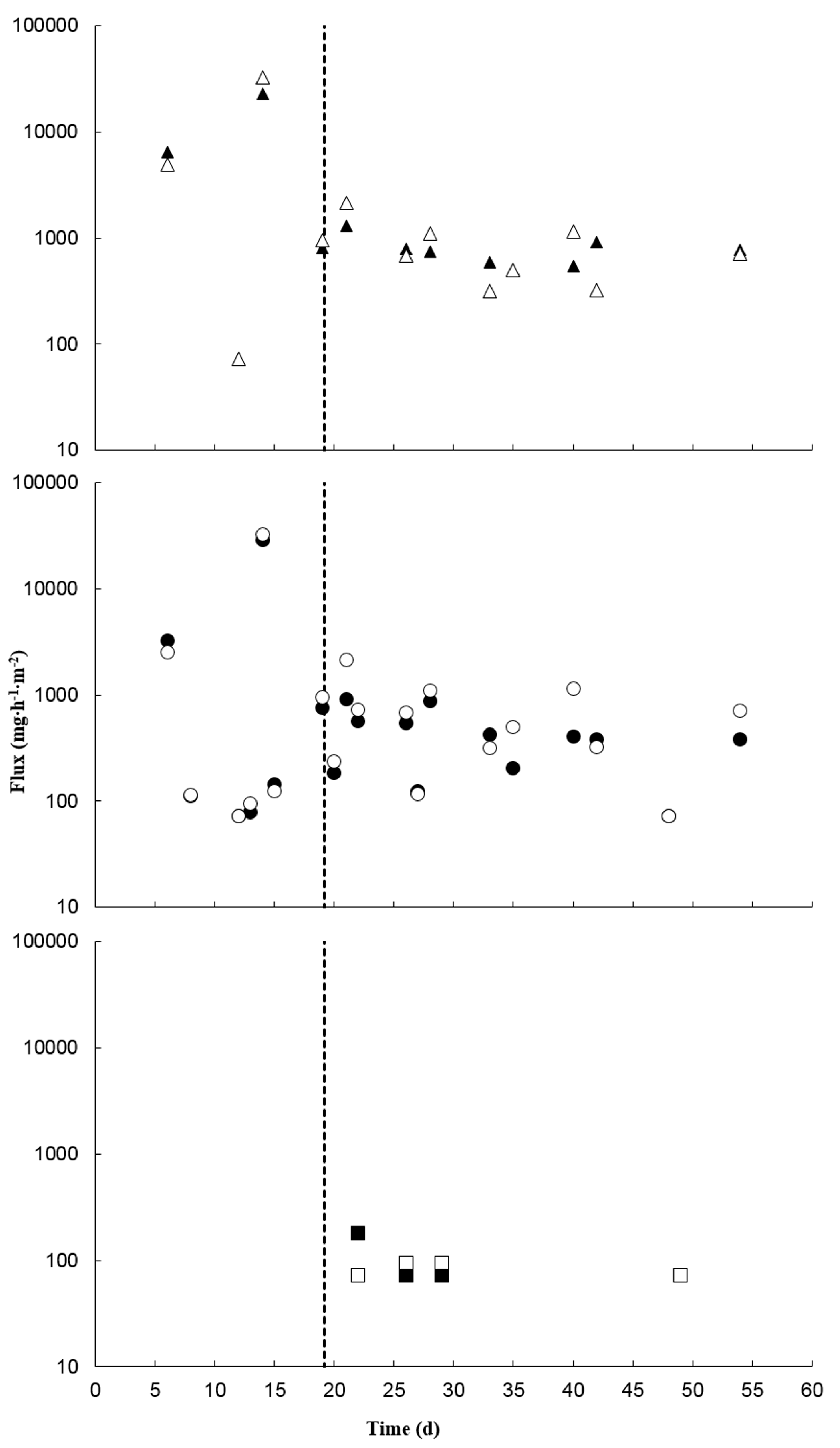
3.2. Treatment Effects on Ammonia and Hydrogen Sulfide
3.3. Treatment Effects on Volatile Organic Compounds
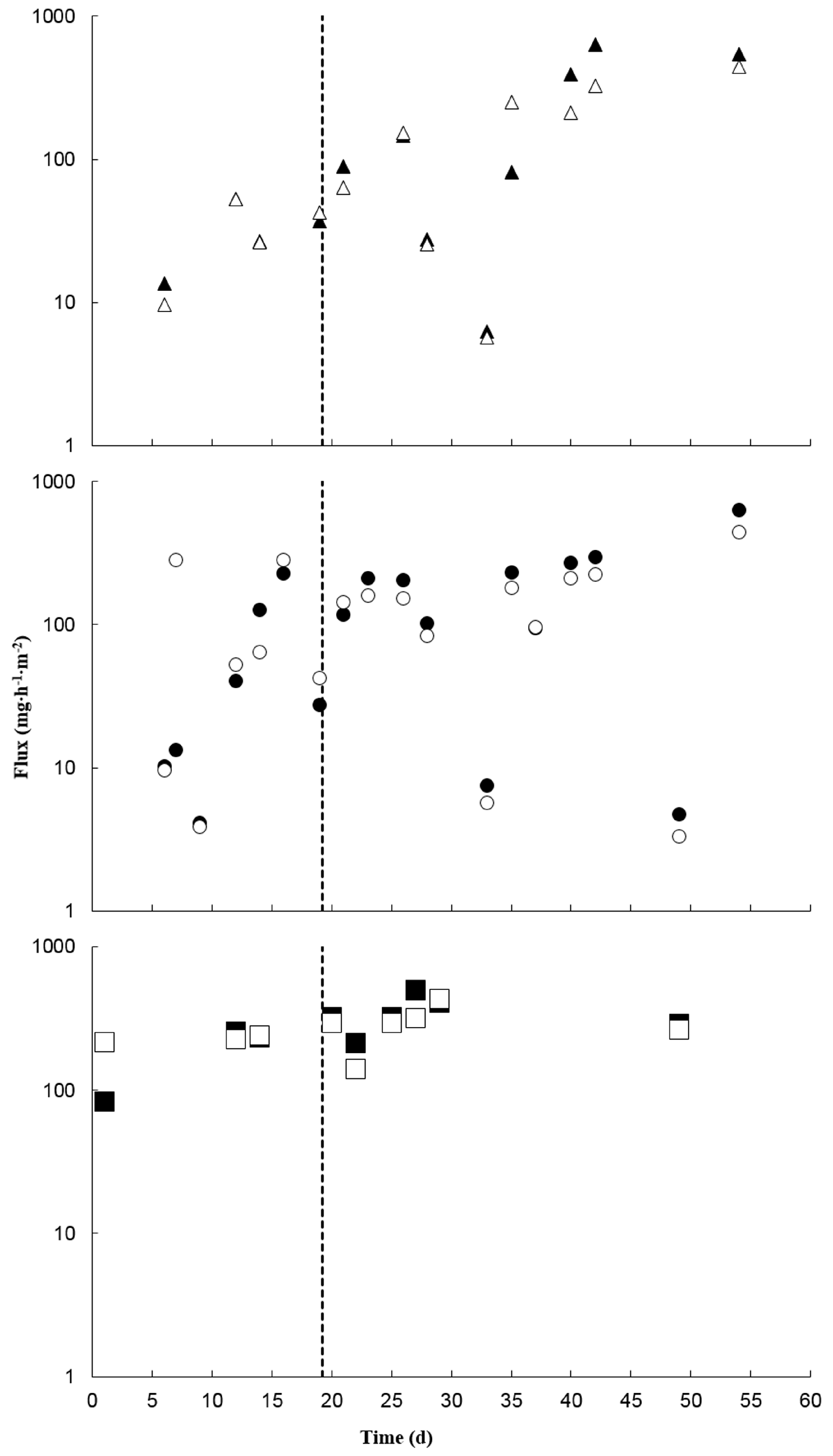
3.4. Treatment Effects on Greenhouse Gases
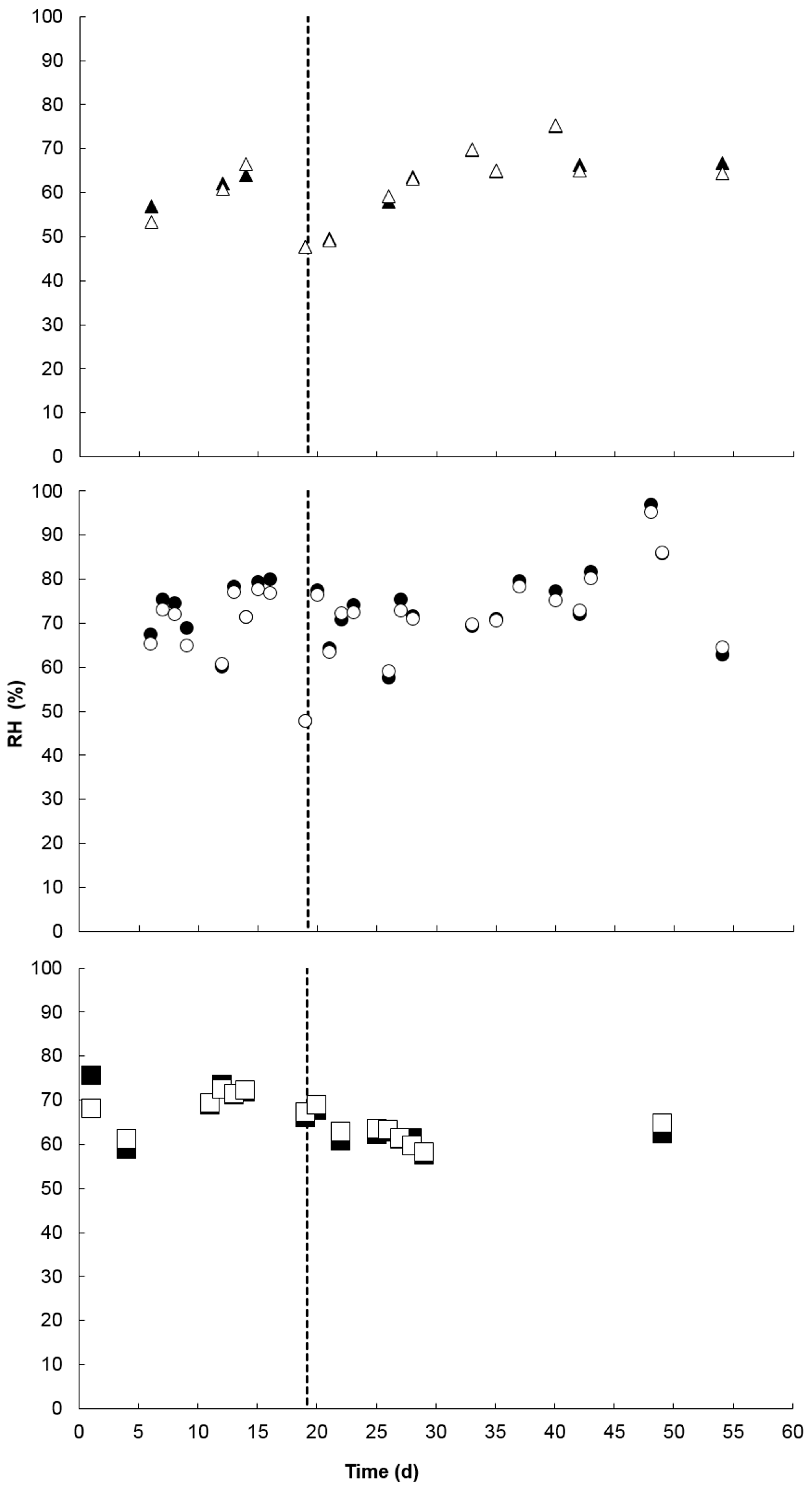
3.5. Treatment Effects on Environmental and Physicochemical Parameters
3.6. Manure Analysis
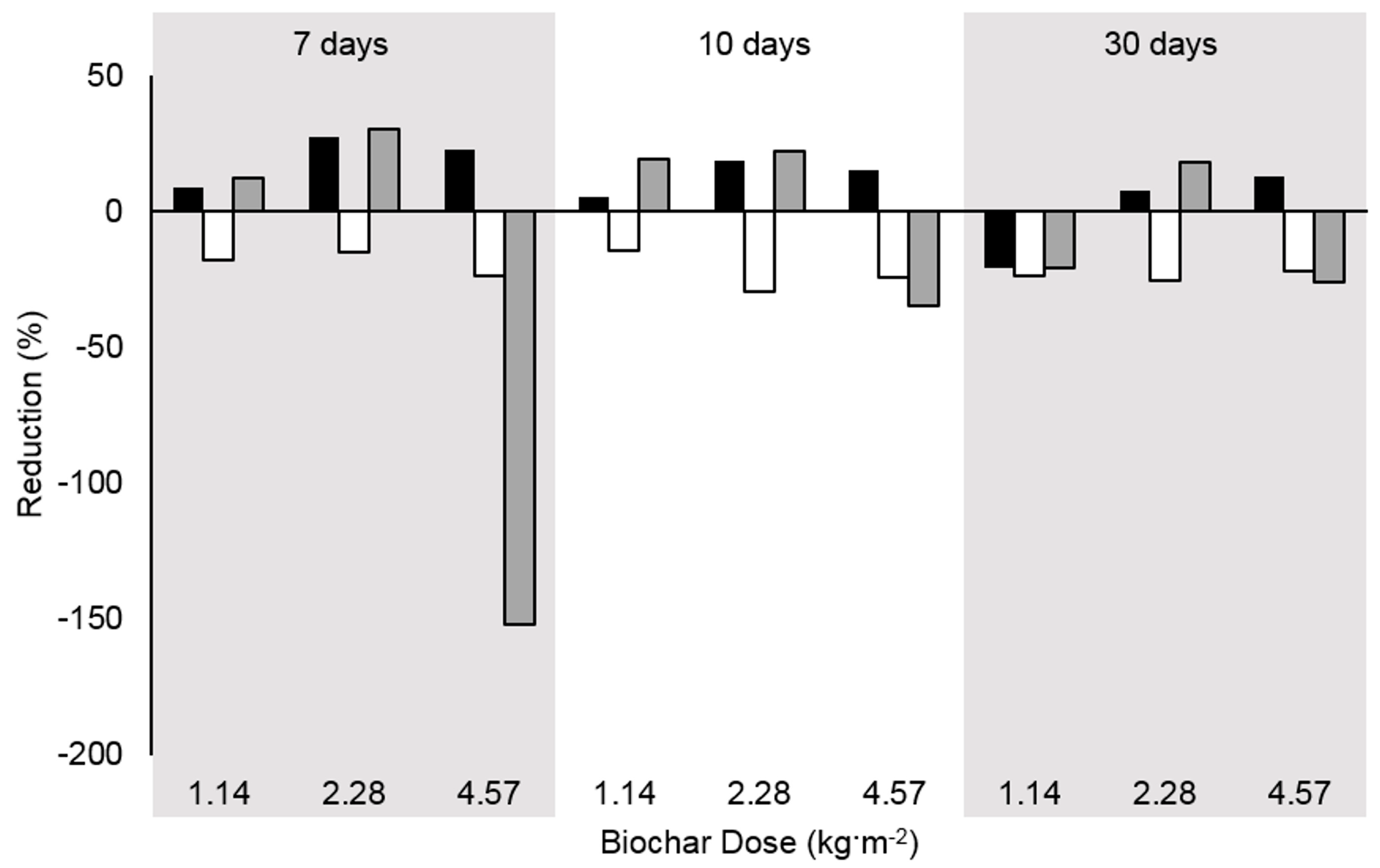
3.7. Effects of Biochar Dose and Time
3.8. Economics of Biochar Treatment
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- The United States Environmental Protection Agency. Sources of Greenhouse Gas Emissions. 2014. Available online: https://www.epa.gov/ghgemissions/sources-greenhouse-gas-emissions (accessed on 28 May 2017).
- Iowa State University Extension and Outreach. Air Management Practices Assessment Tool Website. 2016. Available online: http://www.agronext.iastate.edu/ampat/homepage.html (accessed on 28 May 2017).
- Maurer, D.L.; Koziel, J.A.; Harmon, J.D.; Hoff, S.J.; Rieck-Hinz, A.M.; Andersen, D.S. Summary of performance data for technologies to control gaseous, odor, and particulate emissions from livestock operations: Air management practices assessment tool (AMPAT). Data Brief 2016, 7, 1413–1429. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.; Sarswat, A.; Ok, Y.S.; Pittman, C.U., Jr. Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and sustainable absorbent—A critical review. Bioresour. Technol. 2014, 160, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Gai, X.; Wang, H.; Liu, J.; Zhai, L.; Liu, S.; Ren, T.; Liu, H. Effects of feedstock and pyrolysis temperature on biochar adsorption of ammonium and nitrate. PLoS ONE 2014, 9, e113888. [Google Scholar] [CrossRef] [PubMed]
- Ro, K.; Lima, I.M.; Reddy, G.B.; Jackson, M.A.; Gao, B. Removing gaseous NH3 using biochar as an adsorbent. Agriculture 2015, 5, 991–1002. [Google Scholar] [CrossRef]
- Jindo, K.; Suto, K.; Matsumoto, K.; Garcia, C.; Sonoki, T.; Sanchez-Monedero, M.A. Chemical and biochemical characterisation of biochar-blended composts prepared from poultry manure. Bioresour. Technol. 2012, 110, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Czekala, W.; Malinska, K.; Caceres, R.; Janczak, D.; Dach, J.; Lewicki, A. Co-composting of poultry manure mixtures amended with biochar—The effect of biochar on temperature and C-CO2 emissions. Bioresour. Technol. 2016, 200, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, G.; Sun, H.; Zhou, S.; Zou, G. Straw biochar hastens organic matter degradation and produces nutrient-rich compost. Bioresour. Technol. 2016, 200, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Steiner, C.; Das, K.C.; Melear, N.; Lakly, D. Reducing nitrogen loss during poultry litter composting using biochar. J. Environ. Qual. 2010, 39, 1236–1246. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Yuan, W.; Ju, X. Short report: Effects of biochar addition on manure composting and associated N2O emissions. J. Sustain. Bioenergy Syst. 2015, 5, 56–61. [Google Scholar] [CrossRef]
- Dias, B.O.; Silva, C.A.; Higashikawa, F.S.; Roig, A.; Sanchez-Monedero, M.A. Use of biochar as bulking agent for the composting of poultry manure: Effect on organic matter degradation and humification. Bioresour. Technol. 2010, 101, 1239–1246. [Google Scholar] [CrossRef] [PubMed]
- Jindo, K.; Sanchez-Monedero, M.A.; Hernandez, T.; Garcia, C.; Furukawa, T.; Matsumoto, K.; Sonoki, T.; Bastida, F. Biochar influences the microbial community structure during manure composting with agricultural wastes. Sci. Total Environ. 2012, 416, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Thangarajan, R.; Bolan, N.S.; Sarkar, B.; Khan, N.; Ok, Y.S.; Naidu, R. Biochar-induced concomitant decrease in ammonia volatilization and increase in nitrogen use efficiency by wheat. Chemosphere 2016, 142, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Komnitsas, K.A.; Zaharaki, D. Morphology of modified biochar and its potential for phenol removal from aqueous solutions. Front. Environ. Sci. 2016, 4, 1–11. [Google Scholar] [CrossRef]
- Rogovska, N.; Laird, D.; Cruse, R.; Fleming, P.; Parkin, T.; Meek, D. Impact of biochar on manure carbon stabilization and greenhouse gas emissions. Soil Biol. Biochem. 2011, 75, 871–879. [Google Scholar] [CrossRef]
- Troy, S.M.; Lawlor, P.G.; O’Flynn, C.J.; Healy, M.G. Impact of biochar addition to soil on greenhouse gas emissions following pig manure application. Soil Biol. Biochem. 2013, 60, 173–181. [Google Scholar] [CrossRef]
- Wang, C.; Lu, H.; Dong, D.; Deng, H.; Strong, P.J.; Wang, H.; Wu, W. Insight into the effects of biochar on manure composting: Evidence supporting the relationship between N2O emissions and denitrifying community. Environ. Sci. Technol. 2013, 47, 7341–7349. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.A.; de Neergaard, A.; Jensen, L.S. Potential of aeration flow rate and bio-char addition to reduce greenhouse gas and ammonia emissions during manure composting. Chemosphere 2014, 97, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Kim, H.; Yoo, G. Effects of biochar addition on CO2 and N2O emissions following fertilizer application to a cultivated grassland soil. PLoS ONE 2015, 10, e0126841. [Google Scholar] [CrossRef] [PubMed]
- Brennan, R.B.; Healy, M.G.; Fenton, O.; Lanigan, G.J. The effect of chemical amendments used for phosphorus abatement on greenhouse gas and ammonia emissions from dairy cattle slurry: Synergies and pollution swapping. PLoS ONE 2015, 10, e0111965. [Google Scholar] [CrossRef] [PubMed]
- Maurer, D.L.; Koziel, J.A.; Bruning, K.; Parker, D.B. Pilot-scale testing of renewable biocatalyst for swine manure treatment and mitigation of odorous VOCs, ammonia and hydrogen sulfide emissions. Atmos. Environ. 2017, 150, 313–321. [Google Scholar] [CrossRef]
- MidWest Plan Service (MWPS). Structures and Environment Handbook, MWPS-1, 11th ed.; Iowa State University: Ames, IA, USA, 1983; ISBN 0-89373-057-2. [Google Scholar]
- Da Silva Batista, A.P.; Van Weelden, M.; Andersen, D.S. Impact of temperature and mixing on methane production rates of swine manures obtained from deep pit storages. ASABE Paper 131619616. In Proceedings of the ASABE Annual International Meeting, Kansas City, MO, USA, 21–24 July 2013. [Google Scholar]
- Zhang, S.; Cai, L.; Koziel, J.A.; Hoff, S.J.; Schmidt, D.R.; Clanton, C.J.; Jacobson, L.D.; Parker, D.B.; Heber, A.J. Field air sampling and simultaneous chemical and sensory analysis of livestock odorants with sorbent tubes and GC-MS/olfactometry. Sens. Actuators B 2010, 146, 427–432. [Google Scholar] [CrossRef]
- Andersen, K.B.; Hansen, M.J.; Feilberg, A. Minimisation of artefact formation of dimethyl disulphide during sampling and analysis of methanethiol in air using solid sorbent materials. J. Chromatogr. A 2012, 1245, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Koziel, J.A.; Zhang, S.; Heber, A.J.; Cortus, E.L.; Parker, D.B.; Hoff, S.J.; Sun, G.; Heathcote, K.Y.; Jacobson, L.D.; et al. Odor and odorous chemical emissions from animal buildings: Part 3—Chemical emissions. Trans. ASABE 2015, 58, 1349–1359. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists. Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists International: Rockville, MD, USA, 2000. [Google Scholar]
- American Public Health Association. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA, 1998. [Google Scholar]
- Sethupathi, S.; Zhang, M.; Rajapaksha, A.U.; Lee, S.R.; Nor, N.M.; Mohamed, A.R.; Al-Wabel, M.; Lee, S.S.; Ok, Y.S. Biochars as potential adsorbers of CH4, CO2 and H2S. Sustainability 2017, 9, 121. [Google Scholar] [CrossRef]
- Wang, Q.; Fang, W.; Yan, D.; Han, D.; Li, Y.; Ouyang, C.; Guo, M.; Cao, A. The effects of biochar amendment on dimethyl disulfide emission and efficiency against soil-borne pests. Water Air Soil Pollut. 2016, 227, 98. [Google Scholar] [CrossRef]
- Shang, J.; He, W.; Fan, C. Adsorption of dimethyl trisulfide from aqueous solution on a low-cost adsorbent: Thermally activated pinecone. Chin. J. Oceanol. Limnol. 2015, 33, 169–175. [Google Scholar] [CrossRef]
- Das, L.; Kolar, P.; Classen, J.J.; Osborne, J.A. Adsorbents from pine wood via K2CO3-assisted low temperature carbonization for adsorption of p-cresol. Ind. Crops Prod. 2013, 45, 215–222. [Google Scholar] [CrossRef]
- Fitzgerald, S.; Kolar, P.; Classen, J.; Boyette, M.; Das, L. Swine manure char as an adsorbent for mitigation of p-cresol. Environ. Prog. Sustain. Energy 2015, 34, 125–131. [Google Scholar] [CrossRef]
- Yang, K.; Yang, J.; Jiang, Y.; Wu, W.; Lin, D. Correlations and adsorption mechanisms of aromatic compounds on a high heat temperature treated bamboo biochar. Environ. Pollut. 2016, 210, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Tang, J.; Zhang, R.; Wu, Q.; Gong, M. Effects of biochar application on soil methane emission at different soil moisture levels. Biol. Fertil. Soils 2013, 49, 119–128. [Google Scholar] [CrossRef]
- Clough, T.J.; Condron, L.M.; Kammann, C.; Muller, C. A review of biochar and soil nitrogen dynamics. Agronomy 2013, 3, 275–293. [Google Scholar] [CrossRef]
- Jirka, S.; Tomlinson, T. 2013 State of the Biochar Industry: A Survey of Commercial Activity in the Biochar Field. A Report by the International Biochar Initiative. 2014. Available online: http://www.biochar-international.org/sites/default/files/State_of_the_Biochar_Industry_2013.pdf (accessed on 28 May 2017).
- Balsari, P.; Dinuccio, E.; Gioelli, F. A low cost solution for ammonia emission abatement from slurry storage. Int. Congr. Ser. 2006, 1293, 323–326. [Google Scholar] [CrossRef]
- Heber, A.J.; Ni, J.Q.; Lim, T.T.; Diehl, C.A.; Sutton, A.L.; Duggirala, R.K.; Haymore, B.L.; Kelly, D.T.; Adamchuk, V.I. Effect of a manure additive on ammonia emission from swine finishing buildings. Trans. ASAE 2000, 43, 1895–1902. [Google Scholar] [CrossRef]
- Heber, A.J.; Ni, J.; Sutton, A.L.; Patterson, J.A.; Fakhoury, K.; Kelly, D.; Shao, P. Laboratory Testing of Commercial Manure Additives for Swine Odor Control; Final Report; USDA-ARS-National Swine Research and Information Center: Ames, IA, USA, 2001. [Google Scholar]
- Moreno, L.; Predicala, B.; Nemati, M. Laboratory, semi-pilot and room scale study of nitrite and molybdate mediated control of H2S emission from swine manure. Bioresour. Technol. 2010, 101, 2141–2151. [Google Scholar] [CrossRef] [PubMed]
- US Inflation Calculator. 2015. Available online: http://www.usinflationcalculator.com/ (accessed on 28 May 2017).



| Reference | Scale and Duration (d) | Treated Source | Gaseous Emissions Reduction (%) | |||||
|---|---|---|---|---|---|---|---|---|
| NH3 | H2S | CH4 | CO2 | N2O | Phenol | |||
| Jindo, 2012 [7] | field, 150 | poultry manure composting | NA | NA | NA | NA | NA | NA |
| Czekala, 2015 [8] | laboratory, 45 | poultry manure composting | NA | NA | NA | −7 | NA | NA |
| Zhang, 2015 [9] | laboratory, 105 | swine manure composting | NA | NA | NA | NA | NA | NA |
| Steiner, 2010 [10] | pilot, 42 | poultry litter composting | 47–55 | 58–71 | NA | NS | NA | NA |
| Jia, 2015 [11] | review | manure composting | reduced | NA | NA | NA | reduced | NA |
| Dias, 2010 [12] | field, 210 | poultry manure composting | NA | NA | NA | NA | NA | NA |
| Jindo, 2012 [13] | field, 85 | cattle or poultry manure composting | NA | NA | NA | NA | NA | NA |
| Mandal, 2016 [14] | laboratory, 30 | poultry manure in soil | 41–77 | NA | NA | NA | NA | NA |
| Komnitsas, 2016 [15] | laboratory, 1 | wastewater | NA | NA | NA | NA | NA | 10-51 |
| Rogovska, 2011 [16] | laboratory, 500 | swine manure in soil | NA | NA | NA | 33–78 | NS | NA |
| Troy, 2013 [17] | laboratory, 28 | swine manure in soil | NA | NA | NS | −45-NS | −79–68 | NA |
| Wang, 2013 [18] | pilot, 82 | swine manure composting | NA | NA | NA | NA | 26 | NA |
| Chowdhury, 2014 [19] | laboratory, 31 | poultry manure composting | 35–43 | NA | NS | NS | NS | NA |
| Chen, 2015 [20] | field, 548 | compost and urea in soil | NA | NA | NA | NS | −49-NS | NA |
| Brennan, 2015 [21] | laboratory, 15 | cattle slurry in soil | 77 | NA | NS | 84 | 63 | NA |
| Biochar Dose (kg·m2) | Manure Type | Manure Additions During Trial | Trial Length (d) | Replications | |
|---|---|---|---|---|---|
| Trial 1 | 2.28 | crusted | no | 30 | n = 2 treated; n = 1 control |
| Trial 1 | 2.28 | uncrusted | no | 30 | n = 2 treated; n = 1 control |
| Trial 2 | 4.56 | crusted | yes 2x | 30 | n = 3 |
| Trial 3 | 1.14 | fresh and crusted | yes 4x | 35 | n = 3 |
| Trial 3 | 2.28 | fresh and crusted | yes 4x | 35 | n = 3 |
| Manure Type | TS | VS | Volatility * | COD | pH | NH4-N | TKN | PO4-P | TP | |
|---|---|---|---|---|---|---|---|---|---|---|
| (%) | (%) | (%) | (mg·L−1) | (mg·L−1) | (mg·L−1) | (mg·L−1) | (mg·L−1) | |||
| (1) Deep Pit | Control | 8.7 | 6.4 | 73.6 | 40,400 | 7.9 | 8300 | 10,400 | 173 | 400 |
| Treatment | 8.5 | 6.3 | 74.1 | 37,000 | 8 | 8500 | 10,600 | 187 | 360 | |
| (2) Outdoor Lagoon | Control | 4.4 | 3.5 | 79.5 | 26,800 | 8.2 | 7600 | 8450 | 241 | 294 |
| Treatment | 4.6 | 3.7 | 80.4 | 26,000 | 8.2 | 7600 | 8650 | 259 | 326 | |
| (3) Deep Pit | Control | 7.9 | 5.9 | 74.7 | 37,000 | 7.8 | 7700 | 9700 | 150 | 353 |
| Treatment | 7.7 | 5.7 | 74 | 37,800 | 7.8 | 7900 | 9900 | 130 | 387 |
| Reference | Additive | Additive Cost (USD) | ||
|---|---|---|---|---|
| Marketed Pig−1 | m−2·Year−1 | Pig Space−1·Year−1 | ||
| This Study | 1.14 kg·m−2 Biochar | 0.08–4.29 | 0.29–15.92 | 0.21–11.84 |
| This Study | 2.28 kg·m−2 Biochar | 0.15–8.57 | 0.57–31.84 | 0.42–23.67 |
| This Study | 4.56 kg·m−2 Biochar | 0.30–17.14 | 1.14–63.68 | 0.84–47.34 |
| Maurer et al. [22] | 2.28 kg·m−2 SBP/CaO2 | 1.45 a | 2.96 | 2.19 b |
| Maurer et al. [22] | 4.57 kg·m−2 SBP/CaO2 | 2.90 a | 5.93 | 4.39 b |
| Maurer et al. [22] | 22.8 kg·m−2 SBP/CaO2 | 14.53 a | 29.62 | 21.95 b |
| Maurer et al. [22] | 45.7 kg·m−2 SBP/CaO2 | 29.06 a | 59.33 | 43.90 b |
| Heber et al. [40] | Alliance | 0.69 | 2.55 b | 1.89 |
| Moreno et al. [42] | Sodium Molybdate | 0.45 c | 1.98 b | 1.46 d |
| Balsari et al. [39] | Leca Balls | 0.01 e | 0.06 f | 0.05 b |
| Heber et al. [41] g | AgriKlenz Plus | 0.57 | 2.14 | 1.59 |
| Heber et al. [41] g | Alken Clear-Flo | 18.18 | 67.51 | 50.18 |
| Heber et al. [41] g | AWL-80 | 0.41 | 1.52 | 1.13 |
| Heber et al. [41] g | Biocharge Dry | 0.94 | 3.48 | 2.59 |
| Heber et al. [41] g | BMT | 14.87 | 55.24 | 41.05 |
| Heber et al. [41] g | EM Waste Treatment | 8.27 | 30.72 | 22.84 |
| Heber et al. [41] g | GT-2000OC BC-2000AF | 12.15 | 45.12 | 33.53 |
| Heber et al. [41] g | Inhibodor | 1.78 | 6.61 | 4.91 |
| Heber et al. [41] g | Krystal Air | 1.11 | 4.13 | 3.07 |
| Heber et al. [41] g | Manure Management Plus | 2.95 | 10.94 | 8.13 |
| Heber et al. [41] g | Peroxy Odor Control | 0.16 | 0.59 | 0.44 |
| Heber et al. [41] g | SMOC | 7.96 | 29.56 | 21.97 |
| Heber et al. [41] g | ZymPlex | 0.82 | 3.05 | 2.27 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maurer, D.L.; Koziel, J.A.; Kalus, K.; Andersen, D.S.; Opalinski, S. Pilot-Scale Testing of Non-Activated Biochar for Swine Manure Treatment and Mitigation of Ammonia, Hydrogen Sulfide, Odorous Volatile Organic Compounds (VOCs), and Greenhouse Gas Emissions. Sustainability 2017, 9, 929. https://doi.org/10.3390/su9060929
Maurer DL, Koziel JA, Kalus K, Andersen DS, Opalinski S. Pilot-Scale Testing of Non-Activated Biochar for Swine Manure Treatment and Mitigation of Ammonia, Hydrogen Sulfide, Odorous Volatile Organic Compounds (VOCs), and Greenhouse Gas Emissions. Sustainability. 2017; 9(6):929. https://doi.org/10.3390/su9060929
Chicago/Turabian StyleMaurer, Devin L., Jacek A. Koziel, Kajetan Kalus, Daniel S. Andersen, and Sebastian Opalinski. 2017. "Pilot-Scale Testing of Non-Activated Biochar for Swine Manure Treatment and Mitigation of Ammonia, Hydrogen Sulfide, Odorous Volatile Organic Compounds (VOCs), and Greenhouse Gas Emissions" Sustainability 9, no. 6: 929. https://doi.org/10.3390/su9060929