Characteristics of Pollutants and Microbial Communities Obtained in Simulated Lysimeters of Swine Carcasses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Lysimeter Operation
2.3. Analytical Methods
2.4. Microbial Analysis
3. Results and Discussion
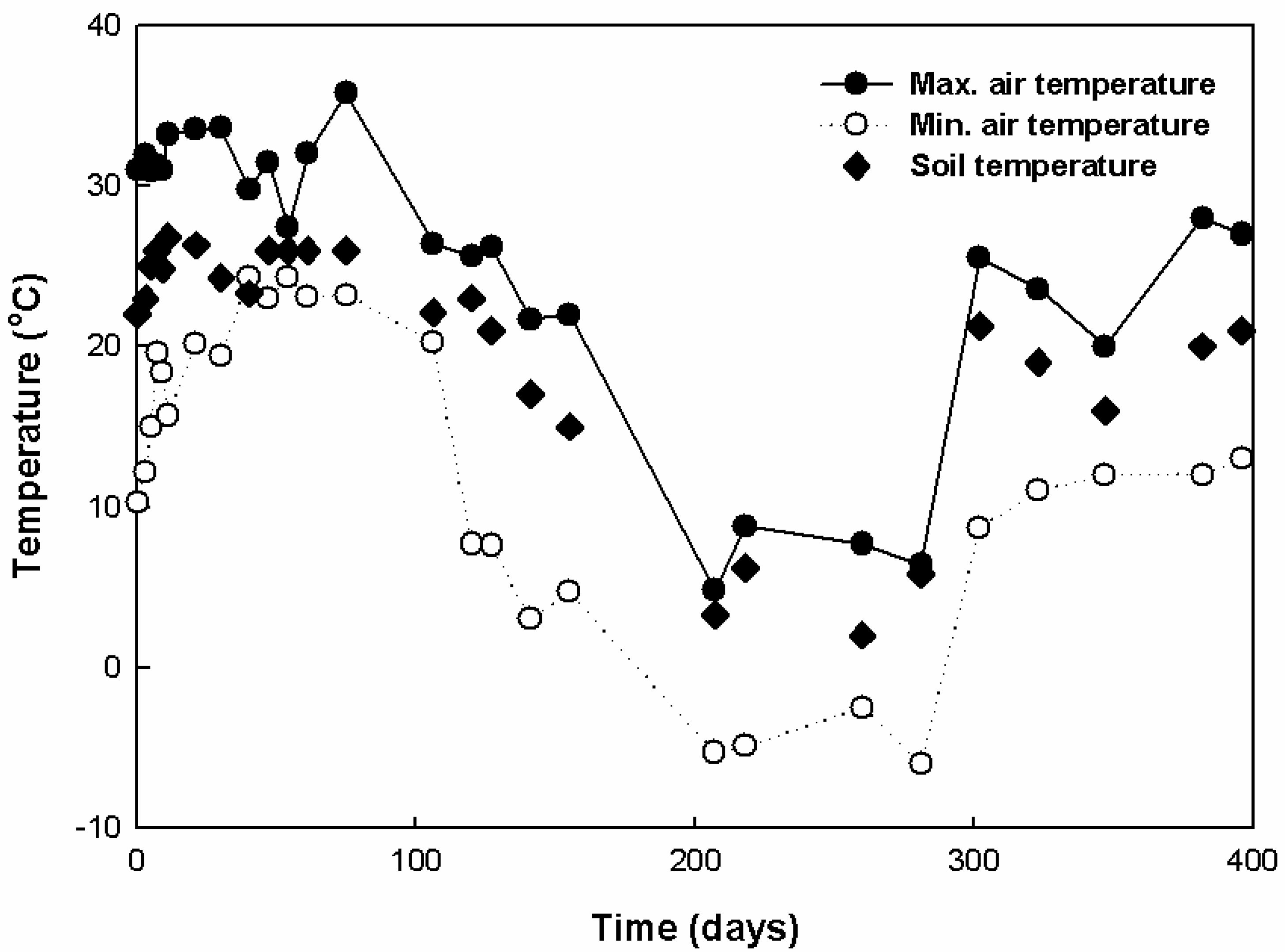
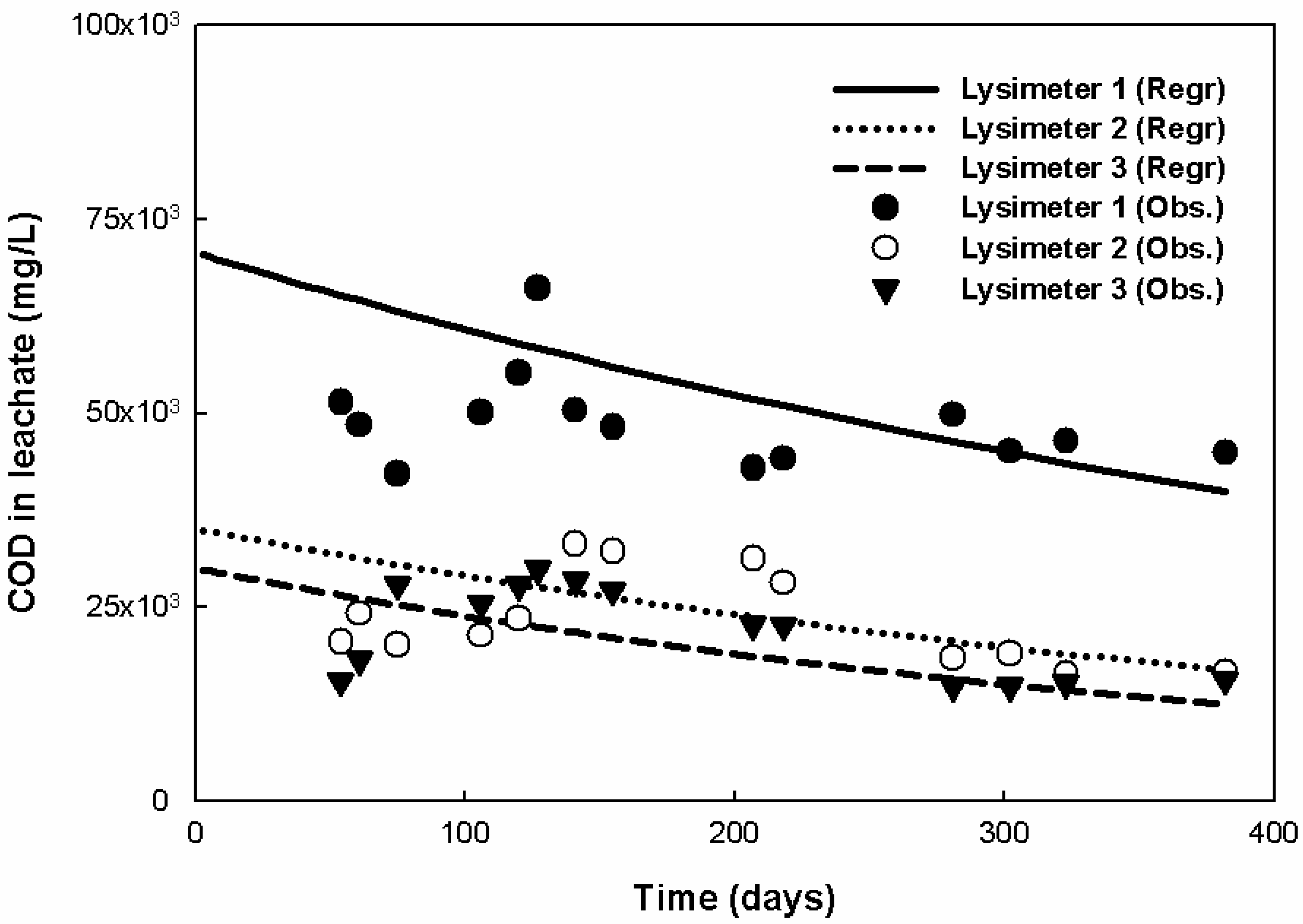
3.1. Characteristics of Leachate Production
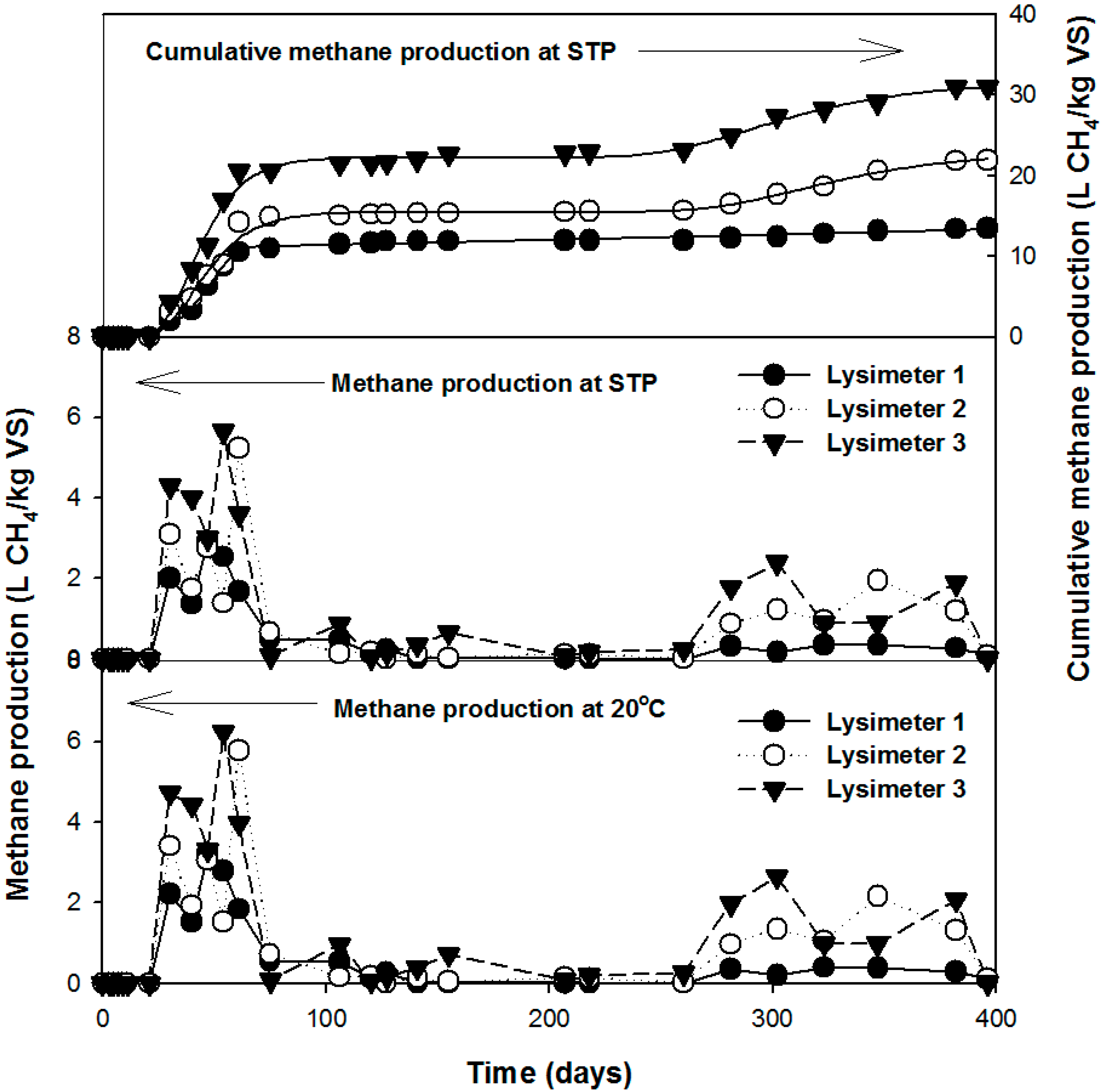
3.2. Characteristics of Gas Production
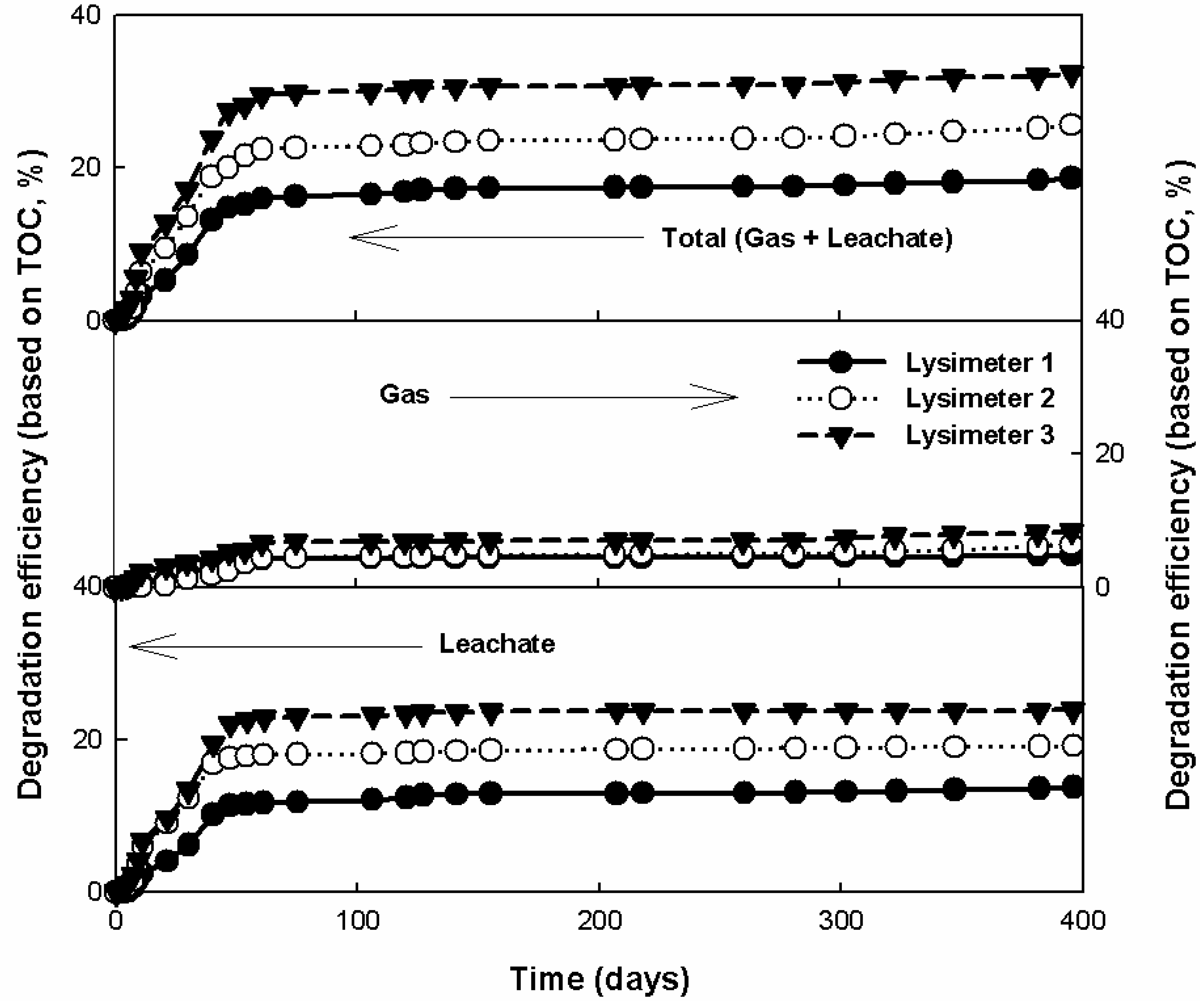
3.3. Comparison of the Leachate and Gas Production Characteristics
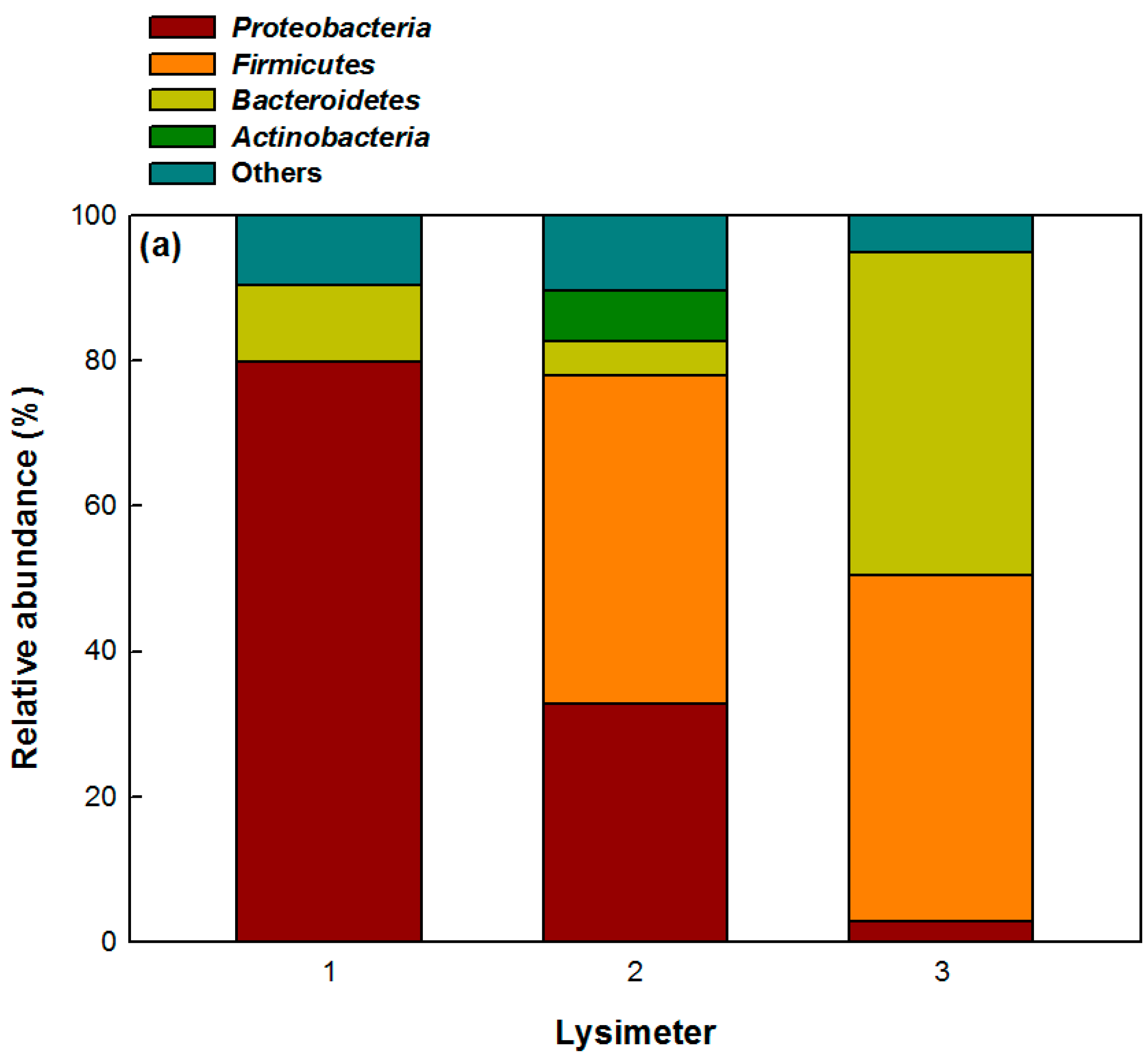
3.4. Bacteria and Archaea Communities in Lysimeters
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ministry of Agriculture Food and Rural Affairs (MAFRA). Foot-and-Mouth Disease Standard Operating Procedure (SOP); University States Department of Agriculture: Riverdale, MA, USA, 2012.
- Choi, N.C.; Choi, E.J.; Kim, B.J.; Kim, S.B.; Park, J.A.; Park, C.Y. Characterization of water quality and the aerobic bacterial population in leachate derived from animal carcass disposal. J. Eng. Geol. 2013, 23, 37–46. [Google Scholar] [CrossRef]
- Cho, P.H. Detection of foot-and-mouth disease virus and coxsakievirus in the soil and leachate of modeled carcass burial sites. Korean J. Vet. Serv. 2012, 35, 255–261. [Google Scholar] [CrossRef]
- Gwyther, C.L.; Williams, A.P.; Golyshin, P.N.; Edward-Jones, G.; Jones, D.L. The environmental and biosecurity characteristics of livestock carcass disposal methods: A review. Waste Manag. 2011, 31, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Kim, G.H. Cost analysis for the carcass burial construction. Korean Soc. Soil Groundw. Environ. 2013, 18, 137–147. [Google Scholar] [CrossRef]
- Pratt, D.L. Environmental Impact of Livestock Mortalities Burial. Master’s Dissertation, The University of Saskatchewan, Saskatoon, SK, Canada, 2009. [Google Scholar]
- Kang, M.A.; An, Y.S. Behavior of refractory organic matter in leachate from landfill contaminated by foot-and-mouth disease. J. Eng. Geol. 2013, 23, 427–434. [Google Scholar] [CrossRef]
- Park, J.A.; Choi, N.C.; Kim, S.B. Analysis of microbial communities in animal carcass disposal soils. J. Korean Soc. Environ. Eng. 2013, 25, 503–508. [Google Scholar] [CrossRef]
- Yuan, Q.; Samuel, E.S.; Bartelt-Hunt, S.L. Methane and carbon dioxide production from simulated anaerobic degradation of cattle carcasses. Waste Manag. 2012, 32, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Snow, D.D.; Bartelt-Hunt, S.L. Potential water quality impacts originating from land burial of cattle carcasses. Sci. Total Environ. 2013, 456–457, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.E.; Kim, M.S.; Choi, B.W.; Sohn, H.Y. Organic matter analysis and physicochemical properties of leachate from a foot-and-mouth disease landfill site. Korean J. Microbiol. Biotechnol. 2012, 40, 128–134. [Google Scholar] [CrossRef]
- Vinten, A.; Smith, H.; Watson, C.; Fenlon, D.; Ritchie, R. Assessment of Risks of Water Contamination with E. coli, Salmonella and Cryptosporidium from Burial of Animal Carcasses Using Artificially Drained Field Burial Plots; Macaulay Institute: Aberdeen, UK, 2008. [Google Scholar]
- Kim, J.K.; Han, S.K.; Kim, G.H.; Kim, J.T.; Lee, C.Y. Biodegradation characteristics of organic matters in swine carcasses under different initial operating conditions of simulated anaerobic lysimeter. J. Mater. Cycles Waste Manag. 2017, 19, 118–123. [Google Scholar] [CrossRef]
- Eaton, A.D.; Clesceri, L.S.; Rice, E.W.; Greenberg, A.E.; Franson, M.A.H. Standard Methods for the Examination of Water and Wastewater, 21th ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Kafle, G.K.; Kim, S.H.; Sung, K.I. Ensiling of fish industry waste for biogas production: A lab scale evaluation of biochemical methane potential (BMP) and kinetics. Bioresour. Technol. 2013, 127, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kang, H.J.; Lee, Y.H.; Lee, T.J.; Han, K.; Choi, Y.; Park, H.D. Monitoring bacterial community structure and variability in time scale in full-scale anaerobic digesters. J. Environ. Monitor. 2012, 14, 1893–1905. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; He, Q.; Ma, Y.; Wang, X.; Peng, W. A mesophilic anaerobic digester for treating food waste: Process stability and microbial community analysis using pyrosequencing. Microb. Cell Fact. 2016, 15, 65. [Google Scholar] [CrossRef] [PubMed]
- Rafizul, I.M.; Howlader, M.K.; Alamgir, M. Construction and evaluation of simulated pilot scale landfill lysimeter in Bangladesh. Waste Manag. 2012, 32, 2068–2079. [Google Scholar] [CrossRef] [PubMed]
- Sanphoti, N.; Towprayoon, S.; Chaiprasert, P.; Nopharatana, A. The effects of leachate recirculation with supplemental water addition on methane production and waste decomposition in a simulated tropical landfill. J. Environ. Manag. 2006, 81, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Christensen, T.H.; Cossu, R.; Stegmann, R. Sanitary Landfilling: Process, Technology and Environmental Impacts; Academic Press: London, UK, 1989. [Google Scholar]
- Tatsi, A.A.; Zouboulis, A.I. A field investigation of the quantity and quality of leachate from a municipal solid waste landfill in a Mediterranean climate (Thessaloniki, Greece). Adv. Environ. Res. 2002, 6, 207–219. [Google Scholar] [CrossRef]
- Clement, B. Physico-chemical characteristics of 25 French landfill leachate. In Proceedings of the Sardinia 95—5th International Landfill Symposium, Cagliari, Italy, 2–6 October 1995; pp. 315–325.
- Chen, P.H. Assessment of leachates from sanitary landfill: Impact of age, rainfall and treatment. Environ. Int. 1996, 22, 225–237. [Google Scholar] [CrossRef]
- Carter, D.O.; Yellowlees, D.; Tibbett, M. Cadaver decomposition in terrestrial ecosystems. Naturwissenschaften 2007, 94, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Skujins, J.J.; McLaren, A.D. Enzyme reaction rates at limited water activities. Science 1967, 158, 1569–1570. [Google Scholar] [CrossRef] [PubMed]
- Khanal, S.K. Anaerobic Biotechnology for Bioenergy Production; Wiley-Blackwell: Ames, IA, USA, 2008. [Google Scholar]
- Luo, K.; Yang, Q.; Li, X.; Yang, G.; Liu, Y.; Wang, D.; Zheng, W.; Zeng, G. Hydrolysis kinetics in anaerobic digestion of waste activated sludge enhanced by α-amylase. Biochem. Eng. J. 2012, 62, 17–21. [Google Scholar] [CrossRef]
- Mou, Z.; Schutz, C.; Kjeldsen, P. Evaluating the methane generation rate constant (k value) of low-organic waste at Danish landfills. Waste Manag. 2015, 35, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Aghdam, E.F.; Scheutz, C.; Kjeldsen, P. Assessment of methane production from shredder waste in landfills: The influence of temperature, moisture and metals. Waste Manag. 2016, in press. [Google Scholar] [CrossRef]
- Li, C.; Mőrtelmaier, C.; Winter, J.; Gallert, C. Effect of moisture of municipal biowaste on start-up and efficiency of mesophilic and thermophilic dry anaerobic digestion. Bioresour. Technol. 2014, 168, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, S.; Miyahara, T.; Noike, T. Effect of moisture content on anaerobic digestion of dewatered sludge: Ammonia inhibition to carbohydrate removal and methane production. Water Sci. Technol. 2000, 41, 119–127. [Google Scholar] [PubMed]
- Matsufuji, Y.; Tachifuji, A.; Matsugu, H. Maa Balance in Anaerobic and Semiaerobic Landfill Type, Sustainable Landfilling; Cossu, R., van der Sloot, H., Eds.; International Waste Working Group: Padova, Italy, 2014; pp. 106–117. [Google Scholar]
- Jiang, X.; Mira, D.; Cluff, D.L. The combustion mitigation of methane as a non-CO2 greenhouse gas. Prog. Energy Combust. Sci. 2016, in press. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2007: The Physical Science Basis; Cambridge University Press: Cambridge, UK, 2007.
- Mosier, A.R.; Duxbury, J.M.; Heinemeyer, O.; Minami, K.; Johnson, D.E. Mitigating agricultural emissions of methane. Clim. Chang. 1998, 40, 39–80. [Google Scholar] [CrossRef]
- Wirth, R.; Kovăcs, E.; Marŏti, G.; Bagi, Z.; Rȁkhely, G.; Kovăcs, K.L. Characterization of a biogas-producing microbial community by short-read next generation DNA sequencing. Biotechnol. Biofuels 2012, 5, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziganshin, A.M.; Liebetrau, J.; Prorter, J.; Kleinsteuber, S. Microbial community structure and dynamics during anaerobic digestion of various agricultural waste materials. Bioenergy Biofuels 2013, 97, 5161–5174. [Google Scholar] [CrossRef] [PubMed]
- Goux, X.; Calusinka, M.; Lemaigre, S.; Marynowska, M.; Klocke, M.; Udelhoven, T.; Benizri, E.; Delfosse, P. Microbial community dynamics in replicate anaerobic digesters exposed sequentially to increasing organic loading rate, acidosis, and process recovery. Biotechnol. Biofuels 2013, 8, 122. [Google Scholar] [CrossRef] [PubMed]
- National Agricultural Biosecurity Centre. Carcass Disposal: A Comprehensive Review; Report Written for the USDA Animal and Plant Health Inspection Service; Kansas State University: Manhattan, KS, USA, 2004.
- Franke-Whittle, I.H.; Walter, A.; Ebner, C.; Insam, H. Investigation into the effect of high concentration of volatile fatty acids in anaerobic digestion on methanogenic communities. Waste Manag. 2014, 34, 2080–2089. [Google Scholar] [CrossRef] [PubMed]
- Schlűter, A.; Bekel, T.; Diaz, N.N.; Dondrup, M.; Eichelaub, R.; Gartemann, K.H.; Krahn, I.; Krause, L.; Krőmeke, H.; Kruse, O.; et al. The metagenome of a biogas-producing microbial community of a production-scale biogas plant fermenter analysed by the 454-pyrosequencing technology. J. Biotechnol. 2008, 136, 77–90. [Google Scholar] [CrossRef] [PubMed]
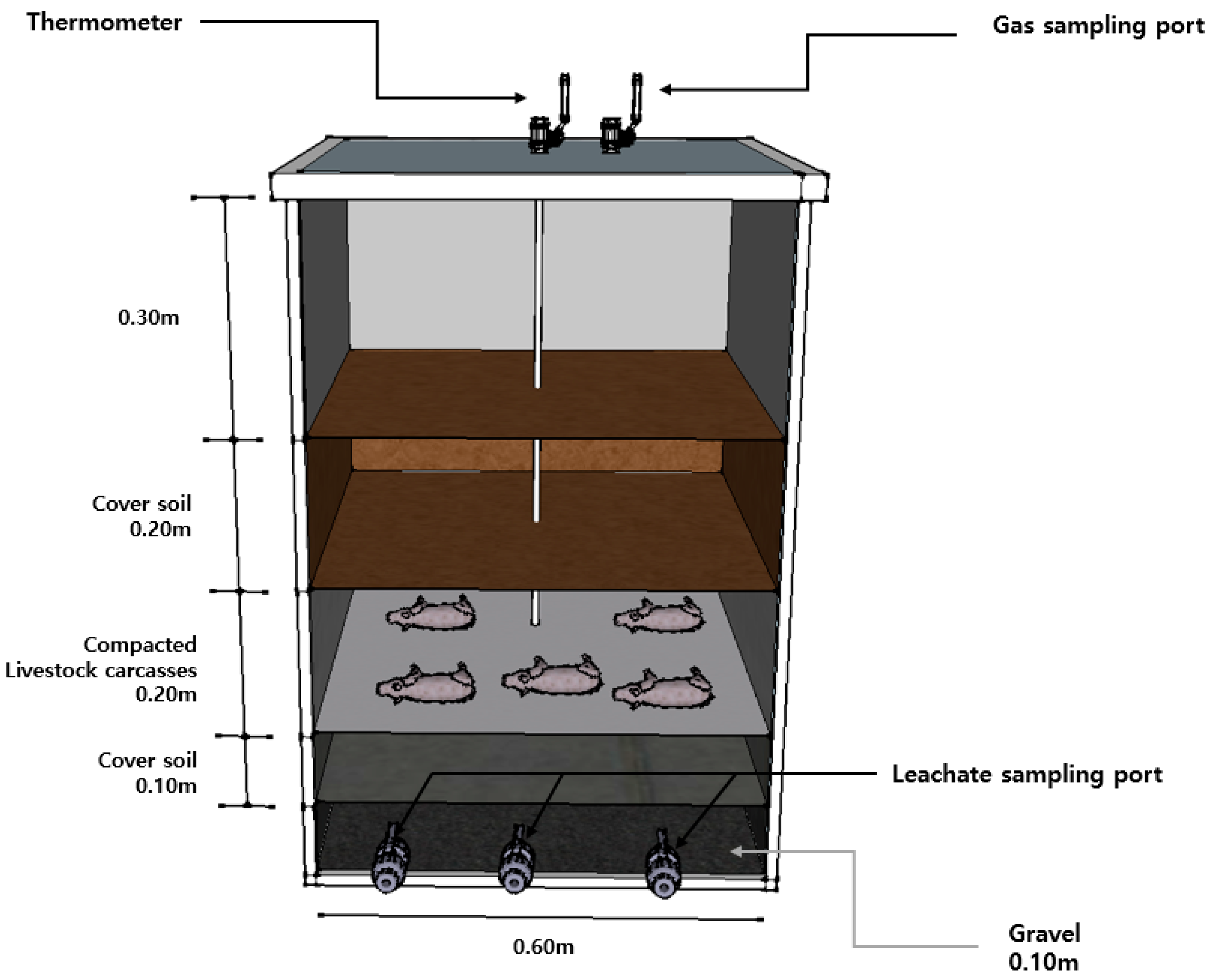


| Parameters | Lysimeter 1 | Lysimeter 2 | Lysimeter 3 |
|---|---|---|---|
| Substrate | Swine Carcasses (5 heads) | ||
| Water content (%, v/v) | 30% | 30% | 40% |
| Anaerobic sludge (%, w/w) | × | 0.04 | 0.04 |
| Item | Lysimeter 1 | Lysimeter 2 | Lysimeter 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Min. | Max. | Avg. | Min. | Max. | Avg. | Min. | Max. | Avg. | |
| pH | 6.1 | 8.3 | 7.5 ± 0.7 | 6.1 | 8.5 | 7.6 ± 0.8 | 6.2 | 8.8 | 7.8 ± 0.9 |
| TS (mg/L) | 5970 | 31,150 | 20,868 ± 8654.5 | 3580 | 15,480 | 8837 ± 4471.3 | 1380 | 12,610 | 7311 ± 3217.2 |
| VS (mg/L) | 3160 | 22,550 | 15,320 ± 6321.4 | 1770 | 10,290 | 5167 ± 3541.2 | 500 | 8190 | 4383 ± 2477.3 |
| COD (mg/L) | 965 | 88,980 | 45,611 ± 19,831.5 | 953 | 38,651 | 21,301 ± 9675.5 | 813 | 32,492 | 20,092 ± 8280.7 |
| BOD (mg/L) | 858 | 77,089 | 27,960 ± 17,230.9 | 811 | 30,904 | 11,359 ± 7165.6 | 617 | 18,615 | 8537 ± 4912.4 |
| TOC (mg/L) | 513 | 36,864 | 12,827 ± 8489.5 | 487 | 14,749 | 5456 ± 4203.5 | 413 | 12,096 | 3789 ± 2753.5 |
| BOD/COD | 0.38 | 0.89 | 0.62 ± 0.16 | 0.23 | 0.85 | 0.55 ± 0.19 | 0.10 | 0.76 | 0.43 ± 0.19 |
| TOC/COD | 0.13 | 0.60 | 0.31 ± 0.15 | 0.09 | 0.77 | 0.31 ± 0.21 | 0.07 | 0.61 | 0.23 ± 0.18 |
| TKN (mg/L) | 1051 | 2541 | 1831 ± 506.4 | 851 | 2002 | 1337 ± 429.9 | 441 | 1751 | 1025 ± 427.6 |
| NH4+-N (mg/L) | 515 | 983 | 802 ± 138.3 | 422 | 917 | 645.1 ± 156.8 | 244 | 802 | 493 ± 166.1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.-M.; Han, S.-K.; Lee, C.-Y. Characteristics of Pollutants and Microbial Communities Obtained in Simulated Lysimeters of Swine Carcasses. Sustainability 2017, 9, 471. https://doi.org/10.3390/su9030471
Choi J-M, Han S-K, Lee C-Y. Characteristics of Pollutants and Microbial Communities Obtained in Simulated Lysimeters of Swine Carcasses. Sustainability. 2017; 9(3):471. https://doi.org/10.3390/su9030471
Chicago/Turabian StyleChoi, Jae-Min, Sun-Kee Han, and Chae-Young Lee. 2017. "Characteristics of Pollutants and Microbial Communities Obtained in Simulated Lysimeters of Swine Carcasses" Sustainability 9, no. 3: 471. https://doi.org/10.3390/su9030471





