Sustainability of Constructed Wetland under the Impact of Aquatic Organisms Overloading
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Location and Sample Collection
2.2. Water Quality Analysis
2.3. Pathogen Analysis
2.4. PCR-DGGE Analysis of Microbial Communities
2.5. Statistical Analysis of Water Quality and Pathogens
2.6. Statistical Analyses of DGGE Patterns
3. Results and Discussion
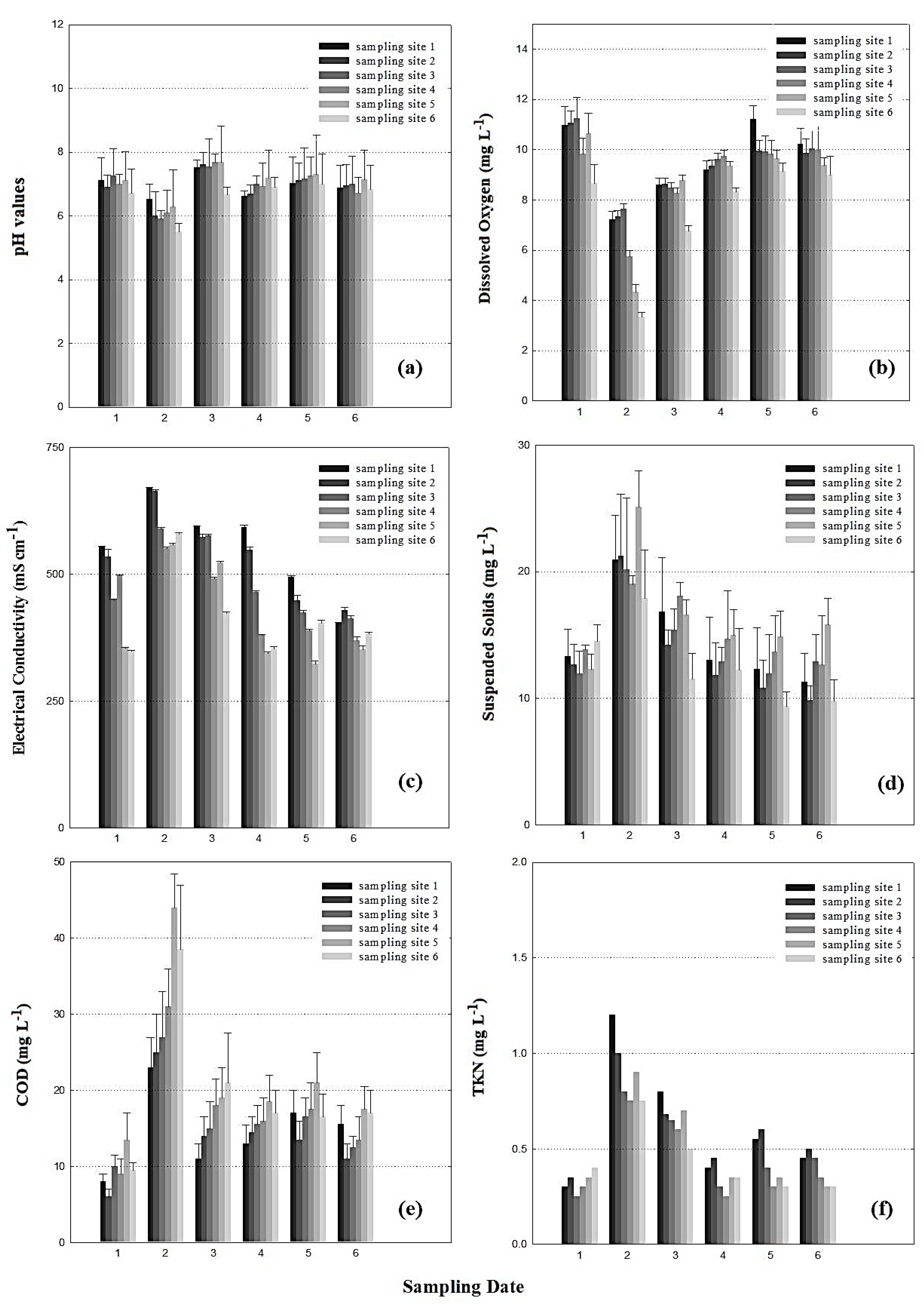
3.1. Water Quality
3.2. Pathogen Amounts and Distribution
3.3. Microbial Community Structure Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wu, H.; Zhang, J.; Ngo, H.H.; Guo, W.; Hu, Z.; Liang, S.; Fan, J.; Liu, H. A review on the sustainability of constructed wetlands for wastewater treatment: Design and operation. Bioresour. Technol. 2015, 175, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Saeed, T.; Sun, G. A review on nitrogen and organics removal mechanisms in subsurface flow constructed wetlands: Dependency on environmental parameters, operating conditions and supporting media. J. Environ. Manag. 2012, 112, 429–448. [Google Scholar] [CrossRef] [PubMed]
- Avelar, F.F.; Matos, A.T.; Matos, M.P.; Borges, A.C. Coliform bacteria removal from sewage in constructed wetlands planted with Mentha aquatica. Environ. Technol. 2014, 35, 2095–2103. [Google Scholar] [CrossRef] [PubMed]
- Badhe, N.; Saha, S.; Biswas, R.; Nandy, T. Role of algal biofilm in improving the performance of free surface, up-flow constructed wetland. Bioresour. Technol. 2014, 169, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.J.; Zheng, D.; Deng, L.W.; Wen, Q.; Liu, Y. Comparison of constructed wetland and stabilization pond for the treatment of digested effluent of swine wastewater. Environ. Technol. 2014, 35, 2660–2669. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Zhang, F.; Yao, S.; Liu, Y.; Chen, J. Application of Pseudomonas flava WD-3 for sewage treatment in constructed wetland in winter. Environ. Technol. 2015, 36, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wallace, S.; Brix, H.; Kuschk, P.; Kirui, W.K.; Masi, F.; Dong, R. Treatment of industrial effluents in constructed wetlands: Challenges, operational strategies and overall performance. Environ. Pollut. 2015, 201, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, H.; Ye, C.; Chen, X.; Xie, B.; Huang, C.; Zhang, J.; Xu, M. Effects of plant species on soil microbial processes and CH4 emission from constructed wetlands. Environ. Pollut. 2013, 174, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Mackintosh, T.J.; Davis, J.A.; Thompson, R.M. Impacts of multiple stressors on ecosystem function: Leaf decomposition in constructed urban wetlands. Environ. Pollut. 2016, 208, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Nicomrat, D.; Dick, W.A.; Tuovinen, O.H. Assessment of the microbial community in a constructed wetland that receives acid coal mine drainage. Microbial. Ecol. 2006, 51, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; Kelley, T.R. Characterization of microbial communities in a pilot-scale constructed wetland using PLFA and PCR-DGGE analyses. J. Environ. Sci. Health Part A 2007, 42, 1639–1647. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.P.; Gehder, M.; Legge, R.L. Assessment of changes in the microbial community of constructed wetland mesocosms in response to acid mine drainage exposure. Water Res. 2008, 42, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Calheiros, C.S.; Duque, A.F.; Moura, A.; Henriques, I.S.; Correia, A.; Rangel, A.O.; Castro, P.M. Substrate effect on bacterial communities from constructed wetlands planted with Typha latifolia treating industrial wastewater. Ecol. Eng. 2009, 35, 744–753. [Google Scholar] [CrossRef]
- Truu, M.; Juhanson, J.; Truu, J. Microbial biomass, activity and community composition in constructed wetlands. Sci. Total Environ. 2009, 407, 3958–3971. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhou, Q.; Tao, M.; Wang, Y.; Jiang, L.; Wu, Z. Comparative study of microbial community structure in different filter media of constructed wetland. J. Environ. Sci. 2010, 22, 127–133. [Google Scholar] [CrossRef]
- Ramond, J.B.; Welz, P.J.; Cowan, D.A.; Burton, S.G. Microbial community structure stability, a key parameter in monitoring the development of constructed wetland mesocosms during start-up. Res. Microbiol. 2012, 163, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Mulling, B.T.; Soeter, A.M.; Van Der Geest, H.G.; Admiraal, W. Changes in the planktonic microbial community during residence in a surface flow constructed wetland used for tertiary wastewater treatment. Sci. Total Environ. 2014, 466–467, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; Wu, Y.; Pi, N.; Tam, N.F.Y. Investigation of microbial community structure in constructed mangrove microcosms receiving wastewater-borne polycyclic aromatic hydrocarbons (PAHs) and polybrominated diphenyl ethers (PBDEs). Environ. Pollut. 2014, 187, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Taiwan Wetland Conservation Association. Available online: http://ppt.cc/HfCDl (accessed on 18 May 2017).
- American Public Health Association. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- U.S. Environmental Protection Agency. Method 1604: Total Coliforms and Escherichia coli in Water by Membrane Filtration Using a Simultaneous Detection Technique (MI Medium); USEPA Office of Water: Washington, DC, USA, 2002.
- Chang, B.V.; Liu, J.H.; Liao, C.S. Aerobic degradation of bisphenol-A and its derivatives in river sediment. Environ. Technol. 2014, 35, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Nübel, U.; Engelen, B.; Felske, A.; Snaidr, J.; Wieshuber, A.; Amann, R.I.; Ludwig, W.; Backhaus, H. Sequence heterogeneities of genes encoding 16S rRNAs in Paenibacillus. polymyxa detected by temperature gradient gel electrophoresis. J. Bacteriol. 1996, 178, 5636–5643. [Google Scholar] [CrossRef] [PubMed]
- Cremonesi, L.; Firpo, S.; Ferrari, M.; Righetti, P.G.; Gelfi, C. Double-gradient DGGE for optimized detection of DNA point mutations. Biotechniques 1997, 22, 326–330. [Google Scholar] [PubMed]
- Eichner, C.A.; Erb, R.W.; Timmis, K.N.; Wagner-Döbler, I. Thermal gradient gel electrophoresis analysis of bioprotection from pollutant shocks in the activated sludge microbial community. Appl. Environ. Microbiol. 1999, 65, 102–109. [Google Scholar] [PubMed]
- Johnson, S.C. Hierarchical clustering schemes. Psychometrika 1967, 32, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Wolfowitz, J. The minimum distance method. Ann. Math. Stat. 1957, 28, 75–88. [Google Scholar] [CrossRef]
- Anderberg, M.R. Cluster Analysis for Applications: Probability and Mathematical Statistics: A Series of Monographs and Textbooks; Academic Press: New York, NY, USA, 2014. [Google Scholar]
- Nübel, U.; Garcia-Pichel, F.; Kühl, M.; Muyzer, G. Quantifying microbial diversity: Morphotypes, 16S rRNA genes, and carotenoids of oxygenic phototrophs in microbial mats. Appl. Environ. Microbiol. 1999, 65, 422–430. [Google Scholar] [PubMed]
- Dice, L.R. Measures of the amount of ecologic association between species. Ecology 1945, 16, 297–302. [Google Scholar] [CrossRef]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of function. J. Am. Stat. Assoc. 1963, 58, 236–244. [Google Scholar]
- Simpson, E.H. Measurement of diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Perkins, J.; Hunter, C. Removal of enteric bacteria in a surface flow constructed wetland in Yorkshire, England. Water Res. 2000, 34, 1941–1947. [Google Scholar] [CrossRef]
- Karim, M.R.; Manshadi, F.D.; Karpiscak, M.M.; Gerba, C.P. The persistence and removal of enteric pathogens in constructed wetlands. Water Res. 2004, 38, 1831–1837. [Google Scholar] [CrossRef] [PubMed]
- García, M.; Soto, F.; González, J.M.; Bécares, E.A. Comparison of bacterial removal efficiencies in constructed wetlands and algae-based systems. Ecol. Eng. 2008, 32, 238–243. [Google Scholar] [CrossRef]


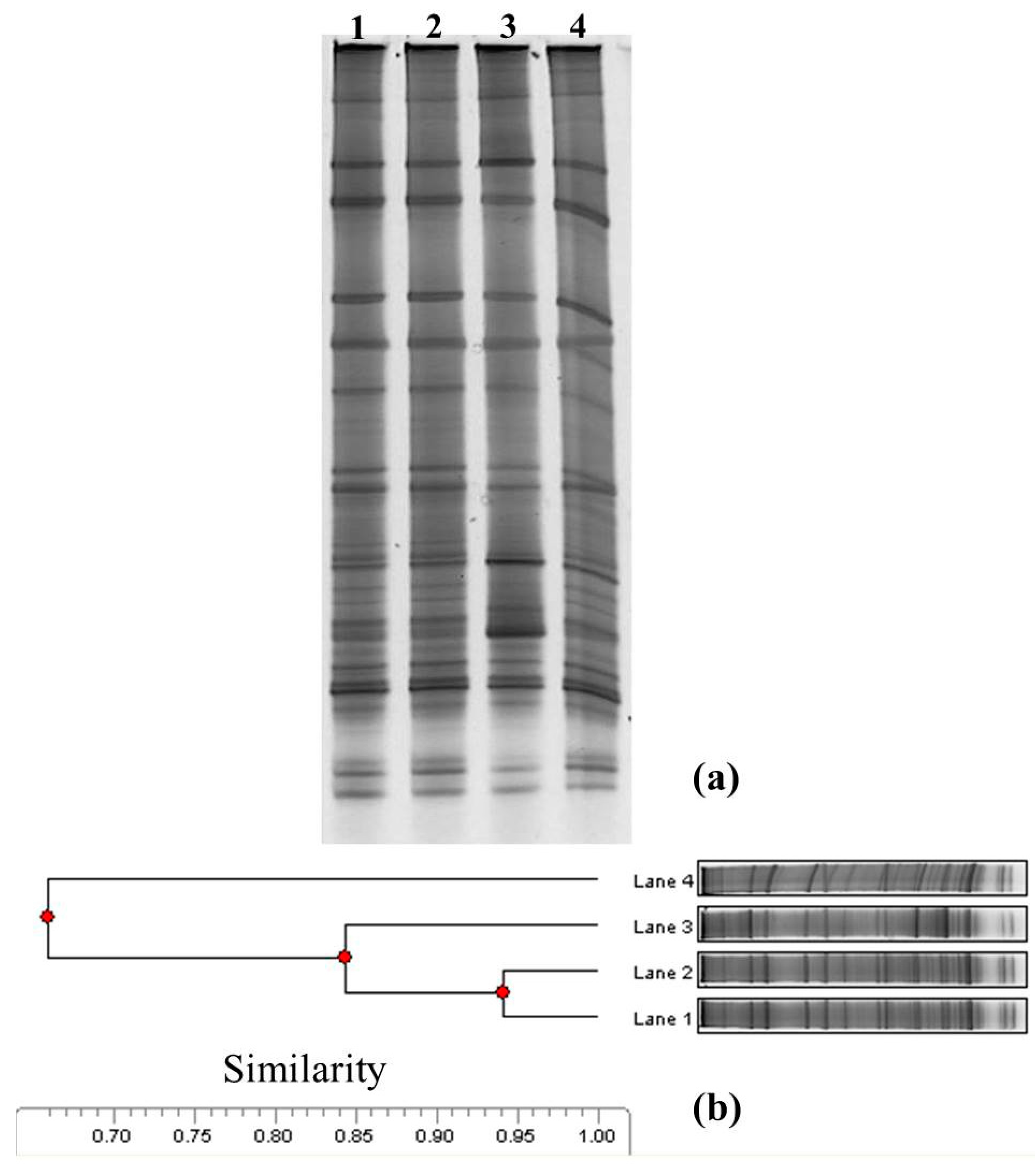
| Sampling Dates | R | H | S |
|---|---|---|---|
| 1 (lane 1, before the impact) | 34 | 4.69 | 0.05 |
| 2 (lane 2, under the impact) | 36 | 4.71 | 0.05 |
| 3 (lane 3, after the impact for 6 months) | 33 | 4.42 | 0.06 |
| 4 (lane 4, after the impact for 1 year) | 31 | 4.57 | 0.05 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.-C.; Jan, M.-Y.; Lin, K.-L.; Chao, S.-L.; Liao, C.-S. Sustainability of Constructed Wetland under the Impact of Aquatic Organisms Overloading. Sustainability 2017, 9, 863. https://doi.org/10.3390/su9050863
Chen S-C, Jan M-Y, Lin K-L, Chao S-L, Liao C-S. Sustainability of Constructed Wetland under the Impact of Aquatic Organisms Overloading. Sustainability. 2017; 9(5):863. https://doi.org/10.3390/su9050863
Chicago/Turabian StyleChen, Shih-Chieh, Ming-Young Jan, Kuo-Liang Lin, Sung-Lin Chao, and Chien-Sen Liao. 2017. "Sustainability of Constructed Wetland under the Impact of Aquatic Organisms Overloading" Sustainability 9, no. 5: 863. https://doi.org/10.3390/su9050863






