Using Green Water Farm to Improve Ecological Restoration
Abstract
:1. Introduction
2. Materials and Methods
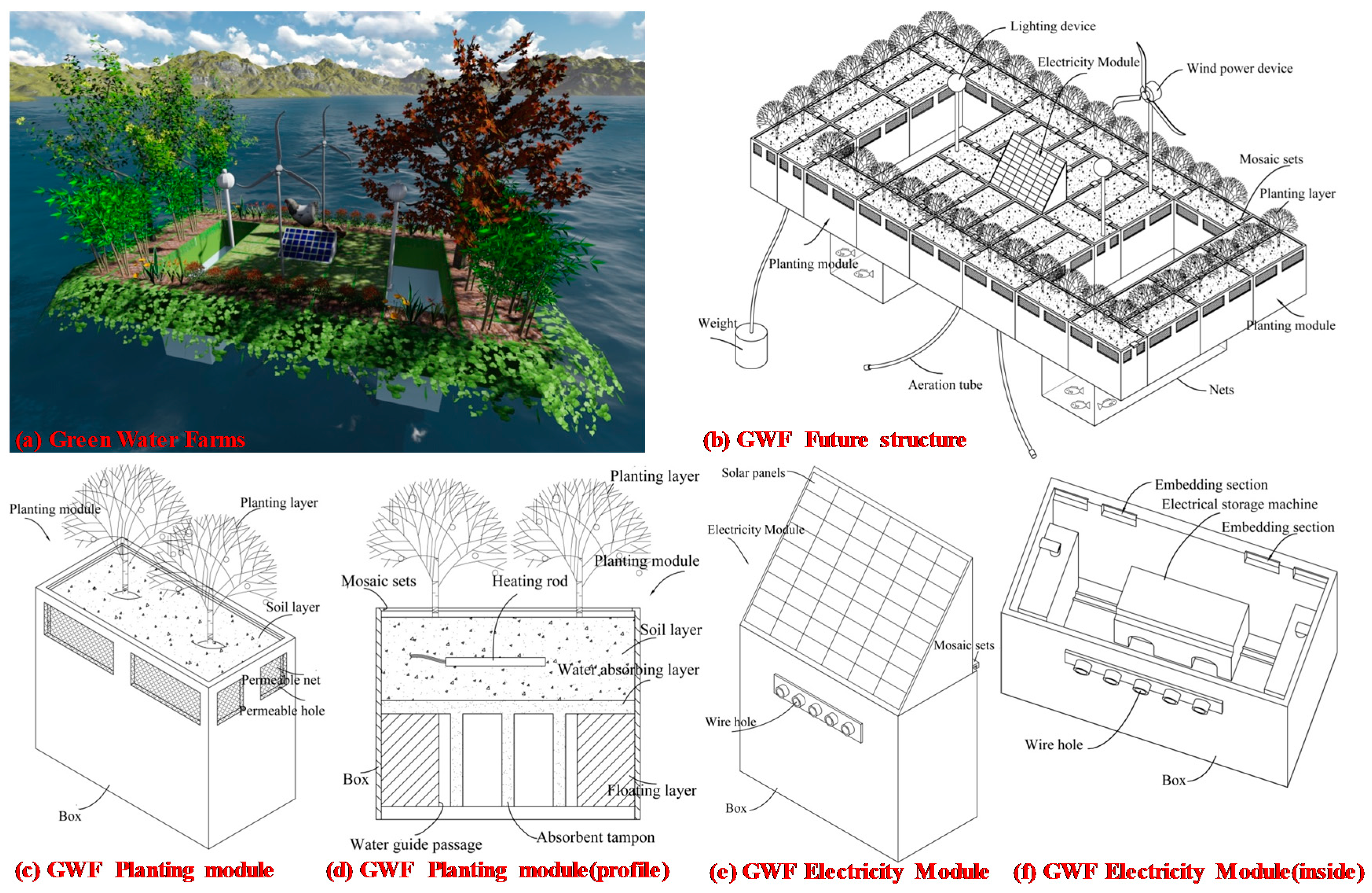
2.1. GWF Structure
2.2. Measuring Instruments and Research Materials
2.2.1. Water Quality Measurement Equipment
2.2.2. Ecological Environment Instrumentation
2.2.3. Solar Power Supply Equipment
2.3. Physical Environment Instrumentation
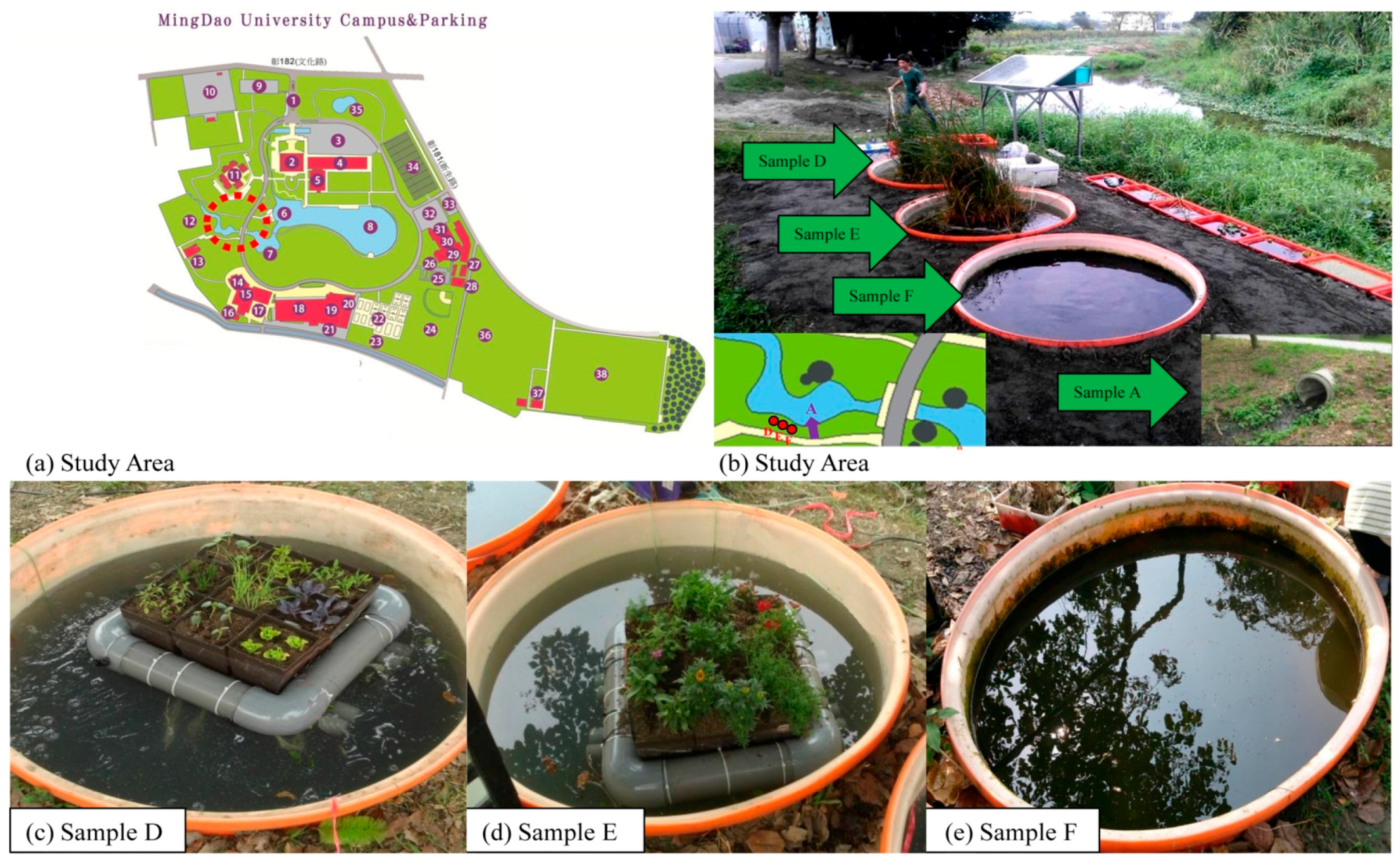
2.4. Water Body Grouping and Water Quality Research
2.5. Ecological Investigation Method
2.6. Statistical Analysis
3. Results
3.1. Climatic Conditions
3.2. Benefit of Water Quality Improvement
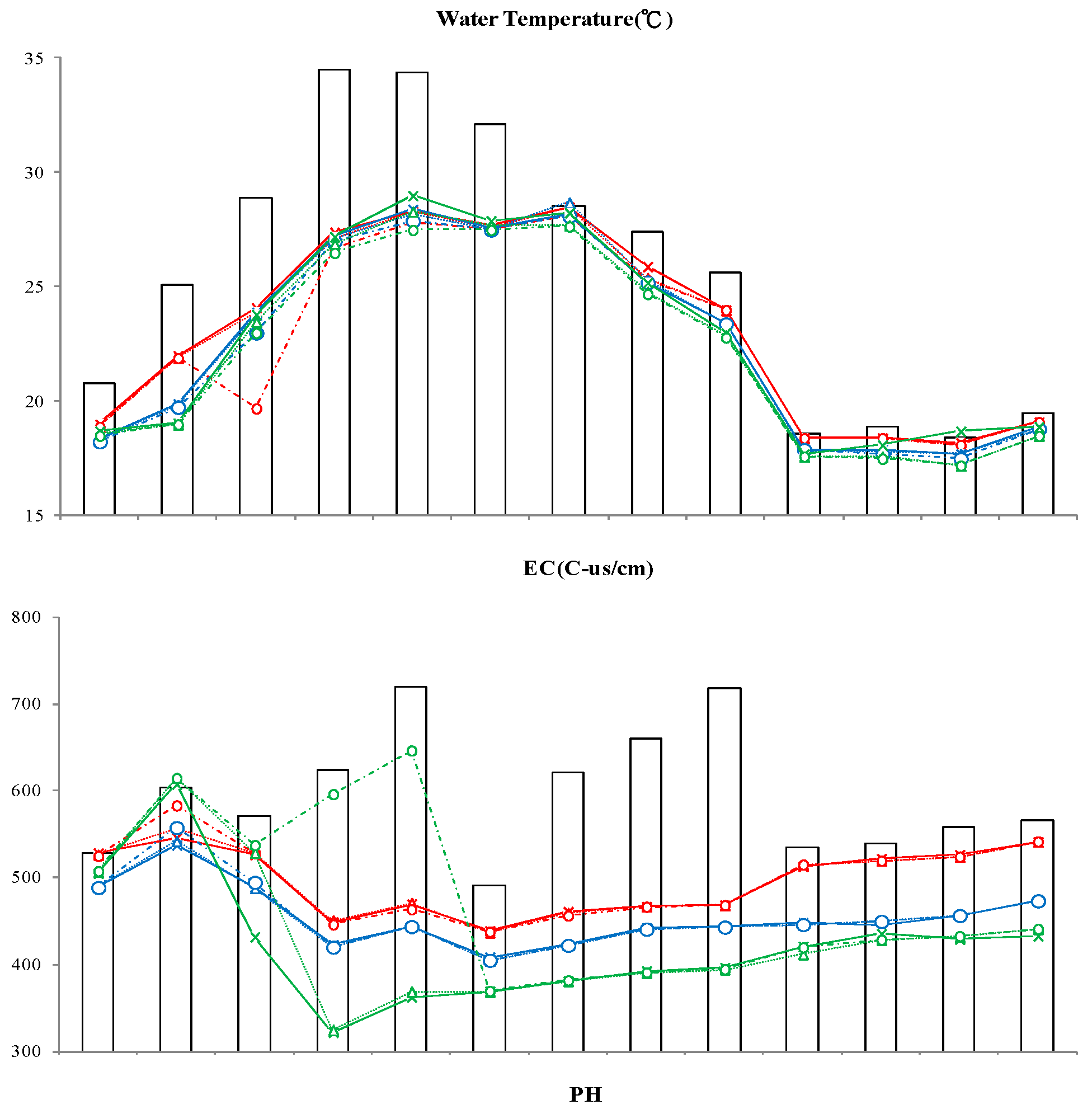
3.2.1. Water Temperature
3.2.2. Electrical Conductivity (EC)
3.2.3. Potential of Hydrogen (pH)
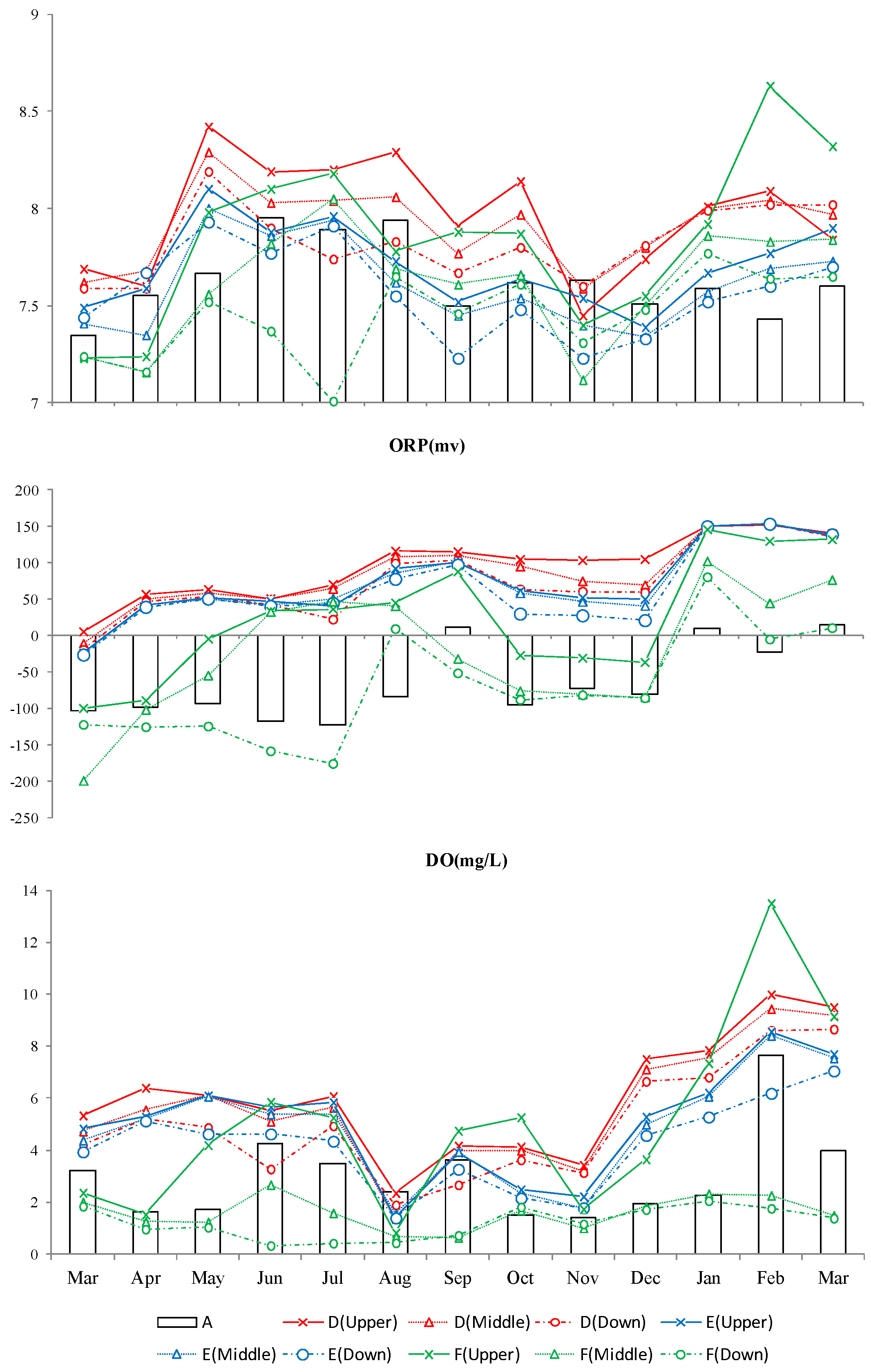
3.2.4. Oxidation-Reduction Potential (ORP)
3.2.5. Dissolved Oxygen (DO)
3.3. Ecological Conservation Benefit
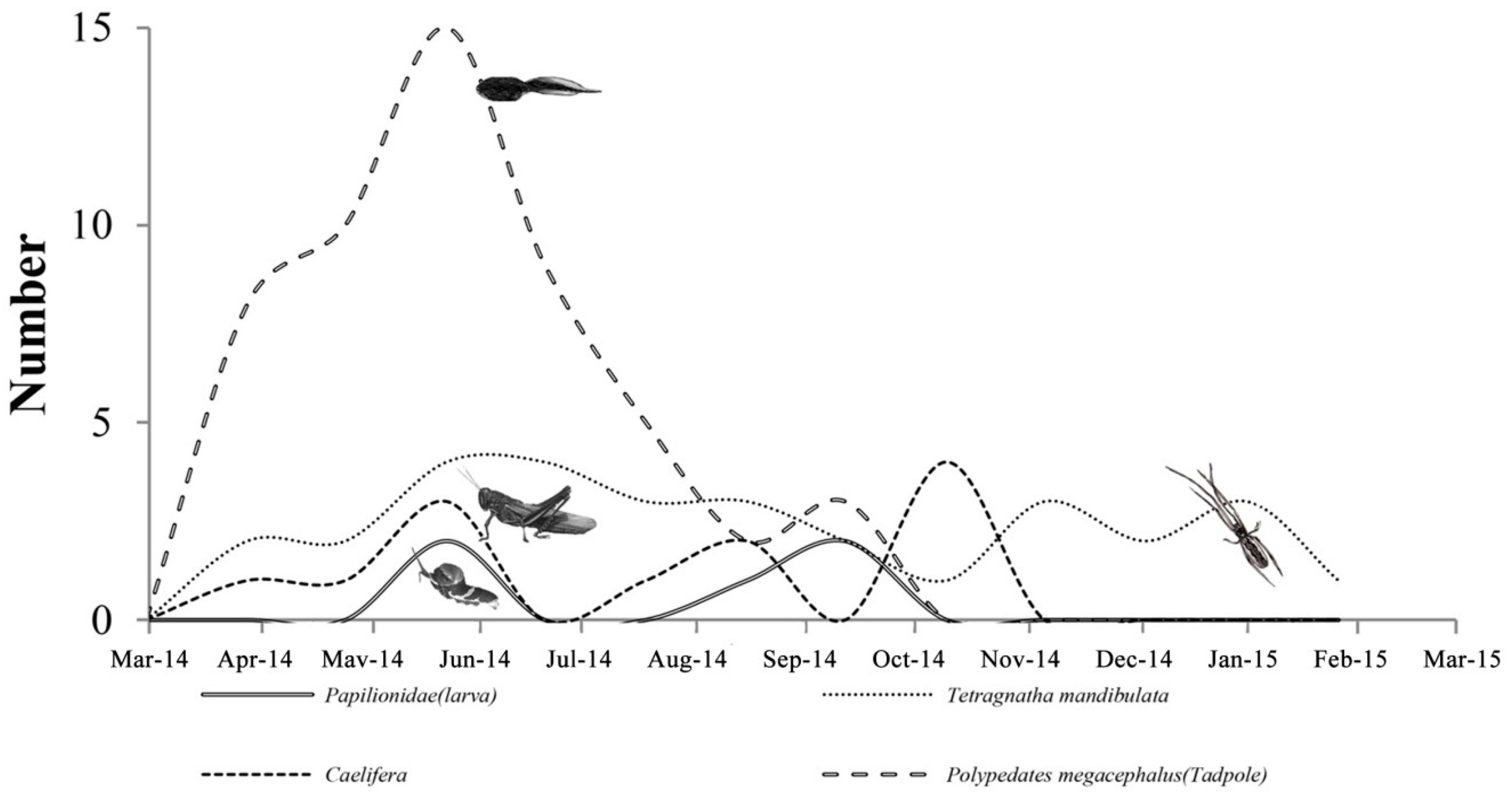
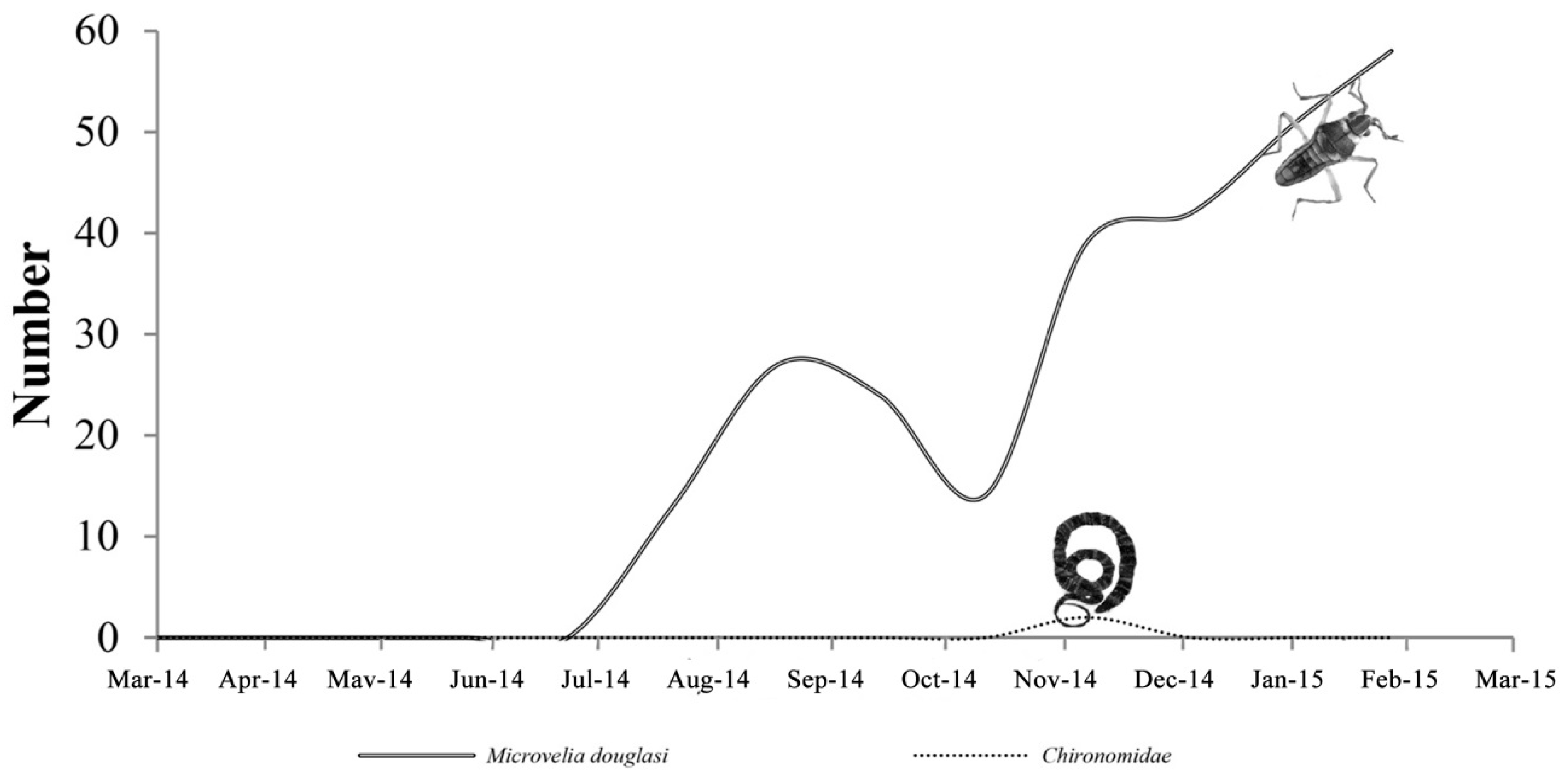
3.3.1. Biological Change of Sample D
3.3.2. Biological Change of Sample E
3.3.3. Biological Change of Sample F
3.4. Statistical Analysis Results
4. Discussion and Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shih, P.K.; Chang, W.L. Surveying the growth of aquatic macrophyte and water purification on artificial floating island. J. Chin. Agric. Eng. 2006, 29, 1–13. [Google Scholar]
- Huang, C.H. Effects of Floating Beds on Water Purification in Salt Marsh Type of Constructed Wetlands; Department of Marine Biotechnology and Resources, National Sun Yat-sen University: Kaohsiung, Taiwan, 2013. (In Chinese) [Google Scholar]
- Artificial Floating Island Study Group. Guideline for Installation of Artificial Floating Island; Water Resources Environment Technology Center (WEC): Tokyo, Japan, 2000. (In Japanese) [Google Scholar]
- Will, G.C.; Crawford, G.I. Evaluated and floating nest structures for Canada geese. J. Wildl. Manag. 1970, 34, 583–586. [Google Scholar] [CrossRef]
- Hirose, T. Introduction of Applied Ecological Engineering; Shinzansha: Tokyo, Japan, 1997; Volume 70. (In Japanese) [Google Scholar]
- Environmental Protection Administration, R.O.C. Water Quality Monitoring Project and Its Significance. 2015. Available online: http://ecological.epa.gov.tw/Taiwan.aspx?Num=06 (accessed on 30 June 2016).
- Liu, H. Environmental Greening Foundation. Introduction of Artificial Floating Island on T Drunken Moon Lake in Taiwan University. 2009. Available online: http://www.hsiliu.org.tw/ (accessed on 28 February 2016).
- Nakamura, K.; Mueller, G. Review of the performance of the artificial floating island as a restoration tool for aquatic environments. In Proceedings of the World Environmental and Water Resources Congress, Honolulu, HI, USA, 12–16 May 2008; pp. 1–10. [Google Scholar]
- Wen, L.; Recknagel, F. In situ removal of dissolved phosphorus in irrigation drainage water by planted floats: Preliminary results from growth chamber experiment. Agric. Ecosyst. Environ. 2002, 90, 9–15. [Google Scholar] [CrossRef]
- Kamble, R.; Patil, D. Artificial Floating Island: Solution to River Water Pollution in India. Case Study: River in Pune City. In Proceedings of the International Conference on Environmental, Biomedical and Biotechnology, Dubai, UAE, 4–5 August 2012; Volume 41, pp. 136–140. [Google Scholar]
- Bernie, M. The ability of vegetated floating Islands to improve water quality in natural and constructed wetlands: A review. Water Pract. Technol. 2012, 7. [Google Scholar] [CrossRef]
- Liu, X.; Li, J. The Progress of Chinese Aquatic Ecological Restoration; Hanghzou Dianzi University: Hangzhou, China, 2013. [Google Scholar]
- Dai, W.C.; Chiang, H. Effectiveness of improving lake water quality through artificial floating island system. J. Chin. Agric. Eng. 2008, 54, 26–34. [Google Scholar]
- Chang, Y.H.; Wu, B.Y.; Lai, C.F. The effect of a green energy landscape fountain on water quality improvement. Ecol. Eng. 2014, 73, 201–208. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, C.; Ding, J.; Hou, A.; Li, Y.; Niu, B.; Su, X.; Xu, Y.; Laws, E. The 2007 water crisis in Wuxi, China: Analysis of the origin. J. Hazard. Mater. 2010, 182, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.L.; Ku, C.R.; Chang, Y.H. Water quality improvement with artificial floating islands. Ecol. Eng. 2015, 74, 371–375. [Google Scholar] [CrossRef]
- Keigo, N. Artificial floating island technical Isaono. Civ. Technol. Inf. 1999, 41, 26–31. [Google Scholar]
- Kalff, J. Limnology: Inland Water Ecosystems; Prentice Hall: Upper Saddle River, NJ, USA, 2001. [Google Scholar]
- Wang, Z.Q.; Shi, Y.; Cheng, M.M.; He, C.G.; Zhuang, J.; Sheng, L.X. Purification of small-scale stagnant water in urban regions: Human-powered water lift and bank-based constructed wetland. Ecol. Eng. 2015, 83, 108–111. [Google Scholar] [CrossRef]
- Carolin, G.; Christina, V.H.; Christian, A. Optimizing environmental measures for landscape multifunctionality: Effectiveness, efficiency and recommendations for agri-environmental programs. J. Environ. Manag. 2015, 151, 243–257. [Google Scholar]
- Fager, L.F.; York, J.C. Floating islands for waterfowl in Arizona. Soil Conserv. 1975, 41, 4–5. [Google Scholar]
- Payne, N.F. Techniques for Wildlife Habitat Management of Wetlands; McGraw-Hill, Inc.: New York, NY, USA, 1992. [Google Scholar]
- Hiraoka, T. Utilization of artificial floating objects as nest Platforms by little Grebes and Eurasian, Central Japan. J. Yamashina Inst. Ornithol. 1996, 28, 108–112. [Google Scholar] [CrossRef]
- Momose, H.; Funakubo, S.; Kibe, N.; Nakamura, K.; Fujiwara, N.; Tanaka, T. Use of different types of planted floating islands by water birds. Environ. Syst. Res. 1998, 26, 45–53. (In Japanese) [Google Scholar] [CrossRef]
- Pei, K. Activity Rhythm of the Spinous Country Rat (Niviventer coxingi) in Taiwan. Zool. Stud. 1995, 34, 55–58. [Google Scholar]
- Wang, L.; Eagles, P. Some theoretical considerations: From landscape ecology to waterscape ecology. Acta Ecol. Sin. 2009, 29, 176–181. [Google Scholar]
- Yao, K.; Song, S.; Zhang, Z.; Xu, J.; Zhang, R.; Liu, J.; Cheng, L.; Liu, J. Vegetation characteristics and water purification by artificial floating island. Afr. J. Biotechnol. 2011, 10, 19119–19125. [Google Scholar]
- Yen, N.; Yen, P.; Chang, Y.H. Artificial floating islands for environmental improvement. Renew. Sustain. Energy Rev. 2015, 47, 616–622. [Google Scholar]
- Evans, M.C.; Carwardine, J.; Fensham, R.J.; Butler, D.W.; Wilson, K.A.; Possingham, H.P.; Martin, T.G. Carbon farming via assisted natural regeneration as a cost-effective mechanism for restoring biodiversity in agricultural landscapes. Environ. Sci. Policy 2015, 50, 114–129. [Google Scholar] [CrossRef]
- Gagné, S.A.; Eigenbrod, F.; Bert, D.G.; Cunnington, G.M.; Olson, L.T.; Smith, A.C.; Fahrig, L. A simple landscape design framework for biodiversity conservation. Landsc. Urban Plan. 2015, 136, 13–27. [Google Scholar]
- Fahrig, L.; Girard, J.; Duro, D.; Pasherb, J.; Smithb, A.; Javorekc, S.; Kinga, D.; Lindsaya, K.F.; Mitchella, S.M.; Tischendorfa, L. Farmlands with smaller crop fields have higher within-field biodiversity. Agric. Ecosyst. Environ. 2015, 200, 219–234. [Google Scholar] [CrossRef]
- Faulwetter, J.L.; Burr, M.D.; Cunningham, A.B.; Stewart, F.M.; Camper, A.K.; Stein, O.R. Floating treatment wetlands for domestic wastewater treatment. Water Sci. Technol. 2011, 64, 2089–2095. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Takemon, Y.; Hori, M. Invertebrate assemblages in relation to habitat types on a floating mat in Mizorogaike Pond, Kyoto, Japan. Limnology 2009, 10, 167–176. [Google Scholar] [CrossRef]
- Song, H.L.; Li, X.N.; Wang, X.J.; Lu, X.W. Enhancing nitrogen removal performance of vegetated floating-bed by adding Hyriopsiscumingii Lea and an artificial medium. Fresenius Environ. Bull. 2011, 20, 2435–2441. [Google Scholar]
- Lee, P.F.; Liang, S.H. The Assess Mode Validation of Animal Ecology Research and Evaluation Techniques; Executive Yuan Environmental Protection Agency: Taipei, Taiwan, 2003.
- Environmental Protection Administration, R.O.C. Effluent Standards. Available online: https://oaout.epa.gov.tw/law/ (accessed on 28 February 2017).
- Chang, Y.H.; Wu, B.Y.; Lai, C.F. A study of the ecological benefits of the green energy landscape fountain. Ecol. Eng. 2015, 75, 128–136. [Google Scholar] [CrossRef]




| Project Sample | Tempe (°C) | DO (mg/L) | EC (C-μS/cm) | pH | OR (mv) | |
|---|---|---|---|---|---|---|
| A | Start | 20.8 | 3.2 | 527.9 | 7.3 | −103 |
| Final | 19.5 | 4 | 566.5 | 7.6 | 14.8 | |
| Difference | 1.3 | 0.8 | 38.6 | 0.3 | 117.8 | |
| D | Start | 18.9 | 4.7 | 526 | 7.6 | −10.9 |
| Final | 19.1 | 9.2 | 541 | 7.9 | 138 | |
| Difference | 0.2 | 4.5 | 15 | 0.3 | 148.9 | |
| E | Start | 18.2 | 4.3 | 488.6 | 7.4 | −24.5 |
| Final | 18.8 | 7.5 | 473.3 | 7.7 | 138.7 | |
| Difference | 0.6 | 3.2 | 15.3 | 0.3 | 163.2 | |
| F | Start | 18.5 | 2 | 507 | 7.2 | −199 |
| Final | 18.5 | 1.5 | 441 | 7.8 | 76.4 | |
| Difference | 0 | 0.5 | 66 | 0.6 | 275.4 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.-H.; Zhuang, T.-J.; Chuang, T.-F.; Wu, B.-Y.; Lu, H.-l.; Chen, P.-Y. Using Green Water Farm to Improve Ecological Restoration. Sustainability 2017, 9, 1896. https://doi.org/10.3390/su9101896
Chang Y-H, Zhuang T-J, Chuang T-F, Wu B-Y, Lu H-l, Chen P-Y. Using Green Water Farm to Improve Ecological Restoration. Sustainability. 2017; 9(10):1896. https://doi.org/10.3390/su9101896
Chicago/Turabian StyleChang, Yuan-Hsiou, Ting-Jie Zhuang, Tsai-Fu Chuang, Bing-Yu Wu, Hsiao-ling Lu, and Pen-Yuan Chen. 2017. "Using Green Water Farm to Improve Ecological Restoration" Sustainability 9, no. 10: 1896. https://doi.org/10.3390/su9101896





