Solid-State Fermentation as a Novel Paradigm for Organic Waste Valorization: A Review
Abstract
:1. Introduction
2. Organic Waste and Its Potential
2.1. Agricultural Organic Waste
2.2. Industrial Organic Waste
2.3. Municipal/Domestic Food Waste
3. General Aspects of Solid State Fermentation
4. Advantages and Challenges of Solid State Fermentation
5. Applications of Solid State Fermentation
5.1. Enzymes Production
5.2. Organic Acids Production
5.3. Biopesticides Production
5.4. Biosurfactant Production
5.5. Bioethanol Production
5.6. Aroma Compounds Production
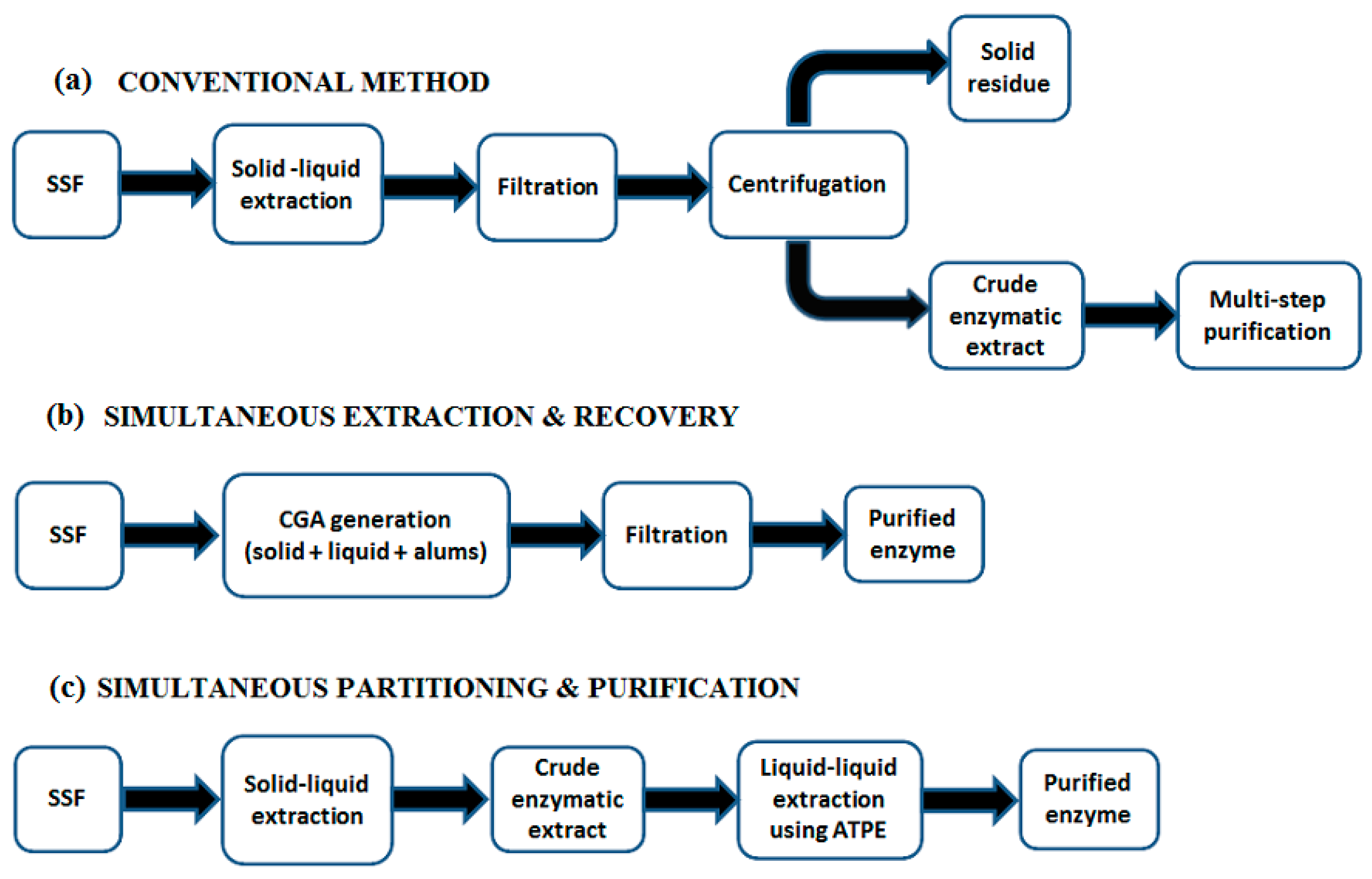
6. Downstream Processing in SSF and Residue Reutilization
7. Economic Viewpoint
8. Future Perspectives and Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mussatto, S.I.; Ballesteros, L.F.; Martins, S.; Teixeira, J.A. Use of Agro-Industrial Wastes in Solid-State Fermentation Processes. In Industrial Waste; InTech: Rijeka, Croatia, 2012; pp. 121–141. [Google Scholar]
- Eco-Cycle. Waste of Energy-Why Incineration Is Bad for Our Economy, Environment and Community; Eco-Cycle: Boulder County, CO, USA, 2011; pp. 1–20. [Google Scholar]
- Sánchez, A.; Artola, A.; Font, X.; Gea, T.; Barrena, R.; Gabriel, D.; Sánchez-Monedero, M.Á.; Roig, A.; Cayuela, M.L.; Mondini, C. Greenhouse gas emissions from organic waste composting. Environ. Chem. Lett. 2015, 13, 223–238. [Google Scholar] [CrossRef]
- Giuntini, E.; Bazzicalupo, M.; Castaldini, M.; Fabiani, A.; Miclaus, N.; Piccolo, R.; Ranalli, G.; Santomassimo, F.; Zanobini, S.; Mengoni, A. Genetic diversity of dinitrogen-fixing bacterial communities in soil amended with olive husks. Ann. Microbiol. 2006, 56, 83–88. [Google Scholar] [CrossRef]
- Thomas, L.; Larroche, C.; Pandey, A. Current developments in solid-state fermentation. Biochem. Eng. J. 2013, 81, 146–161. [Google Scholar] [CrossRef]
- Chen, H.Z.; He, Q. Value-added bioconversion of biomass by solid-state fermentation. J. Chem. Technol. Biotechnol. 2012, 87, 1619–1625. [Google Scholar] [CrossRef]
- Pandey, A. Solid-state fermentation. Biochem. Eng. J. 2003, 13, 81–84. [Google Scholar] [CrossRef]
- Hölker, U.; Lenz, J. Solid-state fermentation—Are there any biotechnological advantages? Curr. Opin. Microbiol. 2005, 8, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Soccol, C.R.; Mitchell, D. New developments in solid state fermentation: I-bioprocesses and products. Process Biochem. 2000, 35, 1153–1169. [Google Scholar] [CrossRef]
- Wang, L.; Yang, S.-T. Chapter 18—Solid State Fermentation and Its Applications. In Bioprocessing for Value-Added Products from Renewable Resources: New Technologies and Applications; Elsevier B.V.: Amsterdam, The Netherlands, 2007; pp. 465–489. [Google Scholar]
- Singhania, R.R.; Patel, A.K.; Soccol, C.R.; Pandey, A. Recent advances in solid-state fermentation. Biochem. Eng. J. 2009, 44, 13–18. [Google Scholar] [CrossRef]
- El-Bakry, M.; Abraham, J.; Cerda, A.; Barrena, R.; Ponsá, S.; Gea, T.; Sánchez, A. From Wastes to High Value Added Products: Novel Aspects of SSF in the Production of Enzymes. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1999–2042. [Google Scholar] [CrossRef]
- Dave, B.R.; Sudhir, A.P.; Pansuriya, M.; Raykundaliya, D.P.; Subramanian, R.B. Utilization of Jatropha deoiled seed cake for production of cellulases under solid-state fermentation. Bioprocess Biosyst. Eng. 2012, 35, 1343–1353. [Google Scholar] [CrossRef] [PubMed]
- Motta, F.L.; Santana, M.H. Solid-state fermentation for humic acids production by a Trichoderma reesei strain using an oil palm empty fruit bunch as the substrate. Appl. Biochem. Biotechnol. 2014, 172, 2205–2217. [Google Scholar] [CrossRef] [PubMed]
- Ohkouchi, Y.; Inoue, Y. Impact of chemical components of organic wastes on l(+)-lactic acid production. Bioresour. Technol. 2007, 98, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zou, H.; Jiang, L.; Yao, J.; Liang, J.; Wang, Q. Semi-solid state fermentation of food waste for production of Bacillus thuringiensis biopesticide. Biotechnol. Bioprocess Eng. 2015, 20, 1123–1132. [Google Scholar] [CrossRef]
- Jooste, T.; García-Aparicio, M.P.; Brienzo, M.; Van Zyl, W.H.; Görgens, J.F. Enzymatic hydrolysis of spent coffee ground. Appl. Biochem. Biotechnol. 2013, 169, 2248–2262. [Google Scholar] [CrossRef] [PubMed]
- Rocha, M.V.P.; de Matos, L.J.B.L.; De Lima, L.P.; Figueiredo, P.M.D.S.; Lucena, I.L.; Fernandes, F.A.N.; Gonçalves, L.R.B. Ultrasound-assisted production of biodiesel and ethanol from spent coffee grounds. Bioresour. Technol. 2014, 167, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Abraham, J.; Gea, T.; Sánchez, A. Substitution of chemical dehairing by proteases from solid-state fermentation of hair wastes. J. Clean. Prod. 2014, 74, 191–198. [Google Scholar] [CrossRef]
- Yazid, N.A.; Barrena, R.; Sánchez, A. Assessment of protease activity in hydrolysed extracts from SSF of hair waste by and indigenous consortium of microorganisms. Waste Manag. 2016, 49, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Wiradimadja, R.; Rusmana, D.; Widjastuti, T.; Mushawwir, A. Chicken Slaughterhouse Waste Utilization (Chicken Feather Meal Treated) as a Source of Protein Animal Feed Ingredients in Broiler Chickens. Lucr. Stiint.—Ser. Zooteh. 2014, 62, 120–124. [Google Scholar]
- Kanagaraj, J.; Velappan, K.C.; Chandra Babu, N.K.; Sadulla, S. Solid wastes generation in the leather industry and its utilization for cleaner environment—A review. J. Sci. Ind. Res. (India) 2006, 65, 541–548. [Google Scholar]
- Rathna, G.S.; Saranya, R.; Kalaiselvam, M. Original Research Article Bioethanol from sawdust using cellulase hydrolysis of Aspergillus ochraceus and fermentation by Saccharomyces cerevisiae. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 733–742. [Google Scholar]
- Ghazi, I.; Fernandez-Arrojo, L.; Gomez De Segura, A.; Alcalde, M.; Plou, F.J.; Ballesteros, A. Beet sugar syrup and molasses as low-cost feedstock for the enzymatic production of fructo-oligosaccharides. J. Agric. Food Chem. 2006, 54, 2964–2968. [Google Scholar] [CrossRef] [PubMed]
- Jayathilakan, K.; Sultana, K.; Radhakrishna, K.; Bawa, A.S. Utilization of byproducts and waste materials from meat, poultry and fish processing industries: A review. J. Food Sci. Technol. 2012, 49, 278–293. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Mendis, E. Bioactive compounds from marine processing byproducts—A review. Food Res. Int. 2006, 39, 383–393. [Google Scholar] [CrossRef]
- Kandra, P.; Challa, M.M.; Kalangi Padma Jyothi, H. Efficient use of shrimp waste: Present and future trends. Appl. Microbiol. Biotechnol. 2012, 93, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Elmekawy, A.; Diels, L.; De Wever, H.; Pant, D. Valorization of cereal based biorefinery byproducts: Reality and expectations. Environ. Sci. Technol. 2013, 47, 9014–9027. [Google Scholar] [CrossRef] [PubMed]
- Embaby, A.M.; Masoud, A.A.; Marey, H.S.; Shaban, N.Z.; Ghonaim, T.M. Raw agro-industrial orange peel waste as a low cost effective inducer for alkaline polygalacturonase production from Bacillus licheniformis SHG10. Springerplus 2014, 3, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.K.; Mishra, S.S.; Kayitesi, E.; Ray, R.C. Microbial-processing of fruit and vegetable wastes for production of vital enzymes and organic acids: Biotechnology and scopes. Environ. Res. 2016, 146, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Mtui, G.Y.S. Recent advances in pretreatment of lignocellulosic wastes and production of value added products. Afr. J. Biotechnol. 2009, 8, 1398–1415. [Google Scholar]
- Govumoni, S.P.; Gentela, J.; Koti, S.; Haragopal, V.; Venkateshwar, S.; Rao, L.V. Original Research Article Extracellular Lignocellulolytic Enzymes by Phanerochaete chrysosporium (MTCC 787) Under Solid-State Fermentation of Agro Wastes. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 700–710. [Google Scholar]
- Ukpai, P.A.; Nnabuchi, M.N. Comparative study of biogas production from cow dung, cow pea and cassava peeling using 45 litres biogas digester. Adv. Appl. Sci. Res. 2012, 3, 1864–1869. [Google Scholar]
- Sebola, M.R.; Tesfagiorgis, H.B.; Muzenda, E. Methane Production from Anaerobic Co-digestion of Cow Dung, Chicken Manure, Pig Manure and Sewage Waste. In Proceedings of the World Congress on Engineering, London, UK, 1–3 July 2015; Volume I, pp. 1–7.
- Adams, T.T.; Eiteman, M.A.; Hanel, B.M. Solid state fermentation of broiler litter for production of biocontrol agents. Bioresour. Technol. 2002, 82, 33–41. [Google Scholar] [CrossRef]
- Botella, C.; Diaz, A.; de Ory, I.; Webb, C.; Blandino, A. Xylanase and pectinase production by Aspergillus awamori on grape pomace in solid state fermentation. Process Biochem. 2007, 42, 98–101. [Google Scholar] [CrossRef]
- Jørgensen, H.; Sanadi, A.R.; Felby, C.; Lange, N.E.K.; Fischer, M.; Ernst, S. Production of ethanol and feed by high dry matter hydrolysis and fermentation of Palm kernel press cake. Appl. Biochem. Biotechnol. 2010, 161, 318–332. [Google Scholar] [CrossRef] [PubMed]
- Gratuito, M.K.B.; Panyathanmaporn, T.; Chumnanklang, R.A.; Sirinuntawittaya, N.; Dutta, A. Production of activated carbon from coconut shell: Optimization using response surface methodology. Bioresour. Technol. 2008, 99, 4887–4895. [Google Scholar] [CrossRef] [PubMed]
- Sapuan, S.M.; Harimi, M.; Maleque, M.A. Mechanical Properties of Epoxy/Coconut Shell Filler Particle Composites. Arab. J. Sci. Eng. 2003, 28, 171–181. [Google Scholar]
- Sunil, K.S.; Vinay, D.R.; Saviraj, A.S.; Naik, P.K. Flexural Behaviour of Coconut Shell/Epoxy Composites Subjected to Accelerated Ageing. Am. J. Mater. Sci. 2015, 5, 126–132. [Google Scholar]
- Behera, S.S.; Ray, R.C. Solid state fermentation for production of microbial cellulases: Recent advances and improvement strategies. Int. J. Biol. Macromol. 2016, 86, 656–669. [Google Scholar] [CrossRef] [PubMed]
- De Castro, R.J.S.; Ohara, A.; Nishide, T.G.; Bagagli, M.P.; Gonçalves Dias, F.F.; Sato, H.H. A versatile system based on substrate formulation using agroindustrial wastes for protease production by Aspergillus niger under solid state fermentation. Biocatal. Agric. Biotechnol. 2015, 4, 678–684. [Google Scholar] [CrossRef]
- Do-Myoung, K.; Eun, J.C.; Ji, W.K.; Yong-Woog, L.; Hwa-Jee, C. Production of cellulases by Penicillium sp. in a solid-state fermentation of oil palm empty fruit bunch. Afr. J. Biotechnol. 2014, 13, 145–155. [Google Scholar]
- Mehboob, N.; Asad, M.J.; Imran, M.; Gulfraz, M.; Wattoo, F.H.; Hadri, S.H.; Asghar, M. Production of lignin peroxidase by Ganoderma leucidum using solid state fermentation. Afr. J. Biotechnol. 2011, 10, 9880–9887. [Google Scholar]
- Graminha, E.B.N.; Gonçalves, A.Z.L.; Pirota, R.D.P.B.; Balsalobre, M.A.A.; Da Silva, R.; Gomes, E. Enzyme production by solid-state fermentation: Application to animal nutrition. Anim. Feed Sci. Technol. 2008, 144, 1–22. [Google Scholar] [CrossRef]
- Chang, J.; Cheng, W.; Yin, Q.; Zuo, R.; Song, A.; Zheng, Q.; Wang, P.; Wang, X.; Liu, J. Effect of steam explosion and microbial fermentation on cellulose and lignin degradation of corn stover. Bioresour. Technol. 2012, 104, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Lio, J.; Wang, T. Solid-state fermentation of soybean and corn processing coproducts for potential feed improvement. J. Agric. Food Chem. 2012, 60, 7702–7709. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.V.; Lee, B.K. Removal of dimethyl sulfide from aqueous solution using cost-effective modified chicken manure biochar produced from slow pyrolysis. Sustainability 2015, 7, 15057–15072. [Google Scholar] [CrossRef]
- Demiral, H.; Demiral, İ. Surface properties of activated carbon prepared from wastes. Surf. Interface Anal. 2008, 40, 612–615. [Google Scholar] [CrossRef]
- Liu, H.; Chen, D.; Zhang, R.; Hang, X.; Li, R.; Shen, Q. Amino Acids Hydrolyzed from Animal Carcasses Are a Good Additive for the Production of Bio-organic Fertilizer. Front. Microbiol. 2016, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Daâssi, D.; Zouari-Mechichi, H.; Frikha, F.; Rodríguez-Couto, S.; Nasri, M.; Mechichi, T. Sawdust waste as a low-cost support-substrate for laccases production and adsorbent for azo dyes decolorization. J. Environ. Heal. Sci. Eng. 2016, 14, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, B.; Kumar, A.G.; Bhavani, P.S.A.; Sekaran, G. Solid-state fermentation for the production of alkaline protease by Bacillus cereus 1173900 using proteinaceous tannery solid waste. Current 2011, 100, 726–730. [Google Scholar]
- Veana, F.; Martínez-Hernández, J.L.; Aguilar, C.N.; Rodríguez-Herrera, R.; Michelena, G. Utilization of molasses and sugar cane bagasse for production of fungal invertase in solid state fermentation using Aspergillus niger GH1. Braz. J. Microbiol. 2014, 45, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, S.; Kumar, G.; Sahgal, M.; Singh, A. Ethanol Production through Saccharomyces Based Fermentation Using Apple Pomace Amended with Molasses. Sugar Tech. 2012, 14, 304–311. [Google Scholar] [CrossRef]
- Sathiyanarayanan, G.; Kiran, G.S.; Selvin, J.; Saibaba, G. Optimization of polyhydroxybutyrate production by marine Bacillus megaterium MSBN04 under solid state culture. Int. J. Biol. Macromol. 2013, 60, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Mandalari, G.; Bisignano, G.; Lo Curto, R.B.; Waldron, K.W.; Faulds, C.B. Production of feruloyl esterases and xylanases by Talaromyces stipitatus and Humicola grisea var. thermoidea on industrial food processing by-products. Bioresour. Technol. 2008, 99, 5130–5133. [Google Scholar] [PubMed]
- Singh, A.; Kuila, A.; Adak, S.; Bishai, M.; Banerjee, R. Utilization of Vegetable Wastes for Bioenergy Generation. Agric. Res. 2012, 1, 213–222. [Google Scholar] [CrossRef]
- Suresh, P.V.; Anil Kumar, P.K. Enhanced degradation of a-chitin materials prepared from shrimp processing byproduct and production of N-acetyl-d-glucosamine by thermoactive chitinases from soil mesophilic fungi. Biodegradation 2012, 23, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Nidheesh, T.; Pal, G.K.; Suresh, P.V. Chitooligomers preparation by chitosanase produced under solid state fermentation using shrimp by-products as substrate. Carbohydr. Polym. 2015, 121, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Esakkiraj, P.; Usha, R.; Palavesam, A.; Immanuel, G. Solid-state production of esterase using fish processing wastes by Bacillus altitudinis AP-MSU. Food Bioprod. Process. 2012, 90, 370–376. [Google Scholar] [CrossRef]
- Uçkun Kiran, E.; Trzcinski, A.P.; Liu, Y. Glucoamylase production from food waste by solid state fermentation and its evaluation in the hydrolysis of domestic food waste. Biofuel Res. J. 2014, 3, 98–105. [Google Scholar] [CrossRef]
- Melikoglu, M.; Lin, C.S.K.; Webb, C. Solid state fermentation of waste bread pieces by Aspergillus awamori: Analysing the effects of airflow rate on enzyme production in packed bed bioreactors. Food Bioprod. Process. 2015, 95, 63–75. [Google Scholar] [CrossRef]
- Cerda, A.; El-Bakry, M.; Gea, T.; Sánchez, A. Long term enhanced solid-state fermentation: Inoculation strategies for amylase production from soy and bread wastes by Thermomyces sp. in a sequential batch operation. J. Environ. Chem. Eng. 2016, 4, 2394–2401. [Google Scholar] [CrossRef]
- Janveja, C.; Rana, S.S.; Soni, S.K. Optimization of valorization of biodegradable kitchen waste biomass for production of fungal cellulase system by statistical modeling. Waste Biomass Valoriz. 2014, 5, 807–821. [Google Scholar] [CrossRef]
- Abdullah, J.J.; Greetham, D.; Pensupa, N.; Tucker, G.A.; Du, C. Optimizing Cellulase Production from Municipal Solid Waste (MSW) using Solid State Fermentation (SSF). J. Fundam. Renew. Energy Appl. 2016, 6, 206. [Google Scholar] [CrossRef]
- Mantzouridou, F.T.; Paraskevopoulou, A.; Lalou, S. Yeast flavour production by solid state fermentation of orange peel waste. Biochem. Eng. J. 2015, 101, 1–8. [Google Scholar] [CrossRef]
- Matsakas, L.; Kekos, D.; Loizidou, M.; Christakopoulos, P. Utilization of household food waste for the production of ethanol at high dry material content. Biotechnol. Biofuels 2014, 7, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Matsakas, L.; Christakopoulos, P. Ethanol production from enzymatically treated dried food waste using enzymes produced on-site. Sustainability 2015, 7, 1446–1458. [Google Scholar] [CrossRef]
- Sun, Z.; Li, M.; Qi, Q.; Gao, C.; Lin, C.S.K. Mixed Food Waste as Renewable Feedstock in Succinic Acid Fermentation. Appl. Biochem. Biotechnol. 2014, 174, 1822–1833. [Google Scholar] [CrossRef] [PubMed]
- Ezejiofor, T.I.N.; Duru, C.I.; Asagbra, A.E.; Ezejiofor, A.N.; Orisakwe, O.E.; Afonne, J.O.; Obi, E. Waste to wealth: Production of oxytetracycline using streptomyces species from household kitchen wastes of agricultural produce. Afr. J. Biotechnol. 2012, 11, 10115–10124. [Google Scholar]
- López-Pérez, M.; Viniegra-González, G. Production of protein and metabolites by yeast grown in solid state fermentation: Present status and perspectives. J. Chem. Technol. Biotechnol. 2016, 91, 1224–1231. [Google Scholar] [CrossRef]
- Martins, S.; Mussatto, S.I.; Martínez-Avila, G.; Montañez-Saenz, J.; Aguilar, C.N.; Teixeira, J.A. Bioactive phenolic compounds: Production and extraction by solid-state fermentation. A review. Biotechnol. Adv. 2011, 29, 365–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, D.; Zhang, R.; Yang, X.; Wu, H.; Xu, D.; Tang, Z.; Shen, Q. Thermostable cellulase production of Aspergillus fumigatus Z5 under solid-state fermentation and its application in degradation of agricultural wastes. Int. Biodeterior. Biodegrad. 2011, 65, 717–725. [Google Scholar] [CrossRef]
- Ávila-Cisneros, N.; Velasco-Lozano, S.; Huerta-Ochoa, S.; Córdova-López, J.; Gimeno, M.; Favela-Torres, E. Production of Thermostable Lipase by Thermomyces lanuginosus on Solid-State Fermentation: Selective Hydrolysis of Sardine Oil. Appl. Biochem. Biotechnol. 2014, 174, 1859–1872. [Google Scholar] [CrossRef] [PubMed]
- Saqib, A.A.N.; Farooq, A.; Iqbal, M.; Hassan, J.U.; Hayat, U.; Baig, S. A thermostable crude endoglucanase produced by aspergillus fumigatus in a novel solid state fermentation process using isolated free water. Enzyme Res. 2012, 2012, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Tsegaye, K.N.; Gessesse, A. Amylase production under solid state fermentation by a bacterial isolate W74. Afr. J. Biotechnol. 2014, 13, 2145–2153. [Google Scholar]
- Afrisham, S.; Badoei-Dalfard, A.; Namaki-Shoushtari, A.; Karami, Z. Characterization of a thermostable, CaCl2-activated and raw-starch hydrolyzing alpha-amylase from Bacillus licheniformis AT70: Production under solid state fermentation by utilizing agricultural wastes. J. Mol. Catal. B Enzym. 2016, 132, 98–106. [Google Scholar] [CrossRef]
- Özdemir, S.; Matpan, F.; Okumus, V.; Dündar, A.; Ulutas, M.S.; Kumru, M. Isolation of a thermophilic Anoxybacillus flavithermus sp. nov. and production of thermostable α-amylase under solid-state fermentation (SSF). Ann. Microbiol. 2012, 62, 1367–1375. [Google Scholar]
- Prajapati, V.S.; Trivedi, U.B.; Patel, K.C. A statistical approach for the production of thermostable and alklophilic alpha-amylase from Bacillus amyloliquefaciens KCP2 under solid-state fermentation. 3 Biotech 2015, 5, 211–220. [Google Scholar] [CrossRef]
- Das, R.K.; Brar, S.K.; Verma, M. Potential use of pulp and paper solid waste for the bio-production of fumaric acid through submerged and solid state fermentation. J. Clean. Prod. 2016, 112, 4435–4444. [Google Scholar] [CrossRef]
- Bhalkar, B.N.; Bedekar, P.A.; Kshirsagar, S.D.; Govindwar, S.P. Solid state fermentation of soybean waste and an up-flow column bioreactor for continuous production of camptothecine by an endophytic fungus: Fusarium oxysporum. RSC Adv. 2016, 6, 56527–56536. [Google Scholar] [CrossRef]
- De Castro, R.J.S.; Sato, H.H. Enzyme Production by Solid State Fermentation: General Aspects and an Analysis of the Physicochemical Characteristics of Substrates for Agro-industrial Wastes Valorization. Waste Biomass Valoriz. 2015, 6, 1085–1093. [Google Scholar] [CrossRef]
- Orzua, M.C.; Mussatto, S.I.; Contreras-Esquivel, J.C.; Rodriguez, R.; de la Garza, H.; Teixeira, J.A.; Aguilar, C.N. Exploitation of agro industrial wastes as immobilization carrier for solid-state fermentation. Ind. Crops Prod. 2009, 30, 24–27. [Google Scholar] [CrossRef]
- Abraham, J.; Gea, T.; Sánchez, A. Potential of the solid-state fermentation of soy fiber residues by native microbial populations for bench-scale alkaline protease production. Biochem. Eng. J. 2013, 74, 15–19. [Google Scholar] [CrossRef]
- Viniegra-González, G.; Favela-Torres, E.; Aguilar, C.N.; Rómero-Gomez, S.D.J.; Díaz-Godínez, G.; Augur, C. Advantages of fungal enzyme production in solid state over liquid fermentation systems. Biochem. Eng. J. 2003, 13, 157–167. [Google Scholar] [CrossRef]
- Hölker, U.; Höfer, M.; Lenz, J. Biotechnological advantages of laboratory-scale solid-state fermentation with fungi. Appl. Microbiol. Biotechnol. 2004, 64, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Gabelle, J.C.; Jourdier, E.; Licht, R.B.; Ben Chaabane, F.; Henaut, I.; Morchain, J.; Augier, F. Impact of rheology on the mass transfer coefficient during the growth phase of Trichoderma reesei in stirred bioreactors. Chem. Eng. Sci. 2012, 75, 408–417. [Google Scholar] [CrossRef]
- De la Cruz Quiroz, R.; Roussos, S.; Hernández, D.; Rodríguez, R.; Castillo, F.; Aguilar, C.N. Challenges and opportunities of the bio-pesticides production by solid-state fermentation: Filamentous fungi as a model. Crit. Rev. Biotechnol. 2015, 35, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Hongzhang, C.; Hongqiang, L.; Liying, L. The inhomogeneity of corn stover and its effects on bioconversion. Biomass Bioenergy 2011, 35, 1940–1945. [Google Scholar] [CrossRef]
- Raghavarao, K.S.M.; Ranganathan, T.; Karanth, N. Some engineering aspects of solid-state fermentation. Biochem. Eng. J. 2003, 13, 127–135. [Google Scholar] [CrossRef]
- Mansour, A.A.; Arnaud, T.; Lu-Chau, T.A.; Fdz-Polanco, M.; Moreira, M.T.; Rivero, J.A.C. Review of solid state fermentation for lignocellulolytic enzyme production: Challenges for environmental applications. Rev. Environ. Sci. Biotechnol. 2016, 15, 31–46. [Google Scholar] [CrossRef]
- Rahardjo, Y.S.P.; Korona, D.; Haemers, S.; Weber, F.J.; Tramper, J.; Rinzema, A. Limitations of membrane cultures as a model solid-state fermentation system. Lett. Appl. Microbiol. 2004, 39, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, R.; Pandey, A.; Binod, P. Solid-state Fermentation for the Production of Poly(hydroxyalkanoates). Chem. Biochem. Eng. Q. 2015, 29, 173–181. [Google Scholar] [CrossRef]
- Ortiz, G.E.; Guitart, M.E.; Cavalitto, S.F.; Albertó, E.O.; Fernández-Lahore, M.; Blasco, M. Characterization, optimization, and scale-up of cellulases production by trichoderma reesei cbs 836.91 in solid-state fermentation using agro-industrial products. Bioprocess Biosyst. Eng. 2015, 38, 2117–2128. [Google Scholar] [PubMed]
- Salihu, A.; Sallau, A.B.; Adamu, A.; Kudu, F.A.; Tajo, M.M.; Bala, T.F.; Yashim, W.D. Utilization of Groundnut Husk as a Solid Substrate for Cellulase Production by Aspergillus niger Using Response Surface Methodology. Waste Biomass Valoriz. 2014, 5, 585–593. [Google Scholar] [CrossRef]
- Sun, H.-Y.; Li, J.; Zhao, P.; Peng, M. Banana peel: A novel substrate for cellulase production under solid-state fermentation. Afr. J. Biotechnol. 2011, 10, 17887–17890. [Google Scholar]
- Zhou, H.; Wang, C.Z.; Ye, J.Z.; Chen, H.X.; Tao, R.; Zhang, Y.S. Solid-state fermentation of Ginkgo biloba L. residue for optimal production of cellulase, protease and the simultaneous detoxification of Ginkgo biloba L. residue using Candida tropicalis and Aspergillus oryzae. Eur. Food Res. Technol. 2015, 240, 379–388. [Google Scholar]
- Bansal, N.; Janveja, C.; Tewari, R.; Soni, R.; Soni, S.K. Highly thermostable and pH-stable cellulases from Aspergillus niger NS-2: Properties and application for cellulose hydrolysis. Appl. Biochem. Biotechnol. 2014, 172, 141–156. [Google Scholar] [CrossRef] [PubMed]
- Dave, B.R.; Parmar, P.; Sudhir, A.; Singal, N.; Subramanian, R.B. Cellulases production under solid state fermentation using agro waste as a substrate and its application in saccharification by Trametes hirsuta NCIM. J. Microbiol. Biotechnol. Food Sci. 2015, 4, 203–208. [Google Scholar] [CrossRef]
- Gasparotto, J.M.; Werle, L.B.; Foletto, E.L.; Kuhn, R.C.; Jahn, S.L.; Mazutti, M.A. Production of Cellulolytic Enzymes and Application of Crude Enzymatic Extract for Saccharification of Lignocellulosic Biomass. Appl. Biochem. Biotechnol. 2015, 175, 560–572. [Google Scholar] [CrossRef] [PubMed]
- Pathak, P.; Bhardwaj, N.K.; Singh, A.K. Production of crude cellulase and xylanase from Trichoderma harzianum PPDDN10 NFCCI-2925 and its application in photocopier waste paper recycling. Appl. Biochem. Biotechnol. 2014, 172, 3776–3797. [Google Scholar] [CrossRef] [PubMed]
- Raghuwanshi, S.; Deswal, D.; Karp, M.; Kuhad, R.C. Bioprocessing of enhanced cellulase production from a mutant of Trichoderma asperellum RCK2011 and its application in hydrolysis of cellulose. Fuel 2014, 124, 183–189. [Google Scholar] [CrossRef]
- Sandhu, S.K.; Oberoi, H.S.; Babbar, N.; Miglani, K.; Chadha, B.S.; Nanda, D.K. Two-Stage Statistical Medium Optimization for Augmented Cellulase Production via Solid-State Fermentation by Newly Isolated Aspergillus niger HN-1 and Application of Crude Cellulase Consortium in Hydrolysis of Rice Straw. J. Agric. Food Chem. 2013, 61, 12653–12661. [Google Scholar] [CrossRef] [PubMed]
- Saratale, G.D.; Kshirsagar, S.D.; Sampange, V.T.; Saratale, R.G.; Oh, S.E.; Govindwar, S.P.; Oh, M.K. Cellulolytic Enzymes Production by Utilizing Agricultural Wastes Under Solid State Fermentation and its Application for Biohydrogen Production. Appl. Biochem. Biotechnol. 2014, 174, 2801–2817. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Dutt, D.; Gautam, A. Production of crude enzyme from Aspergillus nidulans AKB-25 using black gram residue as the substrate and its industrial applications. J. Genet. Eng. Biotechnol. 2016, 14, 107–118. [Google Scholar] [CrossRef]
- Anto, H.; Trivedi, U.B.; Patel, K.C. Glucoamylase production by solid-state fermentation using rice flake manufacturing waste products as substrate. Bioresour. Technol. 2006, 97, 1161–1166. [Google Scholar] [CrossRef] [PubMed]
- Melikoglu, M.; Lin, C.S.K.; Webb, C. Stepwise optimisation of enzyme production in solid state fermentation of waste bread pieces. Food Bioprod. Process. 2013, 91, 638–646. [Google Scholar] [CrossRef]
- Saxena, R.; Singh, R. Amylase production by solid-state fermentation of agro-industrial wastes using Bacillus sp. Braz. J. Microbiol. 2011, 42, 1334–1342. [Google Scholar] [CrossRef] [PubMed]
- Pandi, A.; Ramalingam, S.; Rao, R. Inexpensive α-amylase production and application for fiber splitting in leather processing. RSC Adv. 2016, 6, 33170–33176. [Google Scholar] [CrossRef]
- Singh, S.; Singh, S.; Bali, V.; Sharma, L.; Mangla, J. Production of fungal amylases using cheap, readily available agriresidues, for potential application in textile industry. BioMed Res. Int. 2014, 2014, 1–9. [Google Scholar] [CrossRef] [PubMed]
- De Castro, R.J.S.; Sato, H.H. Production and biochemical characterization of protease from Aspergillus oryzae: An evaluation of the physical-chemical parameters using agroindustrial wastes as supports. Biocatal. Agric. Biotechnol. 2014, 3, 20–25. [Google Scholar] [CrossRef]
- Mahanta, N.; Gupta, A.; Khare, S.K. Production of protease and lipase by solvent tolerant Pseudomonas aeruginosa PseA in solid-state fermentation using Jatropha curcas seed cake as substrate. Bioresour. Technol. 2008, 99, 1729–1735. [Google Scholar] [CrossRef] [PubMed]
- Novelli, P.K.; Barros, M.M.; Fleuri, L.F. Novel inexpensive fungi proteases: Production by solid state fermentation and characterization. Food Chem. 2016, 198, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Prakasham, R.S.; Rao, C.S.; Sarma, P.N. Green gram husk-an inexpensive substrate for alkaline protease production by Bacillus sp. in solid-state fermentation. Bioresour. Technol. 2006, 97, 1449–1454. [Google Scholar] [PubMed]
- Da Silva, R.R.; de Freitas Cabral, T.P.; Rodrigues, A.; Cabral, H. Production and partial characterization of serine and metallo peptidases secreted by Aspergillus fumigatus Fresenius in submerged and solid state fermentation. Braz. J. Microbiol. 2013, 44, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghavan, P.; Lazarus, S.; Vincent, S.G.P. De-hairing protease production by an isolated Bacillus cereus strain AT under solid-state fermentation using cow dung: Biosynthesis and properties. Saudi J. Biol. Sci. 2014, 21, 27–34. [Google Scholar] [CrossRef] [PubMed]
- El-Bakry, M.; Gea, T.; Sánchez, A. Inoculation effect of thermophilic microorganisms on protease production through solid-state fermentation under non-sterile conditions at lab and bench scale (SSF). Bioprocess Biosyst. Eng. 2016, 39, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Ansari, M.W.; Anwar, M.S.; Agrawal, R.; Agrawal, S. Alkaline protease from Thermoactinomyces sp. RS1 mitigates industrial pollution. Protoplasma 2014, 251, 711–718. [Google Scholar] [PubMed]
- Paul, T.; Das, A.; Mandal, A.; Jana, A.; Maity, C.; Adak, A.; Halder, S.K.; DasMohapatra, P.K.; Pati, B.R.; Mondal, K.C. Effective dehairing properties of keratinase from Paenibacillus woosongensis TKB2 obtained under solid state fermentation. Waste Biomass Valoriz. 2014, 5, 97–107. [Google Scholar] [CrossRef]
- Santis-Navarro, A.; Gea, T.; Barrena, R.; Sánchez, A. Production of lipases by solid state fermentation using vegetable oil-refining wastes. Bioresour. Technol. 2011, 102, 10080–10084. [Google Scholar] [CrossRef] [PubMed]
- Silveira, E.A.; Tardioli, P.W.; Farinas, C.S. Valorization of Palm Oil Industrial Waste as Feedstock for Lipase Production. Appl. Biochem. Biotechnol. 2016, 179, 558–571. [Google Scholar] [CrossRef] [PubMed]
- Herculano, P.N.; Moreira, K.A.; Bezerra, R.P.; Porto, T.S.; de Souza-Motta, C.M.; Porto, A.L.F. Potential application of waste from castor bean (Ricinus communis L.) for production for xylanase of interest in the industry. 3 Biotech 2016, 6, 144–154. [Google Scholar]
- Pandya, J.J.; Gupte, A. Production of xylanase under solid-state fermentation by Aspergillus tubingensis JP-1 and its application. Bioprocess Biosyst. Eng. 2012, 35, 769–779. [Google Scholar] [CrossRef] [PubMed]
- Asha Poorna, C.; Prema, P. Production of cellulase-free endoxylanase from novel alkalophilic thermotolerent Bacillus pumilus by solid-state fermentation and its application in wastepaper recycling. Bioresour. Technol. 2007, 98, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Zimbardi, A.L.R.L.; Sehn, C.; Meleiro, L.P.; Souza, F.H.M.; Masui, D.C.; Nozawa, M.S.F.; Guimarães, L.H.S.; Jorge, J.A.; Furriel, R.P.M. Optimization of β-Glucosidase, β-Xylosidase and Xylanase Production by Colletotrichum graminicola under Solid-State Fermentation and Application in Raw Sugarcane Trash Saccharification. Int. J. Mol. Sci. 2013, 14, 2875–2902. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Q.; Chen, X.M.; Chen, T.X.; Xu, X.M.; Jin, Z.Y. Extraction optimization of inulinase obtained by solid state fermentation of Aspergillus ficuum JNSP5-06. Carbohydr. Polym. 2011, 85, 446–451. [Google Scholar] [CrossRef]
- Mazutti, M.; Bender, J.P.; Treichel, H.; Luccio, M. Di Optimization of inulinase production by solid-state fermentation using sugarcane bagasse as substrate. Enzyme Microb. Technol. 2006, 39, 56–59. [Google Scholar] [CrossRef]
- Martínez-Morales, F.; Bertrand, B.; Pasión Nava, A.A.; Tinoco, R.; Acosta-Urdapilleta, L.; Trejo-Hernández, M.R. Production, purification and biochemical characterization of two laccase isoforms produced by Trametes versicolor grown on oak sawdust. Biotechnol. Lett. 2015, 37, 391–396. [Google Scholar] [CrossRef] [PubMed]
- El-Gindy, A.A.; Saad, R.R.; Fawzi, E.M. Purification of β-xylosidase from Aspergillus tamarii using ground oats and a possible application on the fermented hydrolysate by Pichia stipitis. Ann. Microbiol. 2015, 65, 965–974. [Google Scholar] [CrossRef]
- López, J.A.; Lázaro, C.D.C.; Castilho, L.D.R.; Freire, D.M.G.; Castro, A.M.H. De Characterization of multienzyme solutions produced by solid-state fermentation of babassu cake, for use in cold hydrolysis of raw biomass. Biochem. Eng. J. 2013, 77, 231–239. [Google Scholar] [CrossRef]
- Kriaa, M.; Kammoun, R. Producing Aspergillus tubingensis CTM507 Glucose oxidase by Solid state fermentation versus submerged fermentation: Process optimization and enzyme stability by an intermediary metabolite in relation with diauxic growth. J. Chem. Technol. Biotechnol. 2016, 91, 1540–1550. [Google Scholar] [CrossRef]
- Ali, H.K.Q.; Zulkali, M.M.D. Utilization of Agro-Residual Ligno-Cellulosic Substances by Using Solid State Fermentation: A Review. Croat. J. Food Technol. Biotechnol. Nutr. 2011, 6, 5–12. [Google Scholar]
- Bezalwar, P.; Gomashe, A.V.; Sanap, H.M.; Gulhane, P.A. Original Research Article Production and Optimization of Citric Acid by Aspergillus niger using Fruit Pulp Waste Aspergillus niger culture maintenance. Int. J. Curr. Microbiol. Appl. Sci. 2013, 2, 347–352. [Google Scholar]
- Goud, K.H.; Srilakshmi, A.; Kumar, P.A.; Narasimha, G. Citric acid production by aspergillus niger through solid state fermentation using fruit wastes. Biotechnol. Indian J. 2012, 6, 93–96. [Google Scholar]
- Kumar, D.; Jain, V.K.; Shanker, G.; Srivastava, A. Utilization of fruits waste for citric acid production by solid state fermentation. Process Biochem. 2003, 38, 1725–1729. [Google Scholar] [CrossRef]
- Dhillon, G.S.; Brar, S.K.; Verma, M.; Tyagi, R.D. Utilization of different agro-industrial wastes for sustainable bioproduction of citric acid by Aspergillus niger. Biochem. Eng. J. 2011, 54, 83–92. [Google Scholar] [CrossRef]
- Hamdy, H.S. Citric acid production by Aspergillus niger grown on orange peel medium fortified with cane molasses. Ann. Microbiol. 2013, 63, 267–278. [Google Scholar] [CrossRef]
- Karthikeyan, A.; Sivakumar, N. Citric acid production by Koji fermentation using banana peel as a novel substrate. Bioresour. Technol. 2010, 101, 5552–5556. [Google Scholar] [CrossRef] [PubMed]
- Kareem, S.O.; Rahman, R.A. Utilization of banana peels for citric acid production by Aspergillus niger. Agric. Biol. J. N. Am. 2011, 4, 384–387. [Google Scholar] [CrossRef]
- Torrado, A.M.; Cortes, S.; Salgado, J.M.; Max, B.; Rodriguez, N.; Bibbins, B.P.; Converti, A.; Dominguez, J.M. Citric acid production from orange peel wastes by solid-state fermentation. Braz. J. Microbiol. 2011, 42, 394–409. [Google Scholar] [CrossRef] [PubMed]
- Krishna, C. Solid-State Fermentation Systems—An Overview. Crit. Rev. Biotechnol. 2005, 25, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.Z.; Bari, M.N.; Muyibi, S.A.; Jamal, P.; Al-Mamun, A. Development of culture inoculum for scale-up production of citric acid from oil palm empty fruit bunches by Aspergillus niger. Procedia Environ. Sci. 2011, 8, 396–402. [Google Scholar] [CrossRef]
- Yadegary, M.; Hamidi, A.; Alavi, S.A.; Khodaverdi, E.; Sattari, S.; Bagherpour, G.; Yahaghi, E. Citric acid production from sugarcane bagasse through solid state fermentation method using Aspergillus niger mold and optimization of citric acid production by taguchi method. Jundishapur J. Microbiol. 2013, 6, 1–6. [Google Scholar] [CrossRef]
- Prado, F.C.; Vandenberghe, L.P.S.; Woiciechowski, A.L.; Rodrígues-Léon, J.A.; Soccol, C.R. Citric acid production by solid-state fermentation on a semi-pilot scale using different percentages of treated cassava bagasse. Braz. J. Chem. Eng. 2005, 22, 547–555. [Google Scholar] [CrossRef]
- Schneider, M.; Zimmer, G.F.; Cremonese, E.B.; De, C.D.S.S.R.; Corbellini, V.A. By-products from the biodiesel chain as a substrate to citric acid production by solid-state fermentation. Waste Manag. Res. 2014, 32, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Hayihama, S.; Suwazono, W. The Use of Rhizopus sp. mutant for Lactic Acid Production by Solid State Fermentation. KKU Res. J. 2016, 22, 52–58. [Google Scholar]
- John, R.P.; Nampoothiri, K.M.; Pandey, A. Solid-state fermentation for L-lactic acid production from agro wastes using Lactobacillus delbrueckii. Process Biochem. 2006, 41, 759–763. [Google Scholar] [CrossRef]
- Rojan, P.J.; Nampoothiri, K.M.; Nair, A.S.; Pandey, A. L(+)-lactic acid production using Lactobacillus casei in solid-state fermentation. Biotechnol. Lett. 2005, 27, 1685–1688. [Google Scholar] [CrossRef] [PubMed]
- Qi, B.; Yao, R. L-Lactic acid production from Lactobacillus casei by solid state fermentation using rice straw. BioResources 2007, 2, 419–429. [Google Scholar]
- Altaf, M.; Naveena, B.J.; Venkateshwar, M.; Kumar, E.V.; Reddy, G. Single step fermentation of starch to L(+) lactic acid by Lactobacillus amylophilus GV6 in SSF using inexpensive nitrogen sources to replace peptone and yeast extract—Optimization by RSM. Process Biochem. 2006, 41, 465–472. [Google Scholar] [CrossRef]
- Gowdhaman, D.; Sugumaran, K.R.; Ponnusami, V. Optimization of lactic acid production from tea waste by lactobacillus plantarum MTCC 6161 in solid state fermentation by central composite design. Int. J. ChemTech Res. 2012, 4, 143–148. [Google Scholar]
- Ghosh, M.K.; Ghosh, U.K. Utilization of Pine needles as bed material in solid state fermentation for production of Lactic acid by Lactobacillus strains. BioResources 2011, 6, 1556–1575. [Google Scholar]
- Ghosh, U.K.; Ghosh, M.K. Utilization of Wheat Bran as Bed Material in Solid State Bacterial Production of Lactic Acid with Various Nitrogen Sources. Int. J. Biol. Biomol. Agric. Food Biotechnol. Eng. 2012, 6, 90–93. [Google Scholar]
- El-Naggar, N.E.A.; El-Hersh, M.S. Organic acids associated with saccharification of cellulosic wastes during solid-state fermentation. J. Microbiol. 2011, 49, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Mai, H.T.N.; Lee, K.M.; Choi, S.S. Enhanced oxalic acid production from corncob by a methanol-resistant strain of Aspergillus niger using semi solid-sate fermentation. Process Biochem. 2016, 51, 9–15. [Google Scholar] [CrossRef]
- De Vrije, T.; Antoine, N.; Buitelaar, R.M.; Bruckner, S.; Dissevelt, M.; Durand, A.; Gerlagh, M.; Jones, E.E.; Lüth, P.; Oostra, J.; et al. The fungal biocontrol agent Coniothyrium minitans: Production by solid-state fermentation, application and marketing. Appl. Microbiol. Biotechnol. 2001, 56, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Oostra, J.; Tramper, J.; Rinzema, A. Model-based bioreactor selection for large-scale solid-state cultivation of Coniothyrium minitans spores on oats. Enzyme Microb. Technol. 2000, 27, 652–663. [Google Scholar] [CrossRef]
- Viccini, G.; Mannich, M.; Capalbo, D.M.F.; Valdebenito-Sanhueza, R.; Mitchell, D.A. Spore production in solid-state fermentation of rice by Clonostachys rosea, a biopesticide for gray mold of strawberries. Process Biochem. 2007, 42, 275–278. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Zhou, Y.; Ge, Y. Spore Production of Clonostachys rosea in a New Solid-state Fermentation Reactor. Appl. Biochem. Biotechnol. 2014, 174, 2951–2959. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Kumar, P.; Malik, A. Suitability of agricultural by-products as production medium for spore production by Beauveria bassiana HQ917687. Int. J. Recycl. Org. Waste Agric. 2016, 5, 179–184. [Google Scholar] [CrossRef]
- Vijith, C.C.; Thota, S.G.; Vivek, A.T.; Gopinathan, C. Improved Bacillus thuringiensisbased biopesticide production using cheap carbon and nitrogen sources by solid state fermentation technique. IOSR J. Environ. Sci. Food Technol. 2016, 10, 49–53. [Google Scholar]
- Jisha, V.N.; Benjamin, S. Solid-State Fermentation for the Concomitant Production of δ-Endotoxin and Endospore from Bacillus thuringiensis subsp. kurstaki. Adv. Biosci. Biotechnol. 2014, 5, 797–804. [Google Scholar] [CrossRef]
- Smitha, R.B.; Jisha, V.N.; Pradeep, S.; Josh, M.S.; Benjamin, S. Potato flour mediated solid-state fermentation for the enhanced production of Bacillus thuringiensis-toxin. J. Biosci. Bioeng. 2013, 116, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.; Zhou, S.; Wang, Y.; Liu, Z.; Xu, R. Cost-effective production of Bacillus thuringiensis biopesticides by solid-state fermentation using wastewater sludge: Effects of heavy metals. Bioresour. Technol. 2011, 102, 4820–4826. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Qiu, L.; Gong, A.; Cao, Y.; Wang, B. Solid-state Fermentation of Kitchen Waste for Production of Bacillus thuringiensis-based Bio-pesticide. BioResources 2013, 8, 1124–1135. [Google Scholar] [CrossRef]
- Ballardo, C.; Abraham, J.; Barrena, R.; Artola, A.; Gea, T.; Sánchez, A. Valorization of soy waste through SSF for the production of compost enriched with Bacillus thuringiensis with biopesticide properties. J. Environ. Manag. 2016, 169, 126–131. [Google Scholar] [CrossRef] [PubMed]
- De, S.; Malik, S.; Ghosh, A.; Saha, R.; Saha, B. A Review on Natural Surfactants. RSC Adv. 2015, 5, 65757–65767. [Google Scholar] [CrossRef]
- Winterburn, J.B.; Martin, P.J. Foam mitigation and exploitation in biosurfactant production. Biotechnol. Lett. 2012, 34, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Velioglu, Z.; Urek, R.O. Physicochemical and structural characterization of biosurfactant produced by Pleurotus djamor in solid-state fermentation. Biotechnol. Bioprocess Eng. 2016, 21, 430–438. [Google Scholar] [CrossRef]
- Zouari, R.; Ellouze-Chaabouni, S.; Ghribi-Aydi, D. Optimization of Bacillus subtilis SPB1 Biosurfactant Production Under Solid-state Fermentation Using By-products of a Traditional Olive Mill Factory. Achieve Life Sci. 2014, 8, 162–169. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, G.; Luo, Y.; Ran, W.; Shen, Q. Production of lipopeptides by Bacillus amyloliquefaciens XZ-173 in solid state fermentation using soybean flour and rice straw as the substrate. Bioresour. Technol. 2012, 112, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zhang, F.; Wei, Z.; Ran, W.; Shen, Q. The usage of rice straw as a major substrate for the production of surfactin by Bacillus amyloliquefaciens XZ-173 in solid-state fermentation. J. Environ. Manag. 2013, 127, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Slivinski, C.T.; Mallmann, E.; De Araújo, J.M.; Mitchell, D.A.; Krieger, N. Production of surfactin by Bacillus pumilus UFPEDA 448 in solid-state fermentation using a medium based on okara with sugarcane bagasse as a bulking agent. Process Biochem. 2012, 47, 1848–1855. [Google Scholar] [CrossRef]
- Sitohy, M.Z.; Rashad, M.M.; Sharobeem, S.F.; Mahmoud, A.E.; Nooman, M.U.; Kashef, A.S. Al Bioconversion of soy processing waste for production of surfactants. J. Microbiol. 2010, 4, 2811–2821. [Google Scholar]
- Das, K.; Mukherjee, A.K. Comparison of lipopeptide biosurfactants production by Bacillus subtilis strains in submerged and solid state fermentation systems using a cheap carbon source: Some industrial applications of biosurfactants. Process Biochem. 2007, 42, 1191–1199. [Google Scholar] [CrossRef]
- Parekh, V.J.; Pandit, A.B. Solid state fermentation (SSF) for the production of sophorolipids from Starmerella bombicola NRRL Y-17069 using glucose, wheat bran and oleic acid. Curr. Trends Biotechnol. Pharm. 2012, 6, 418–424. [Google Scholar]
- Jimémenz-Peñalver, P.; Gea, T.; Sánchez, A.; Font, X. Production of sophorolipids from winterization oil cake by Solid-state fermentation: Optimization, monitoring and effect of mixing. Biochem. Eng. J. 2016, 115, 93–100. [Google Scholar] [CrossRef]
- Camilios-Neto, D.; Bugay, C.; De Santana-Filho, A.P.; Joslin, T.; De Souza, L.M.; Sassaki, G.L.; Mitchell, D.A.; Krieger, N. Production of rhamnolipids in solid-state cultivation using a mixture of sugarcane bagasse and corn bran supplemented with glycerol and soybean oil. Appl. Microbiol. Biotechnol. 2011, 89, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, L.A.; Toro, M.E.; Vazquez, F.; Correa-Daneri, M.L.; Gouiric, S.C.; Vallejo, M.D. Bioethanol production from grape and sugar beet pomaces by solid-state fermentation. Int. J. Hydrogen Energy 2010, 35, 5914–5917. [Google Scholar] [CrossRef]
- Chintagunta, A.D.; Jacob, S.; Banerjee, R. Integrated bioethanol and biomanure production from potato waste. Waste Manag. 2016, 49, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Yan, J.; Feng, Q.; Li, P.; Zhang, L.; Chang, S.; Li, S. A novel wild-type saccharomyces cerevisiaestrain TSH1 in scaling-up of solid-state fermentation of ethanol from sweet sorghum stalks. PLoS ONE 2014, 9, 94480–94490. [Google Scholar] [CrossRef] [PubMed]
- Uçkun Kiran, E.; Liu, Y. Bioethanol production from mixed food waste by an effective enzymatic pretreatment. Fuel 2015, 159, 463–469. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Xu, J.; Sun, Y.; Yuan, Z.; Xie, J. Consolidated bioprocess for bioethanol production with alkali-pretreated sugarcane bagasse. Appl. Energy 2015, 157, 517–522. [Google Scholar] [CrossRef]
- Ang, S.K.; Adibah, Y.; Abd-Aziz, S.; Madihah, M.S. Potential Uses of Xylanase-Rich Lignocellulolytic Enzymes Cocktail for Oil Palm Trunk (OPT) Degradation and Lignocellulosic Ethanol Production. Energy Fuels 2015, 29, 5103–5116. [Google Scholar] [CrossRef]
- Thomas, L.; Parameswaran, B.; Pandey, A. Hydrolysis of pretreated rice straw by an enzyme cocktail comprising acidic xylanase from Aspergillus sp. for bioethanol production. Renew. Energy 2016, 98, 9–15. [Google Scholar] [CrossRef]
- Suresh, S.V.; Srujana, S.; Muralidharan, A. Production of bioethanol by Solid State Fermentation using paddy straw as a substrate. Int. J. Adv. Res. 2015, 3, 212–215. [Google Scholar]
- Ingale, S.; Joshi, S.J.; Gupte, A. Production of bioethanol using agricultural waste: Banana pseudo stem. Braz. J. Microbiol. 2014, 45, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Pirota, R.D.P.B.; Delabona, P.S.; Farinas, C.S. Simplification of the Biomass to Ethanol Conversion Process by Using the Whole Medium of Filamentous Fungi Cultivated Under Solid-State Fermentation. Bioenergy Res. 2014, 7, 744–752. [Google Scholar] [CrossRef]
- Singhania, R.R.; Saini, R.; Adsul, M.; Saini, J.K.; Mathur, A.; Tuli, D. An integrative process for bio-ethanol production employing SSF produced cellulase without extraction. Biochem. Eng. J. 2015, 102, 45–48. [Google Scholar] [CrossRef]
- Jain, A.; Morlok, C.K.; Henson, J.M. Comparison of solid-state and submerged-state fermentation for the bioprocessing of switchgrass to ethanol and acetate by Clostridium phytofermentans. Appl. Microbiol. Biotechnol. 2013, 97, 905–917. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Li, J.; Li, S.; Du, R.; Jiang, Y.; Fan, G.; Zhao, G.; Chang, S. A cost-effective integrated process to convert solid-state fermented sweet sorghum bagasse into cellulosic ethanol. Appl. Energy 2014, 115, 331–336. [Google Scholar] [CrossRef]
- Yu, M.; Li, J.; Chang, S.; Zhang, L.; Mao, Y.; Cui, T.; Yan, Z.; Luo, C.; Li, S. Bioethanol production using the sodium hydroxide pretreated sweet sorghum bagasse without washing. Fuel 2016, 175, 20–25. [Google Scholar] [CrossRef]
- Izawa, N.; Kudo, M.; Nakamura, Y.; Mizukoshi, H.; Kitada, T.; Sone, T. Production of aroma compounds from whey using Wickerhamomyces pijperi. AMB Express 2015, 5, 23. [Google Scholar] [CrossRef] [PubMed]
- Ben Akacha, N.; Gargouri, M. Microbial and enzymatic technologies used for the production of natural aroma compounds: Synthesis, recovery modeling, and bioprocesses. Food Bioprod. Process. 2015, 94, 675–706. [Google Scholar] [CrossRef]
- Madrera, R.R.; Bedriñana, R.P.; Valles, B.S. Production and characterization of aroma compounds from apple pomace by solid-state fermentation with selected yeasts. LWT Food Sci. Technol. 2015, 64, 1342–1353. [Google Scholar] [CrossRef]
- Aggelopoulos, T.; Katsieris, K.; Bekatorou, A.; Pandey, A.; Banat, I.M.; Koutinas, A.A. Solid state fermentation of food waste mixtures for single cell protein, aroma volatiles and fat production. Food Chem. 2014, 145, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Pedroni Medeiros, A.B.; Christen, P.; Roussos, S.; Gern, J.C.; Soccol, C.R. Coffee residues as substrates for aroma production by Ceratocystis fimbriata in solid state fermentation. Braz. J. Microbiol. 2003, 34, 245–248. [Google Scholar] [CrossRef]
- Medeiros, A.B.P.; Pandey, A.; Vandenberghe, L.P.S.; Pastorel, G.M.; Soccol, C.R. Production and recovery of aroma compounds produced by solid-state fermentation using different adsorbents. Food Technol. Biotechnol. 2006, 44, 47–52. [Google Scholar]
- Soares, M.; Christen, P.; Pandey, A. Fruity flavor production by Ceratocystis fimbriata grown on coffe husk in solid-state fermentation. Process Biochem. 2000, 35, 857–861. [Google Scholar] [CrossRef]
- Rossi, S.C.; Vandenberghe, L.P.S.; Pereira, B.M.P.; Gago, F.D.; Rizzolo, J.A.; Pandey, A.; Soccol, C.R.; Medeiros, A.B.P. Improving fruity aroma production by fungi in SSF using citric pulp. Food Res. Int. 2009, 42, 484–486. [Google Scholar] [CrossRef]
- Medeiros, A.B.P.; Pandey, A.; Christen, P.; Fontoura, P.S.G.; de Freitas, R.J.S.; Soccol, C.R. Aroma compounds produced by Kluyveromyces marxianus in solid state fermentation on a packed bed column bioreactor. World J. Microbiol. Biotechnol. 2001, 17, 767–771. [Google Scholar] [CrossRef]
- De Aráujo, Á.; Pastore, G.M.; Berger, R.G. Production of coconut aroma by fungi cultivation in solid-state fermentation. Appl. Biochem. Biotechnol. 2002, 98–100, 747–751. [Google Scholar]
- Fadel, H.H.M.; Mahmoud, M.G.; Asker, M.M.S.; Lotfy, S.N. Characterization and evaluation of coconut aroma produced by Trichoderma viride EMCC-107 in solid state fermentation on sugarcane bagasse. Electron. J. Biotechnol. 2015, 18, 5–9. [Google Scholar] [CrossRef]
- Da Penha, M.P.; da Rocha Leão, M.H.M.; Leite, S.G.F. Sugarcane bagasse as support for the production of coconut aroma by solid state fermentation (SSF). BioResources 2012, 7, 2366–2375. [Google Scholar] [CrossRef]
- Kabbaj, W.; Breheret, S.; Guimberteau, J.; Talou, T.; Olivier, J.-M.; Bensoussan, M.; Sobal, M.; Roussos, A.S. Comparison of Volatile Compound Production in Fruit Body and in Mycelium of Pleurotus ostreatus Identified by Submerged and Solid-State Cultures. Appl. Biochem. Biotechnol. 2002, 102–103, 463–469. [Google Scholar] [CrossRef]
- Omarini, A.; Dambolena, J.S.; Lucini, E.; Jaramillo Mejía, S.; Albertó, E.; Zygadlo, J.A. Biotransformation of 1,8-cineole by solid-state fermentation of Eucalyptus waste from the essential oil industry using Pleurotus ostreatus and Favolus tenuiculus. Folia Microbiol. 2016, 61, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Zidehsaraei, A.Z.; Moshkelani, M.; Amiri, M.C. An innovative simultaneous glucoamylase extraction and recovery using colloidal gas aphrons. Sep. Purif. Technol. 2009, 67, 8–13. [Google Scholar] [CrossRef]
- Dhillon, G.S.; Brar, S.K.; Kaur, S.; Verma, M. Bioproduction and extraction optimization of citric acid from Aspergillus niger by rotating drum type solid-state bioreactor. Ind. Crops Prod. 2013, 41, 78–84. [Google Scholar] [CrossRef]
- Pirota, R.D.P.B.; Miotto, L.S.; Delabona, P.S.; Farinas, C.S. Improving the extraction conditions of endoglucanase produced by Aspergillus niger under solid-state fermentation. Braz. J. Chem. Eng. 2013, 30, 117–123. [Google Scholar] [CrossRef]
- Arantes, V.; Silva, E.M.; Milagres, A.M.F. Optimal recovery process conditions for manganese-peroxidase obtained by solid-state fermentation of eucalyptus residue using Lentinula edodes. Biomass Bioenergy 2011, 35, 4040–4044. [Google Scholar] [CrossRef]
- Chandra, M.S.; Viswanath, B.; Reddy, B.R. Optimization of extraction of beta-endoglucanase from the fermented bran of Aspergillus niger. Indian J. Microbiol. 2010, 50, S122–S126. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.A.; Mostafa, F.A. Utilization of orange bagasse and molokhia stalk for production of pectinase enzyme. Braz. J. Chem. Eng. 2013, 30, 449–456. [Google Scholar] [CrossRef]
- Rodríguez-Fernández, D.E.; Rodríguez-León, J.A.; de Carvalho, J.C.; Thomaz-Soccol, V.; Parada, J.L.; Soccol, C.R. Recovery of phytase produced by solid-state fermentation on citrus peel. Braz. Arch. Biol. Technol. 2010, 53, 1487–1496. [Google Scholar] [CrossRef]
- Dey, T.B.; Kuhad, R.C. Enhanced production and extraction of phenolic compounds from wheat by solid-state fermentation with Rhizopus oryzae RCK2012. Biotechnol. Rep. 2014, 4, 120–127. [Google Scholar]
- Salariato, D.; Diorio, L.A.; Mouso, N.; Forchiassin, F. Extraction and characterization of polygalacturonase of fomes sclerodermeus produced by solid-state fermentation. Rev. Argent. Microbiol. 2010, 42, 57–62. [Google Scholar] [PubMed]
- Szabo, O.E.; Csiszar, E.; Koczka, B.; Kiss, K. Ultrasonically assisted single stage and multiple extraction of enzymes produced by Aspergillus oryzae on a lignocellulosic substrate with solid-state fermentation. Biomass Bioenergy 2015, 75, 161–169. [Google Scholar] [CrossRef]
- Rezaei, F.; Joh, L.D.; Kashima, H.; Reddy, A.P.; Vandergheynst, J.S. Selection of conditions for cellulase and xylanase extraction from switchgrass colonized by acidothermus cellulolyticus. Appl. Biochem. Biotechnol. 2011, 164, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Poletto, P.; Borsói, C.; Zeni, M.; Moura, M. Downstream processing of pectinase produced by Aspergillus niger in solid state cultivation and its application to fruit juices clarification. Food Sci. Technol. 2015, 35, 391–397. [Google Scholar] [CrossRef]
- Rashad, M.M.; Nooman, M.U.; Ali, M.M.; Mahmoud, A.E. Production, characterization and anticancer activity of Candida bombicola sophorolipids by means of solid state fermentation of sunflower oil cake and soybean oil. Grasas Aceites 2014, 65, 1–11. [Google Scholar] [CrossRef]
- Hu, Y.; Ju, L.K. Purification of lactonic sophorolipids by crystallization. J. Biotechnol. 2001, 87, 263–272. [Google Scholar] [CrossRef]
- Bhavsar, K.; Ravi Kumar, V.; Khire, J.M. Downstream processing of extracellular phytase from Aspergillus niger: Chromatography process vs. aqueous two phase extraction for its simultaneous partitioning and purification. Process Biochem. 2012, 47, 1066–1072. [Google Scholar]
- Kachrimanidou, V.; Kopsahelis, N.; Vlysidis, A.; Papanikolaou, S.; Kookos, I.K.; Monje Martinez, B.; Escrig Rondan, M.C.; Koutinas, A.A. Downstream separation of poly(hydroxyalkanoates) using crude enzyme consortia produced via solid state fermentation integrated in a biorefinery concept. Food Bioprod. Process. 2016, 100, 323–334. [Google Scholar] [CrossRef]
- Narra, M.; Balasubramanian, V. Utilization of solid and liquid waste generated during ethanol fermentation process for production of gaseous fuel through anaerobic digestion—A zero waste approach. Bioresour. Technol. 2015, 180, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, G.S.; Kaur, S.; Sarma, S.J.; Brar, S.K. Integrated process for fungal citric acid fermentation using apple processing wastes and sequential extraction of chitosan from waste stream. Ind. Crops Prod. 2013, 50, 346–351. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Jin, B.; Kelly, J.M. Production of lactic acid from renewable materials by Rhizopus fungi. Biochem. Eng. J. 2007, 35, 251–263. [Google Scholar] [CrossRef]
- Zhuang, J.; Marchant, M.A.; Nokes, S.E.; Strobel, H.J. Economic analysis of cellulase production methods for bio-ethanol. Appl. Eng. Agric. 2007, 23, 679–687. [Google Scholar] [CrossRef]
- De Castro, A.M.; Carvalho, D.F.; Freire, D.M.G.; Castilho, L.D.R. Economic analysis of the production of amylases and other hydrolases by Aspergillus awamori in solid-state fermentation of Babassu Cake. Enzyme Res. 2010, 2010, 1–9. [Google Scholar]

| Category of Organic Waste | Type of Products/Processes | Waste Materials/Residues | Potential Use | References |
|---|---|---|---|---|
| Municipal/domestic food waste | Kitchen waste | Preparation waste, leftover food, sludge, cocoyam peels | Biopesticides, animal feedstuff, organic acids, antibiotics | [15,16] |
| Commercial/marke/hotel | Used coffee grounds, used tea bags, waste bread, leftover, expired foods, sludge | Enzymes, animal feedstuff, biopesticides, bioethanol, bioplastics | [17,18] | |
| Industrial organic waste | Animal products/tannery/slaughterhouse | Skin, hides, fleshing wastes, fats, horns, shaving wastes, bones, liver, intestines | Enzymes, animal feedstuff, glues, surfactants, lubricants, fillers | [19,20,21,22] |
| Paper/wood industry | Pulp, sawdust | Enzymes, bioethanol, biofuels | [23] | |
| Sugar industry | Molasses | Enzymes, fructo-oligosaccharides | [24] | |
| Poultry processing | Skin, blood, fats, hairs, feathers, bones, liver, intestines, wings, trimmed organs | Enzymes, animal feedstuff, biofertilizers | [25] | |
| Marine products processing | Shells, roes, pincers, trimmed parts | Enzymes, bioactive compounds | [26,27] | |
| Cereals and spices processing | Husk, hull, chaff, stalks, residues | Enzymes, activated carbon | [28] | |
| Fruits and vegetables processing | Skin, peels, pomace, fiber, kernel, stones, seeds | Pectinolytic enzymes, biofuels, animal feed, organic acids | [29,30] | |
| Nuts processing | Shells, coir, pith | Biopulping, biochar, activated carbon | [31] | |
| Agricultural organic waste | Corn, wheat, rice, soya, coffee, sugarcane, barley | Fiber, meal, straw, bran, husk, pulp, bagasse | Enzymes, animal feedstuff, biofuels, bioethanol, furfural, chemical feedstock, biopolymers, organic acids | [1,32] |
| Cattle, broiler | Fleshing wastes, dung, litter | Animal glue, animal feed supplement, methane production, biochar, activated carbon, biofertilizers, biopesticide | [33,34,35] | |
| Fruits and vegetables | Seeds, peels, pomace, husks | Enzymes, animal feed, biofuels, compost | [31,36] | |
| Oils and oil seeds | Shells, husks, fibers, sludge, presscake | Bioethanol, enzymes, biofuels, biofertilizers, activated carbon | [37] | |
| Coconuts | Fibers, shell, kernel | Resins, pigments, fillers, mats, activated carbon, tanning materials | [38,39,40] |
| Enzyme | Microorganism | Substrate | Reference |
|---|---|---|---|
| cellulase | T. reesei | Municipal solid waste | [65] |
| Thermoascus aurantiacus | Jatropha deoiled seed cake | [13] | |
| A. niger | kitchen waste | [64] | |
| Penicillium sp. | empty fruit bunches | [43] | |
| T. reesei | wheat bran | [94] | |
| A. niger | groundnut husks | [95] | |
| Trichoderma viridae | banana peel | [96] | |
| Candida tropicalis, A. oryzae | Ginkgo biloba residues | [97] | |
| A. niger | wheat bran | [98] | |
| Trametes hirsuta | wheat bran | [99] | |
| T. reesei | soybean bran | [100] | |
| A. fumigatus | lignocellulosic materials | [73] | |
| T. harzianum | wheat bran | [101] | |
| T. asperellum | lignocellulosic materials | [102] | |
| A. niger | rice straw and wheat bran | [103] | |
| Phanerochaete chrysosporium | grass powder | [104] | |
| A.nidulans | black gram residues | [105] | |
| glucoamylase | Aspergillus sp. | banana peel, pineapple peel | [106] |
| A. awamori | domestic food waste | [61] | |
| A. awamori | bread waste | [62,107] | |
| amylase | Bacillus sp. | mustard oil seed cake | [108] |
| Thermomyces sp. | soy and bread waste | [63] | |
| Bacillus subtilis | wheat bran and wheat rava | [109] | |
| A. fumigatus | wheat bran | [110] | |
| protease | A. niger | wheat bran, soybean meal, cottonseed meal, orange peel | [111] |
| Pseudomonas aeruginosa | Jatropha curcas seed cake | [112] | |
| A. awamori | bread waste | [62,107] | |
| A. oryzae | wheat bran, soya bran | [113] | |
| Bacillus sp. | green gram husk | [114] | |
| A. fumigatus | wheat bran | [115] | |
| B. cereus | cow dung | [116] | |
| Candida tropicalis, A. oryzae | Ginkgo biloba residues | [97] | |
| Thermus sp. | soy fiber | [117] | |
| N.S. | hair waste, activated wastewater sludge | [19] | |
| Thermoactinomyces sp. | agricultural and household waste | [118] | |
| N.S. | hair waste, anaerobic digested wastewater sludge | [20] | |
| keratinase | Paenibacillus woosongensis | dry feathers | [119] |
| lipase | Pseudomonas aeruginosa | Jatropha curcas seed cake | [112] |
| N.S. | vegetable oil refining waste | [120] | |
| A. niger | oil palm waste residues | [121] | |
| xylanase | A. japonicus | castor bean waste | [122] |
| A. tubingensis | wheat straw | [123] | |
| B. pumilus | wheat bran | [124] | |
| T. harzianum | wheat bran | [101] | |
| Colletotrichum graminicola | wheat bran | [125] | |
| A. nidulans | black gram residues | [105] | |
| inulinase | A. ficuum | wheat bran | [126] |
| Kluyveromyces marxianus | sugarcane bagasse | [127] | |
| laccase | Coriolopsis gallica | sawdust waste | [51] |
| Trametes versicolor | oak sawdust | [128] | |
| esterase | B. altitudinis | fish processing waste | [60] |
| lignin peroxidase | Ganoderma leucidum | waste corn cob | [44] |
| invertase | A. niger | molasses and sugarcane bagasse | [53] |
| β-xylosidase | A. tamarii | ground oats | [129] |
| Colletotrichum graminicola | wheat bran | [125] | |
| β-glucosidase | Colletotrichum graminicola | wheat bran | [125] |
| multienzyme protease, xylanase, cellulase, endomylase | A. awamori | babassu cake | [130] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu Yazid, N.; Barrena, R.; Komilis, D.; Sánchez, A. Solid-State Fermentation as a Novel Paradigm for Organic Waste Valorization: A Review. Sustainability 2017, 9, 224. https://doi.org/10.3390/su9020224
Abu Yazid N, Barrena R, Komilis D, Sánchez A. Solid-State Fermentation as a Novel Paradigm for Organic Waste Valorization: A Review. Sustainability. 2017; 9(2):224. https://doi.org/10.3390/su9020224
Chicago/Turabian StyleAbu Yazid, Noraziah, Raquel Barrena, Dimitrios Komilis, and Antoni Sánchez. 2017. "Solid-State Fermentation as a Novel Paradigm for Organic Waste Valorization: A Review" Sustainability 9, no. 2: 224. https://doi.org/10.3390/su9020224







