Management of Animal Carcass Disposal Sites Using a Biochar Permeable Reactive Barrier and Fast Growth Tree (Populus euramericana): A Field Study in Korea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Carcass Disposal Site
2.2. Establishment of Permeable Reactive Barrier
2.3. Planting Poplar Trees
2.4. Installation of Wells
2.5. Soil and Leachate Sampling
2.6. Analytical
2.7. Statistical Analyses
3. Results
3.1. Properties of the Soils
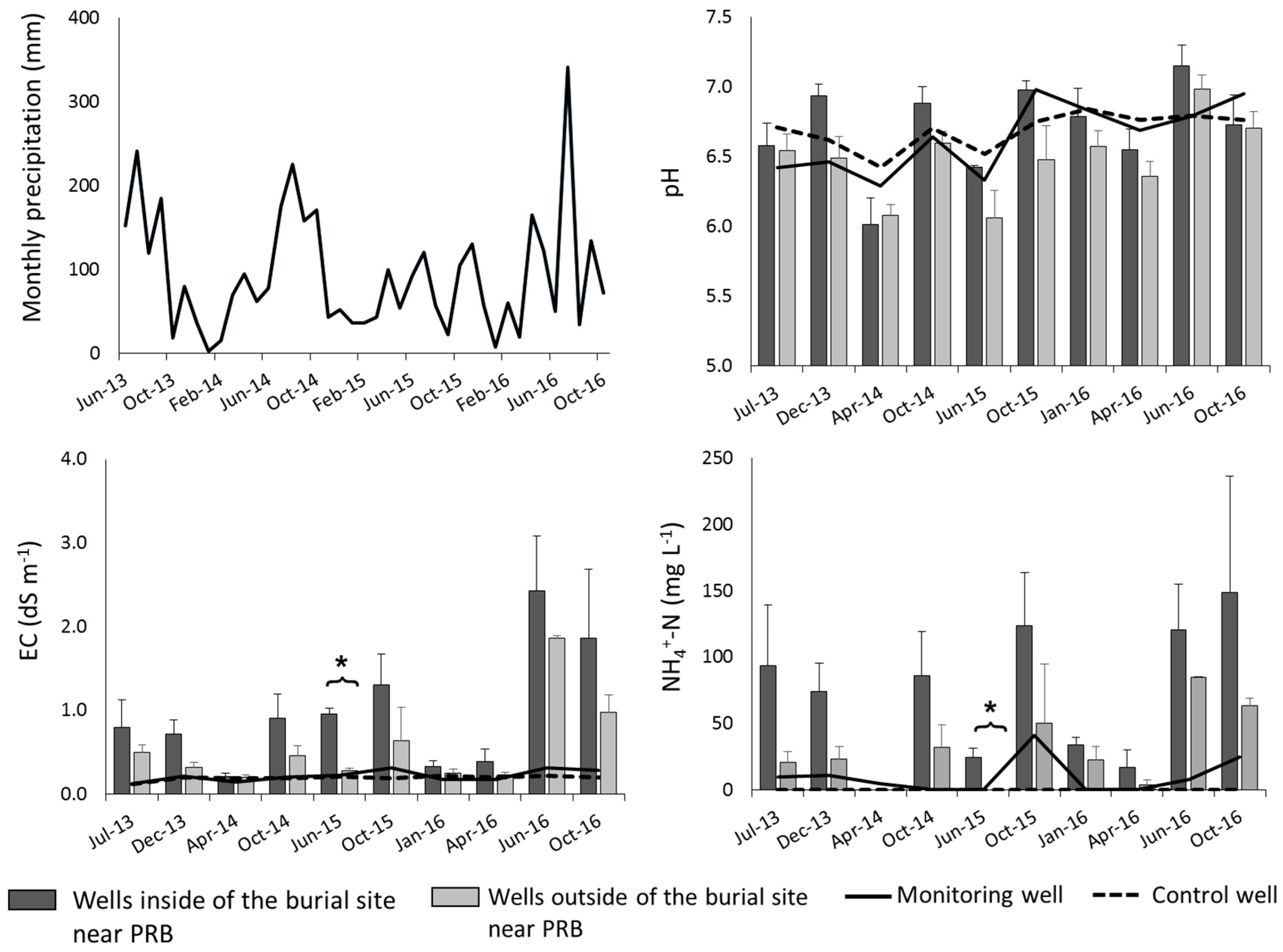
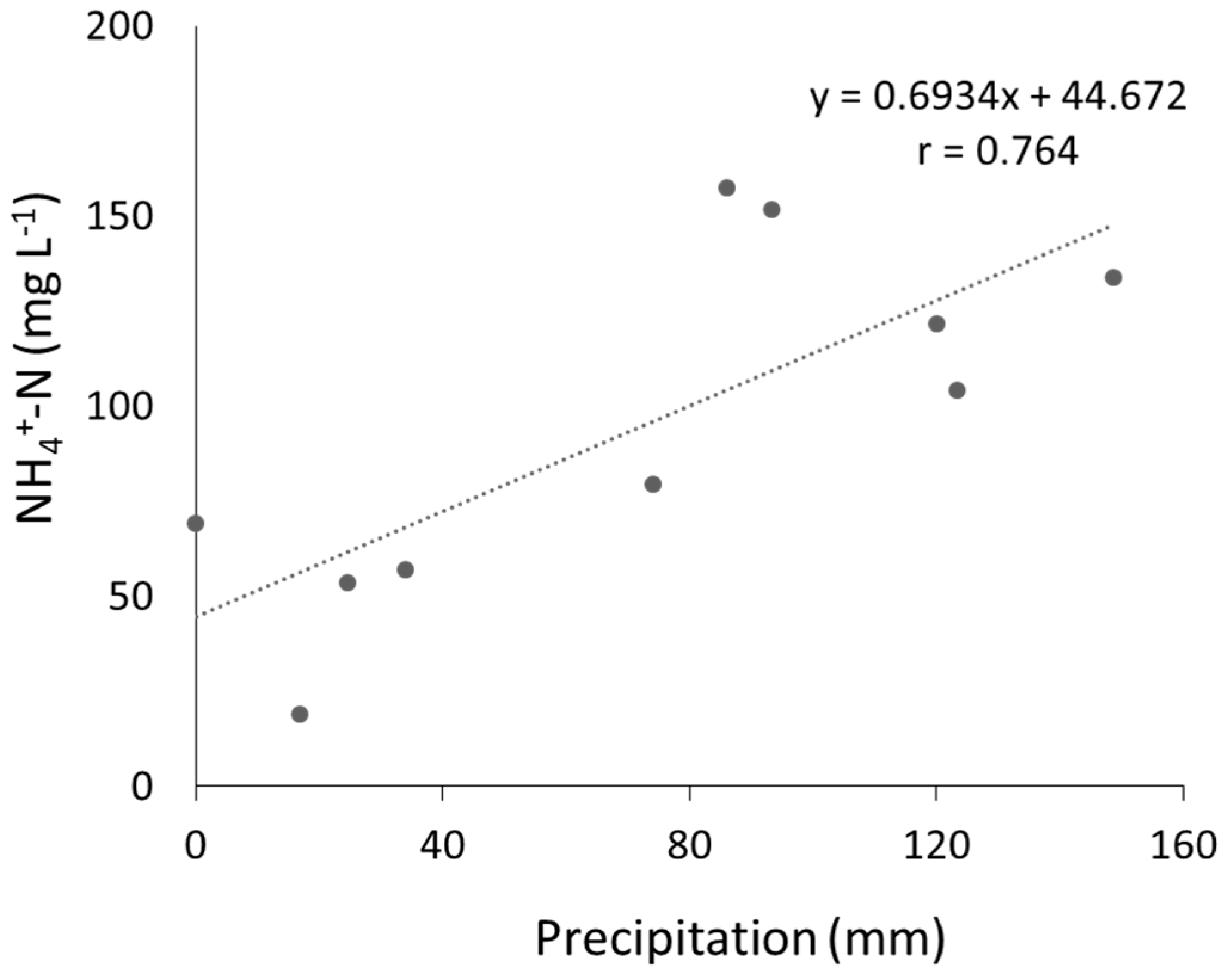
3.2. Leachate Properties
3.3. Rhizosphere Soil Properties
3.4. Plant Growth
4. Discussion
4.1. Impacts of PRB on Soil Properties
4.2. Removal of Leachate Contaminants by PRB
4.3. Phytoremediation Effects
4.4. Improving Removal Efficiency
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cho, H.S.; Kim, G.H. Needs of Biosecurity and Protocols for the Environmental Management of Carcasses Burial. J. Korean Soc. Water Environ. 2012, 28, 305–312. [Google Scholar]
- Kim, M.H.; Kim, G. Analysis of environmental impacts of burial sites. J. Mater. Cycles Waste Manag. 2015, 17, 1–11. [Google Scholar] [CrossRef]
- Choi, N.C.; Choi, E.J.; Kim, B.J.; Park, J.A.; Kim, S.B.; Park, C.Y. Characterization of water quality and the aerobic bacterial population in leachate derived from animal carcass disposal. J. Eng. Geol. 2013, 23, 37–46. [Google Scholar] [CrossRef]
- Gwyther, C.L.; Williams, A.P.; Golyshin, P.N.; Edwards-Jones, G.; Jones, D.L. The environmental and biosecurity characteristics of livestock carcass disposal methods: A review. Waste Manag. 2011, 31, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Snow, D.D.; Bartelt-Hunt, S.L. Potential water quality impacts originating from land burial of cattle carcasses. Sci. Total Environ. 2013, 456–457, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.A.; Kim, M.S.; Choi, B.W.; Sohn, H.Y. Organic matter analysis and physicochemical properties of leachate from a foot-and-mouth disease landfill site. Microbiol. Biotechnol. Lett. 2012, 40, 128–134. [Google Scholar] [CrossRef]
- Scudamore, J.M.; Trevelyan, G.M.; Tas, M.V.; Varley, E.M.; Hickman, G.A.W. Carcass disposal: Lessons from Great Britain following the foot and mouth disease outbreaks of 2001. Rev. Sci. Tech. (Int. Off. Epizoot.) 2002, 21, 775–787. [Google Scholar] [CrossRef]
- MacArthur, A.J.; Milne, J.C. Leachate characteristics and management requirements arising from the foot & mouth operations in Scotland. In Proceedings of the Waste 2002 Integrated Waste Management and Pollution Control: Research, Policy, and Practice, Stratford-upon-Avon, UK, 24–26 September 2002; pp. 305–314.
- Pratt, D.L.; Fonstad, T.A. Livestock mortalities burial leachate chemistry after two years of decomposition. In Proceedings of the American Society of Agricultural and Biological Engineers, Reno, Nevada, USA, 21–24 June 2009.
- Cundy, A.B.; Hopkinson, L.; Whitby, R.L. Use of iron-based technologies in contaminated land and groundwater remediation: A review. Sci. Total Environ. 2008, 400, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Scherer, M.M.; Richter, S.; Valentine, R.L.; Alvarez, P.J. Chemistry and microbiology of permeable reactive barriers for in situ groundwater clean up. Crit. Rev. Environ. Sci. Technol. 2000, 30, 363–411. [Google Scholar] [CrossRef]
- Thiruvenkatachari, R.; Vigneswaran, S.; Naidu, R. Permeable reactive barrier for groundwater remediation. J. Ind. Eng. Chem. 2008, 14, 145–156. [Google Scholar] [CrossRef]
- Patterson, B.M.; Grassi, M.E.; Robertson, B.S.; Davis, G.B.; Smith, A.J.; McKinley, A.J. Use of polymer mats in series for Sequential Reactive Barrier Remediation of Ammonium-contaminated Groundwater: Field evaluation. Environ. Sci. Technol. 2004, 38, 6846–6854. [Google Scholar] [CrossRef] [PubMed]
- Carniato, L.; Schoups, G.; Seuntjens, P.; Van Nooten, T.; Simons, Q.; Bastiaens, L. Predicting longevity of iron permeable reactive barriers using multiple iron deactivation models. J. Contam. Hydrol. 2012, 142, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Bastiaens, L.; Dries, J.; Vos, J.; Simons, Q.; Smet, M.D.; Diels, L. 2 New Developments in PRB Technology-Comparison of different multibarrier concepts designed for treatment of groundwater containing mixed pollutants. IAHS Publ. Ser. Proc. Rep. 2005, 298, 45–51. [Google Scholar]
- Park, J.B.; Lee, S.H.; Lee, J.W.; Lee, C.Y. Lab scale experiments for permeable reactive barriers against contaminated groundwater with ammonium and heavy metals using clinoptilolite (01-29B). J. Hazard. Mater. 2002, 95, 65–79. [Google Scholar] [CrossRef]
- Beesley, L.; Moreno-Jiménez, E.; Gomez-Eyles, J.L.; Harris, E.; Robinson, B.; Sizmur, T. A review of biochar’s potential role in the remediation, revegetation and restoration of contaminated soils. Environ. Pollut. 2011, 159, 3269–3282. [Google Scholar] [CrossRef] [PubMed]
- Major, J.; Steiner, C.; Downie, A.; Lehmann, J. Biochar effects on nutrient leaching. In Biochar for Environmental Management: Science and Technology; Lehmann, J., Joseph, S., Eds.; Earthscan: London, UK, 2009; pp. 271–287. [Google Scholar]
- Srinivasan, P.; Sarmah, A.K. Characterisation of agricultural waste-derived biochars and their sorption potential for sulfamethoxazole in pasture soil: a spectroscopic investigation. Sci. Total Environ. 2015, 502, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Gao, B.; Zimmerman, A.R.; Li, Y.; Ma, L.; Harris, W.G.; Migliaccio, K.W. Physicochemical and sorptive properties of biochars derived from woody and herbaceous biomass. Chemosphere 2015, 134, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, H.; He, L.; Lu, K.; Sarmah, A.; Li, J.; Bolan, N.S.; Pei, J.; Huang, H. Using biochar for remediation of soils contaminated with heavy metals and organic pollutants. Environ. Sci. Pollut. Res. 2013, 20, 8472–8483. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.H.; Kim, K.H. Application of biochar from organic wastes for remediation of leachate from animal carcass. In Proceedings of the Korean Society of Soil Sciences and Fertilizer, Muju, Korea, 20–21 October 2016; p. 318.
- Kim, G.H.; Chowdhury, S.; Bolan, N. Assessing the efficiency of permeable reactive barrier to reduce the groundwater contamination from carcass burial. In Proceedings of the IWA World Water Congress & Exhibition, Brisbane, Australia, 9–14 October 2016.
- Schnoor, J.L.; Light, L.A.; McCutcheon, S.C.; Wolfe, N.L.; Carreia, L.H. Phytoremediation of organic and nutrient contaminants. Environ. Sci. Technol. 1995, 29, 318A–323A. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.D.; Woo, S.Y.; Yeo, J.K.; Koo, Y.B.; Chun, S.H. Sapflow change and growth response of poplar species under swine wastewater irrigation. J. Korean For. Soc. 2009, 98, 740–747. [Google Scholar]
- Justin, M.Z.; Pajk, N.; Zupanc, V.; Zupančič, M. Phytoremediation of landfill leachate and compost wastewater by irrigation of Populus and Salix: biomass and growth response. Waste Manag. 2010, 30, 1032–1042. [Google Scholar] [CrossRef] [PubMed]
- Zalesny, J.A.; Zalesny, R.S.; Coyle, D.R.; Hall, R.B. Growth and biomass of Populus irrigated with landfill leachate. For. Ecol. Manag. 2007, 248, 143–152. [Google Scholar] [CrossRef]
- Dix, M.E.; Klopfenstein, N.B.; Zhang, J.W.; Workman, S.W.; Kim, M.S. Potential use of Populus for phytoremediation of environmental pollution in riparian zones. In Micropropagation, Genetic Engineering, and Molecular Biology of Populus; Klopfenstein, N.B., Chun, Y.W., Kim, M.S., Ahuja, M.A., Dillon, M.C., Carman, R.C., Eskew, L.G., Eds.; Rochy Mountain Forest and Range Experiment Station: Fort Collins, CO, USA, 1997; pp. 206–211. [Google Scholar]
- Licht, L.A.; Isebrands, J.G. Linking phytoremediated pollutant removal to biomass economic opportunities. Biomass Bioenergy 2006, 28, 203–218. [Google Scholar] [CrossRef]
- Ministry of Environment (MOE). Environment Management Guideline of Carcass Burial Sites; Ministry of Environment: Seoul, Korea, 2012.
- Kim, H.S. Rice Hull-Derived Biochar as a Stabilizer for Heavy Metal, an Ameliorant for Reclaimed Tidal Soil and a Material for Growing Substrate. Ph.D. Thesis, University of Seoul, Seoul, Korea, February 2013. [Google Scholar]
- Liang, B.; Lehmann, J.; Solomon, D.; Kinyangi, J.; Grossman, J.; O’Neill, B.; Skjemstad, J.O.; Thies, J.; Luizão, F.J.; Petersen, J.; et al. Black carbon increases cation exchange capacity in soils. Soil Sci. Soc. Am. J. 2006, 70, 1719–1730. [Google Scholar] [CrossRef]
- Dickmann, D.I.; Isebrands, J.G.; Eckenwalder, J.E.; Richardson, J. Poplar Culture in North America; NCR-CNRC; NCR Research Press: Ottawa, ON, Canada, 2001; p. 397. [Google Scholar]
- Gordon, J.C.; Promnitz, L.C. Photosynthetic and enzymatic criteria for the early selection of fast-growing Populus clones. In Tree Physiology and Yield Improvement; Cannell, M.G.R., Last, F.T., Eds.; Academic Press: New York, NY, USA, 1976; pp. 79–97. [Google Scholar]
- Seo, B.H.; Kim, H.S.; Kuppusamy, S.; Kim, K.H.; Kim, K.R. Enhanced Nitrogen and Phosphorus Removal by Woody Plants with Deep-Planting Technique for the Potential Environmental Management of Carcass Burial Sites. Sustainability 2017, 9, 155. [Google Scholar] [CrossRef]
- Heilman, P.E.; Fu-Guang, X. Influence of nitrogen on growth and productivity of short-rotation Populus trichocarpa × Populus deltoides hybrid. Can. J. For. Res. 1993, 23, 1863–1869. [Google Scholar] [CrossRef]
- Yeo, J.K.; Koo, Y.B.; Kim, H.C.; Shin, H.N.; Lee, E.D. Removal of swine wastewater by evapotranspiration at poplar plantation and growth characteristics of poplar clones. Korean Soc. Waste Manag. 2010, 27, 226–233. [Google Scholar]
- Thomas, G.W. Soil pH and soil acidity. In Methods of Soil Analysis Part 3: Chemical Methods, 2nd ed.; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; Soil Science Society of America and American Society of Agronomy: Madison, WI, USA, 1996; pp. 475–490. [Google Scholar]
- Bremner, J.M. Nitrogen-total. Methods of soil analysis. In Methods of Soil Analysis Part 3: Chemical Methods, 2nd ed.; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; Soil Science Society of America and American Society of Agronomy: Madison, WI, USA, 1996; pp. 1085–1121. [Google Scholar]
- Mulvaney, R.L. Nitrogen-Inorganic forms. In Methods of Soil Analysis Part 3: Chemical Methods, 2nd ed.; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; Soil Science Society of America and American Society of Agronomy: Madison, WI, USA, 1996; pp. 869–920. [Google Scholar]
- Frankenberger, W.T.; Tabatabai, M.A.; Adriano, D.C.; Doner, H.E. Bromine, chlorine, & fluorine. In Methods of Soil Analysis Part 3: Chemical Methods, 2nd ed.; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; Soil Science Society of America and American Society of Agronomy: Madison, WI, USA, 1996; pp. 833–867. [Google Scholar]
- Cresser, M.S.; Parsons, J.W. Sulphuric—Perchloric acid digestion of plant material for the determination of nitrogen, phosphorus, potassium, calcium and magnesium. Anal. Chim. Acta 1979, 109, 431–436. [Google Scholar] [CrossRef]
- Kuo, S. Phosphorus. In Methods of Soil Analysis Part 3: Chemical Methods, 2nd ed.; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; Soil Science Society of America and American Society of Agronomy: Madison, WI, USA, 1996; pp. 869–920. [Google Scholar]
- Lawrinenko, M.; Laird, D.A. Anion exchange capacity of biochar. Green Chem. 2015, 17, 4628–4636. [Google Scholar] [CrossRef]
- Sharanowski, B.J.; Walker, E.G.; Anderson, G.S. Insect succession and decomposition patterns on shaded and sunlit carrion in Saskatchewan in three different season. Forensic Sci. Int. 2008, 179, 219–240. [Google Scholar] [CrossRef] [PubMed]
- Linhares, A.X.; de Carvalho, L.M.L. Seasonality of insect succession and pig carcass decomposition in a natural forest area in southeastern Brazil. J. Forensic Sci. 2001, 46, 604–608. [Google Scholar]
- Westphal, L.M.; Isebrands, J.G. Phytoremediation of Chicago’s brown fields: consideration of ecological approaches and social issues. In Proceedings of the Brownfields Conference, Chicago, IL, USA, 20 August 2001.
- Armstrong, A.; Johns, C.; Tubby, I. Effects of spacing and cutting cycle on the yield of poplar grown as an energy crop. Biomass Bioenergy 1999, 17, 305–314. [Google Scholar] [CrossRef]
- Bowersox, T.W.; Ward, W.W. Growth and yield of close-spaced young hybrid poplars. For. Sci. 1976, 22, 449–454. [Google Scholar]
- Laureysens, I.; Bogaert, J.; Blust, R.; Ceulemans, R. Biomass production of 17 poplar clones in a short-rotation coppice culture on a waste disposal site and its relation to soil haracteristics. For. Ecol. Manag. 2004, 187, 295–309. [Google Scholar] [CrossRef]
- Wittmer, R.F.; Immel, M.J. A comparison of five tree species for intensive fiber production. For. Ecol. Manag. 1997, 1, 249–254. [Google Scholar] [CrossRef]
- Kim, D.W.; Yu, S.H.; Chang, S.W.; Lee, J.A. Ecotoxicty assessment of leachate from disposal site for foot-and-mouth disease carcasses. J. Korean Geo-Environ. Soc. 2014, 15, 5–11. [Google Scholar]
- Korea Environment Corporation (KECO). A Study on the Environmental Assessment of Surrounding Area of AI Outbreak; Korean Environment Corporation: Incheon, Korea, 2009. [Google Scholar]
- Environment Agency. Assessing the Groundwater Pollution Potential of Cemetery Developments; Environment Agency: Bristol, UK, 2004.
- Thomas, B.W.; Sharifi, M.; Whalen, J.K.; Chantigny, M.H. Mineralizable nitrogen responds differently to manure type in contrasting soil textures. Soil Sci. Soc. Am. J. 2015, 79, 1396–1405. [Google Scholar] [CrossRef]
- Demirbas, A. Effects of temperature and particle size on bio-char yield from pyrolysis of agricultural residues. J. Anal. Appl. Pyrolysis 2004, 72, 243–248. [Google Scholar] [CrossRef]
- Kizito, S.; Wu, S.; Kirui, W.K.; Lei, M.; Lu, Q.; Bah, H.; Dong, R. Evaluation of slow pyrolyzed wood and rice husks biochar for adsorption of ammonium nitrogen from piggery manure anaerobic digestate slurry. Sci. Total Environ. 2015, 505, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Wen, D.; Ho, Y.S.; Tang, X. Comparative sorption kinetic studies of ammonium onto zeolite. J. Hazard. Mater. 2006, 133, 252–256. [Google Scholar] [CrossRef] [PubMed]


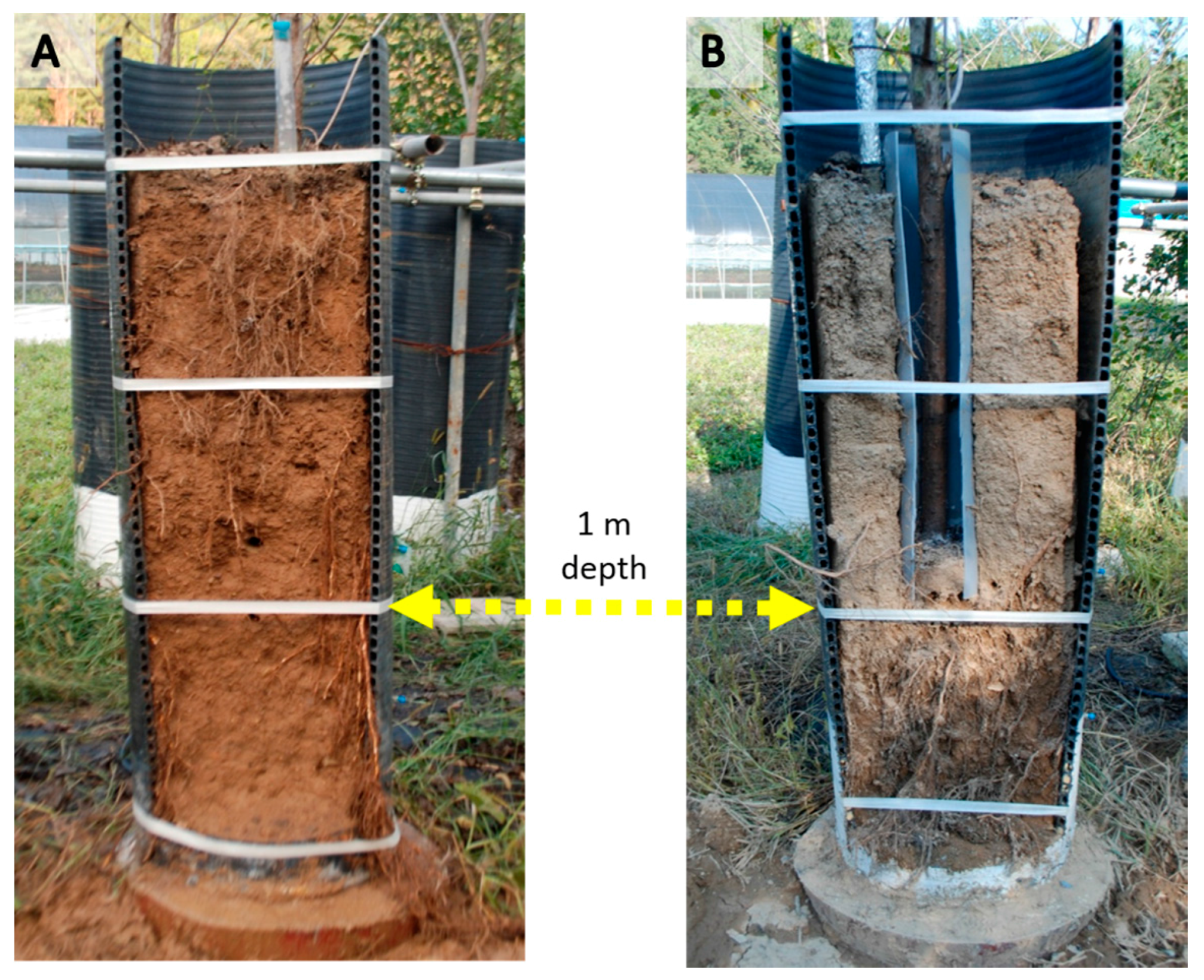

| Threshold Level | Criteria for Judgment |
|---|---|
| Level 1 (high potential) | NH4-N ≥ 10 mg·L−1 or Cl− ≥ 100 mg·L−1 |
| Level 2 (medium potential) | NH4-N ≥ 2 mg·L−1 or Cl− ≥ 25 mg·L−1 or Electrical conductivity (EC) ≥ 800 μS·cm−1 |
| Level 3 (low potential) | NH4-N ≥ 0.1 mg·L−1 and Cl− ≥ 4.0 mg·L−1 and {(Cl−monitoring well/Cl−burial site) ≥ 0.05} and EC ≥ 800 μS·cm−1 |
| Location of Soil Samples | pH (1:5W) | EC (dS·m−1) | OM (%) | T-N (%) | T-P (%) | NH4-N (mg·kg−1) | NO3-N (mg·kg−1) | Cl− (mg·kg−1) |
|---|---|---|---|---|---|---|---|---|
| The soil near PRB inside of the burial site | 4.9 | 0.22 | 12.7 | 0.14 | 651 | 171 | 22.1 | 4.84 |
| The soil near PRB outside of the burial site | 5.5 | 0.06 | 0.61 | 0.03 | 299 | 35.8 | 22.1 | 2.75 |
| The soil near monitoring well | 6.3 | 0.08 | 0.53 | 0.03 | 243 | 38.6 | N.M. 1 | 4.24 |
| DBH (cm) | SH (cm) | DW (kg/Plant) | PAP (ton DW/ha/4 Years) |
|---|---|---|---|
| 22.1 (4.1) | 761.3 (50.4) | 10.7 (4.1) | 85.3 |
| Sampling Year | Total N (g·kg−1) | Total P (g·kg−1) | ||
|---|---|---|---|---|
| Leaf | Stem | Leaf | Stem | |
| 2013 | 22.6 (1.0) a | - | 0.5 (0.1) a | - |
| 2014 | 23.7 (1.1) a | 5.2 (1.1) | 0.4 (<0.1) a | 1.2 (0.1) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, J.-H.; Kim, Y.-N.; Shin, D.-C.; Kim, K.-R.; Kim, K.-H. Management of Animal Carcass Disposal Sites Using a Biochar Permeable Reactive Barrier and Fast Growth Tree (Populus euramericana): A Field Study in Korea. Sustainability 2017, 9, 457. https://doi.org/10.3390/su9030457
Yoon J-H, Kim Y-N, Shin D-C, Kim K-R, Kim K-H. Management of Animal Carcass Disposal Sites Using a Biochar Permeable Reactive Barrier and Fast Growth Tree (Populus euramericana): A Field Study in Korea. Sustainability. 2017; 9(3):457. https://doi.org/10.3390/su9030457
Chicago/Turabian StyleYoon, Jung-Hwan, Young-Nam Kim, Dong-Chun Shin, Kwon-Rae Kim, and Kye-Hoon Kim. 2017. "Management of Animal Carcass Disposal Sites Using a Biochar Permeable Reactive Barrier and Fast Growth Tree (Populus euramericana): A Field Study in Korea" Sustainability 9, no. 3: 457. https://doi.org/10.3390/su9030457






