Integrated Multi-Trophic Recirculating Aquaculture System for Nile Tilapia (Oreochlomis niloticus)
Abstract
:1. Introduction
2. Materials and Methods
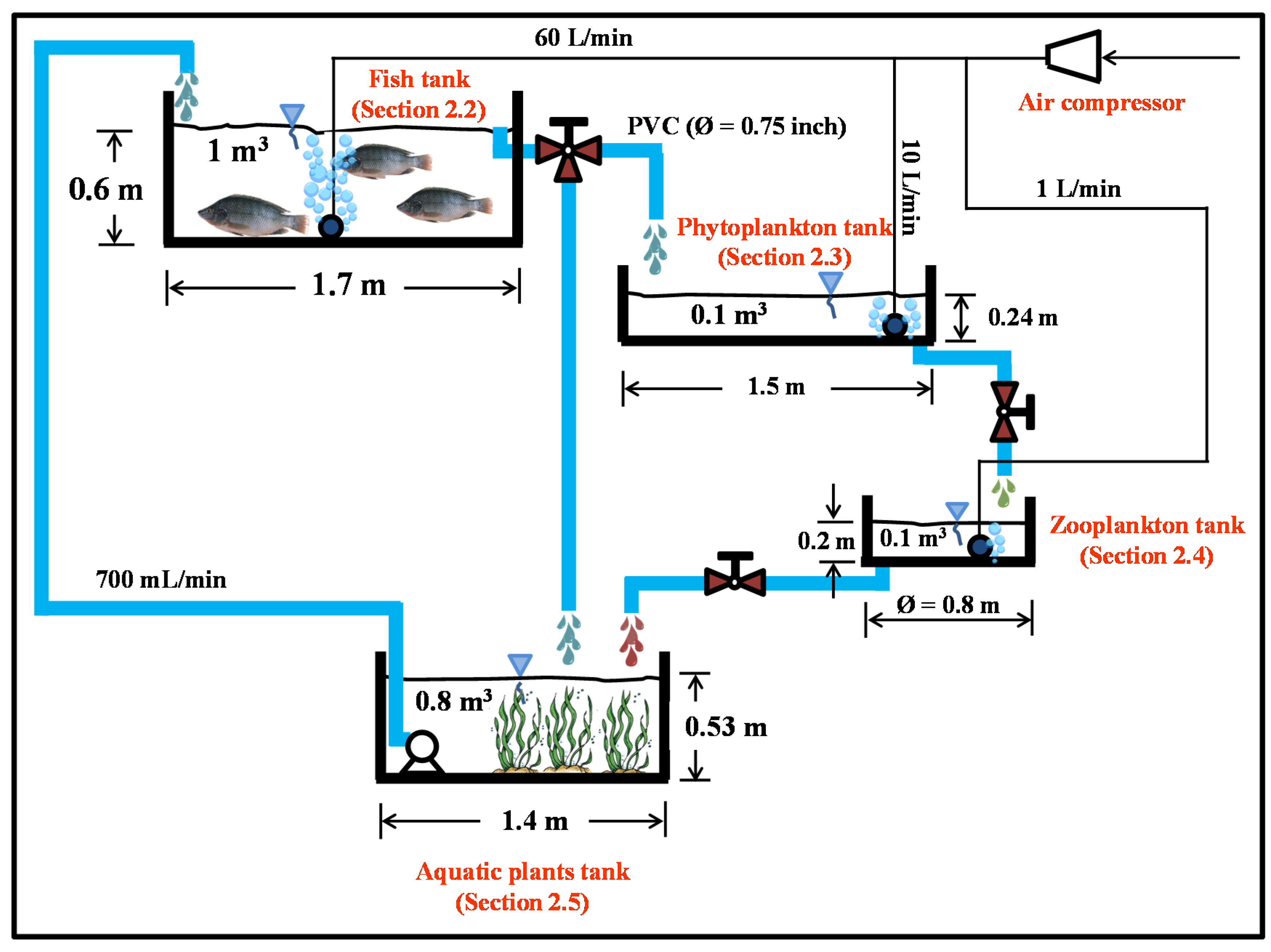
2.1. System Setup
2.2. Fish Tank (Nile Tilapia)
2.3. Phytoplankton Tank (Chlorella sp.)
2.4. Zooplankton Tank (Moina macrocopa)
2.5. Aquatic Plants Tank
3. Results and Discussion
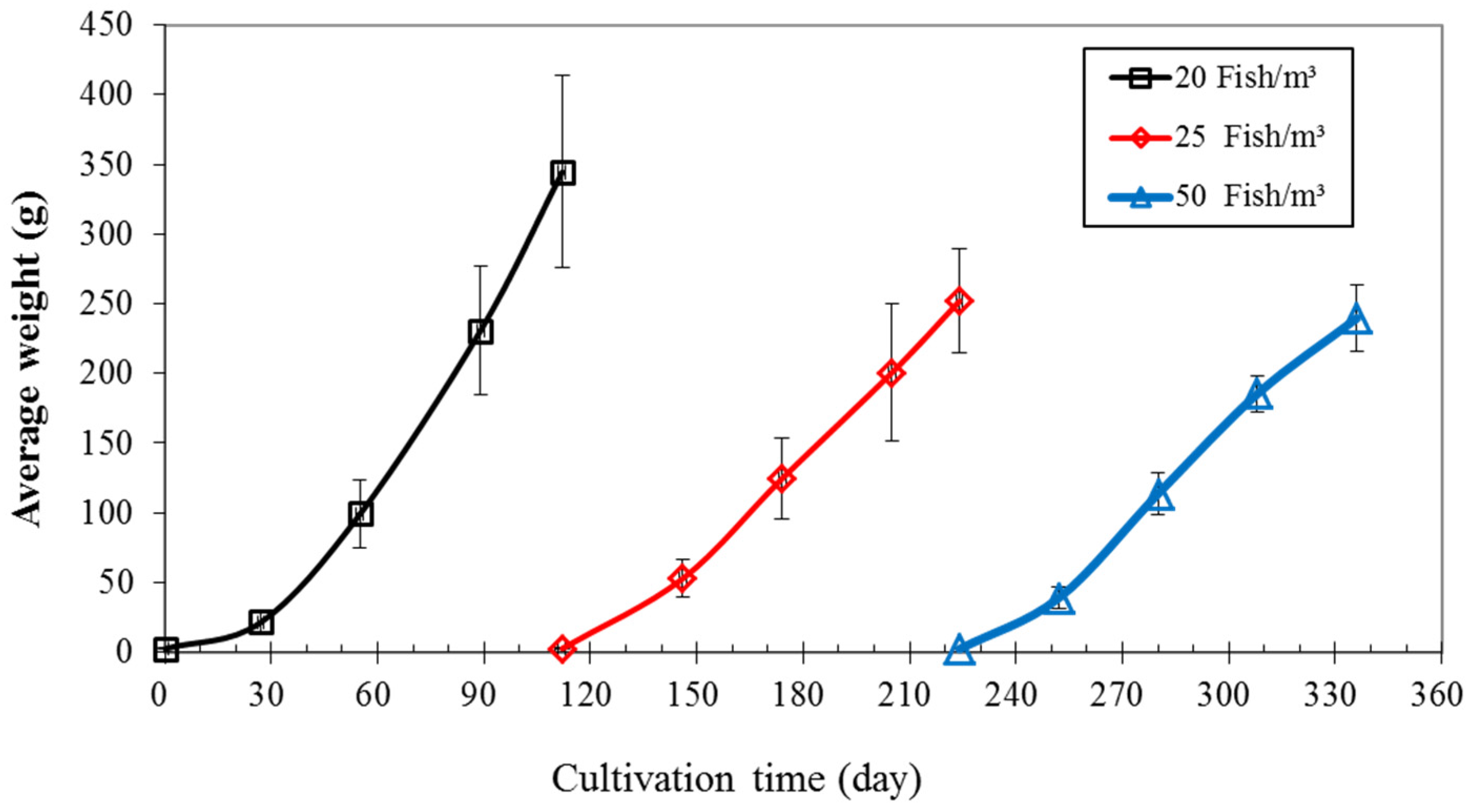
3.1. Growth of Nile Tilapia
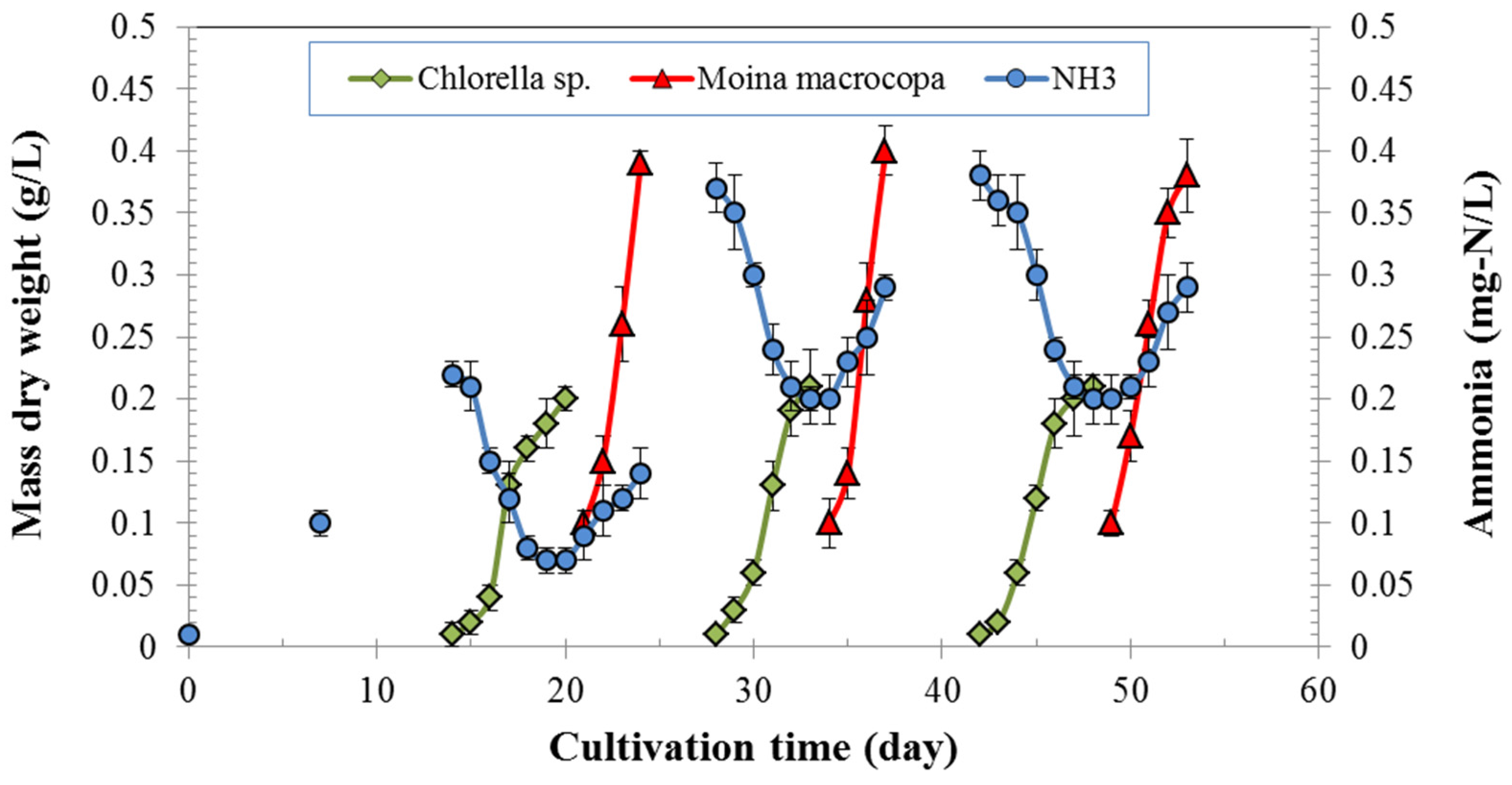
3.2. Growth of Chlorella sp. and Moina macrocopa
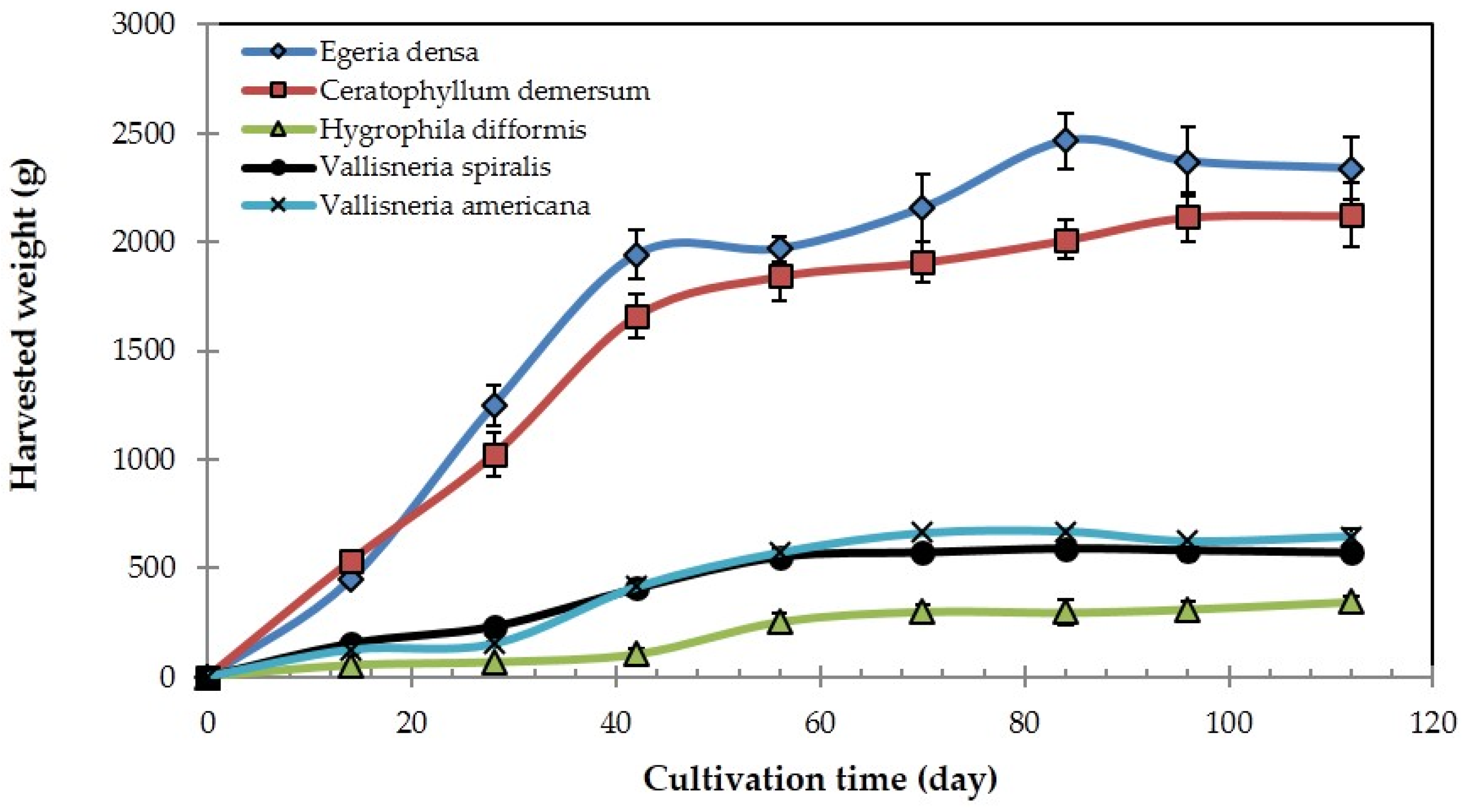
3.3. Growth of Aquatic Plants
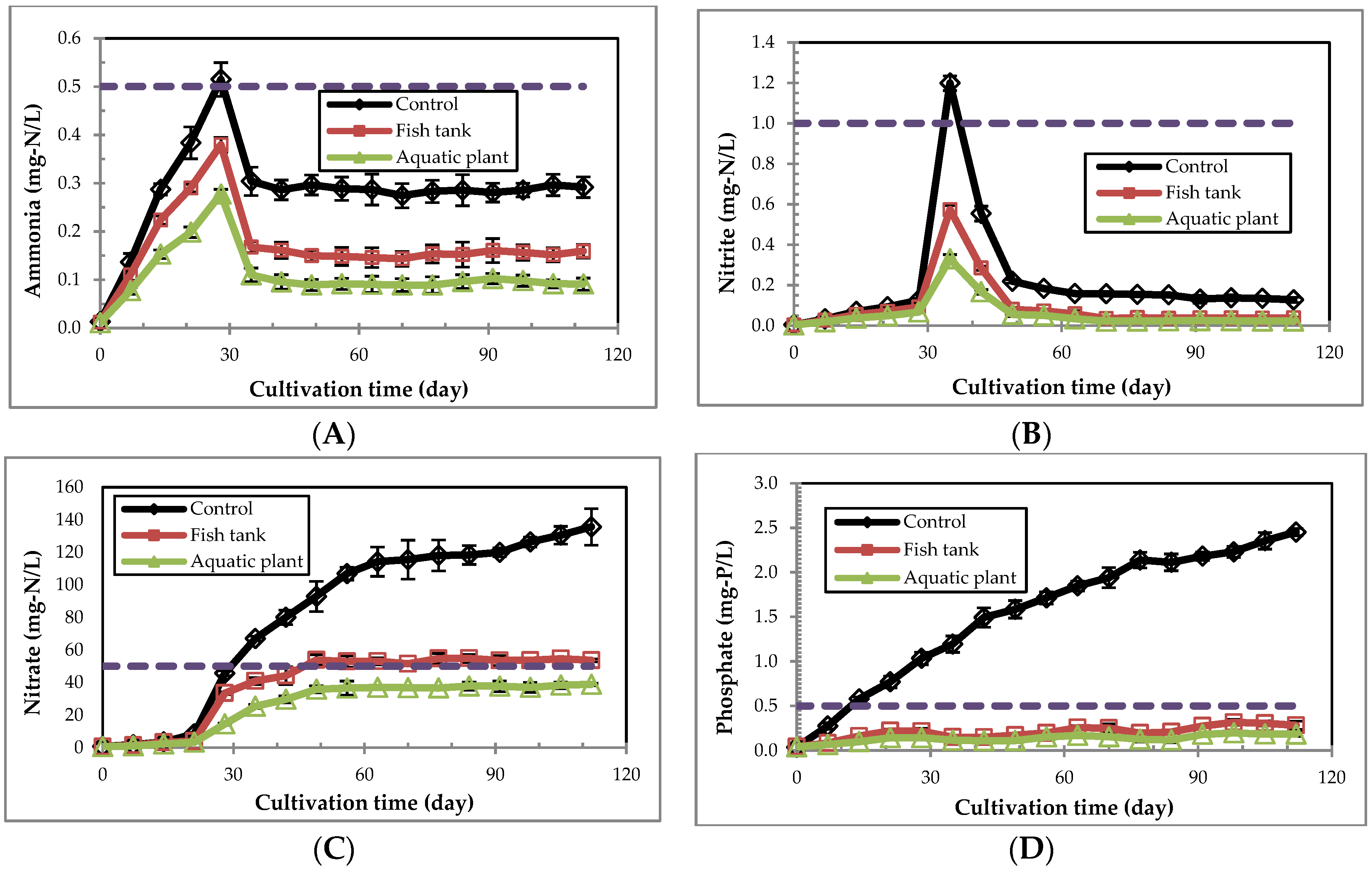
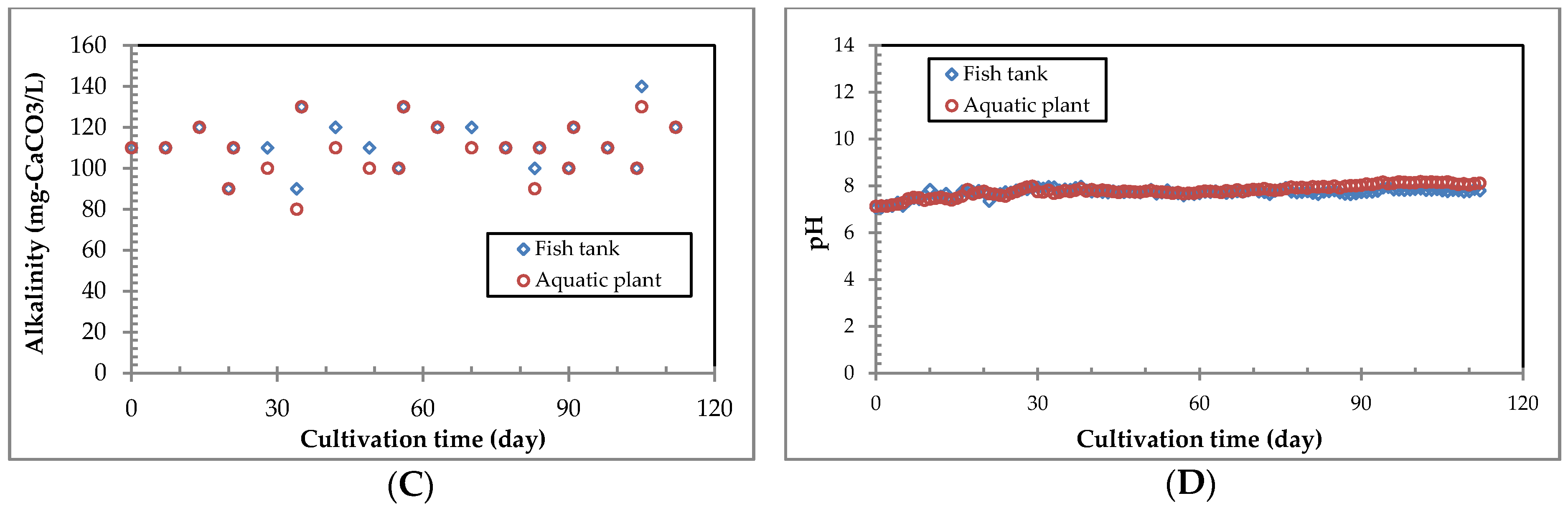
3.4. Water Quality
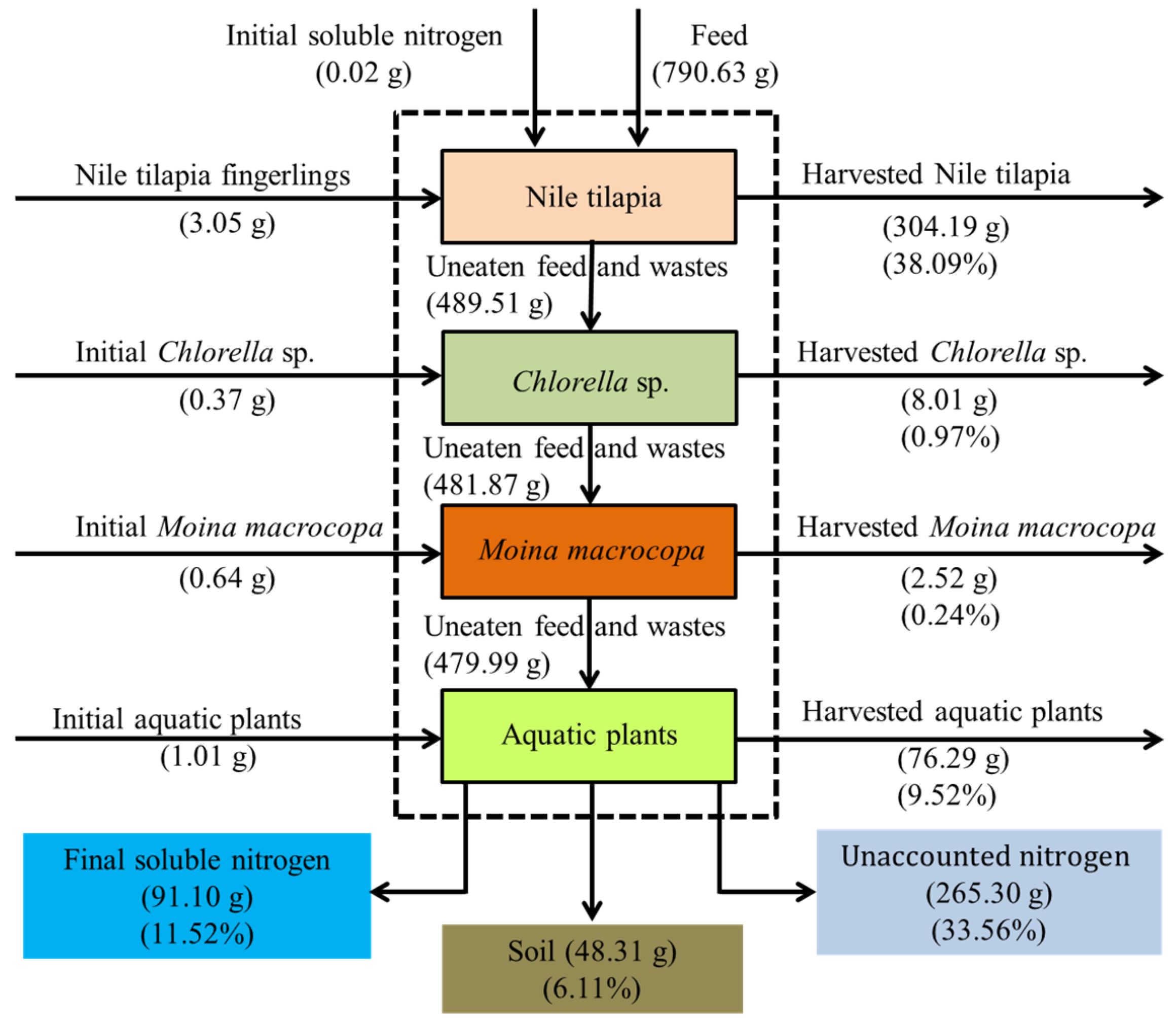
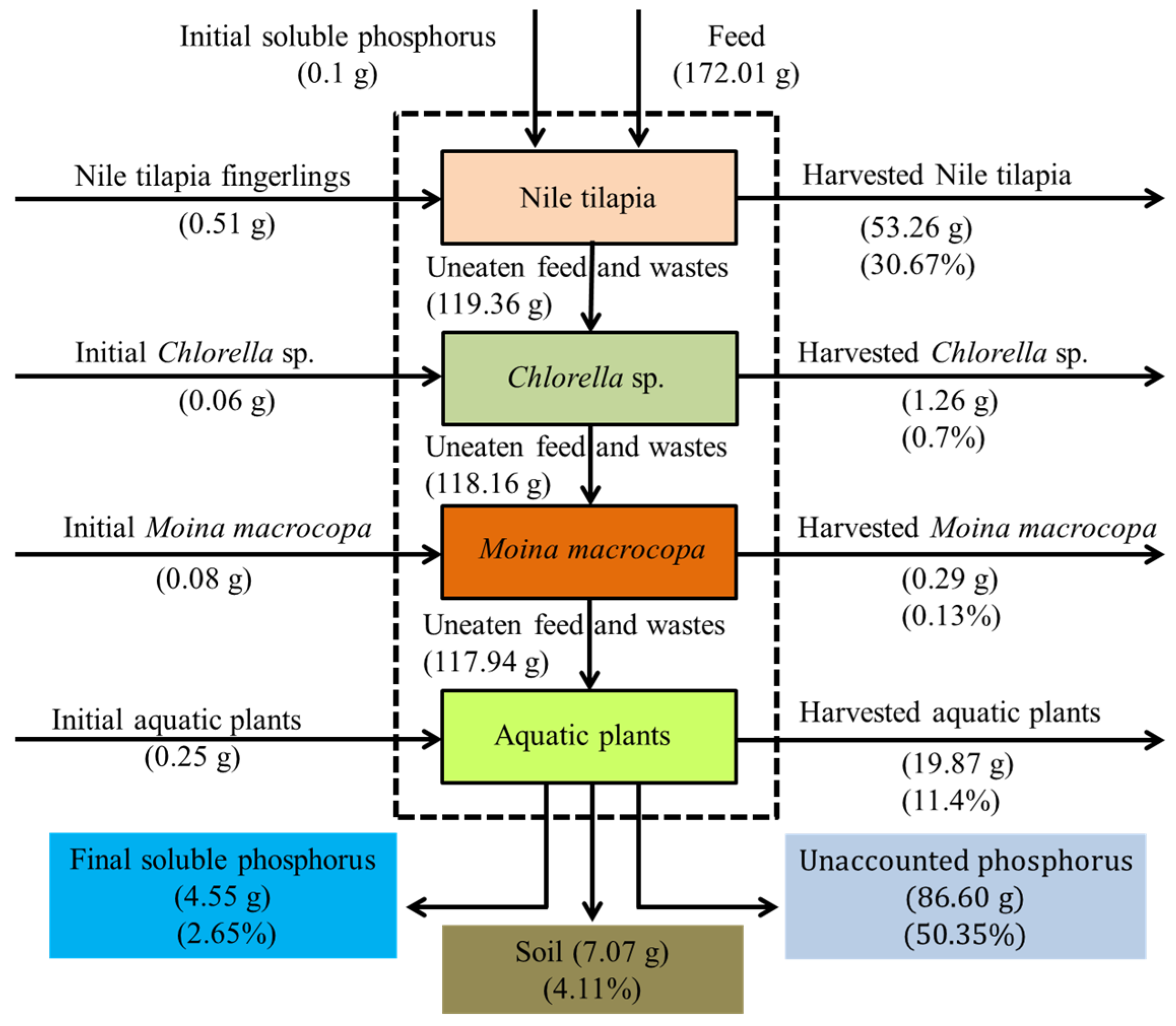
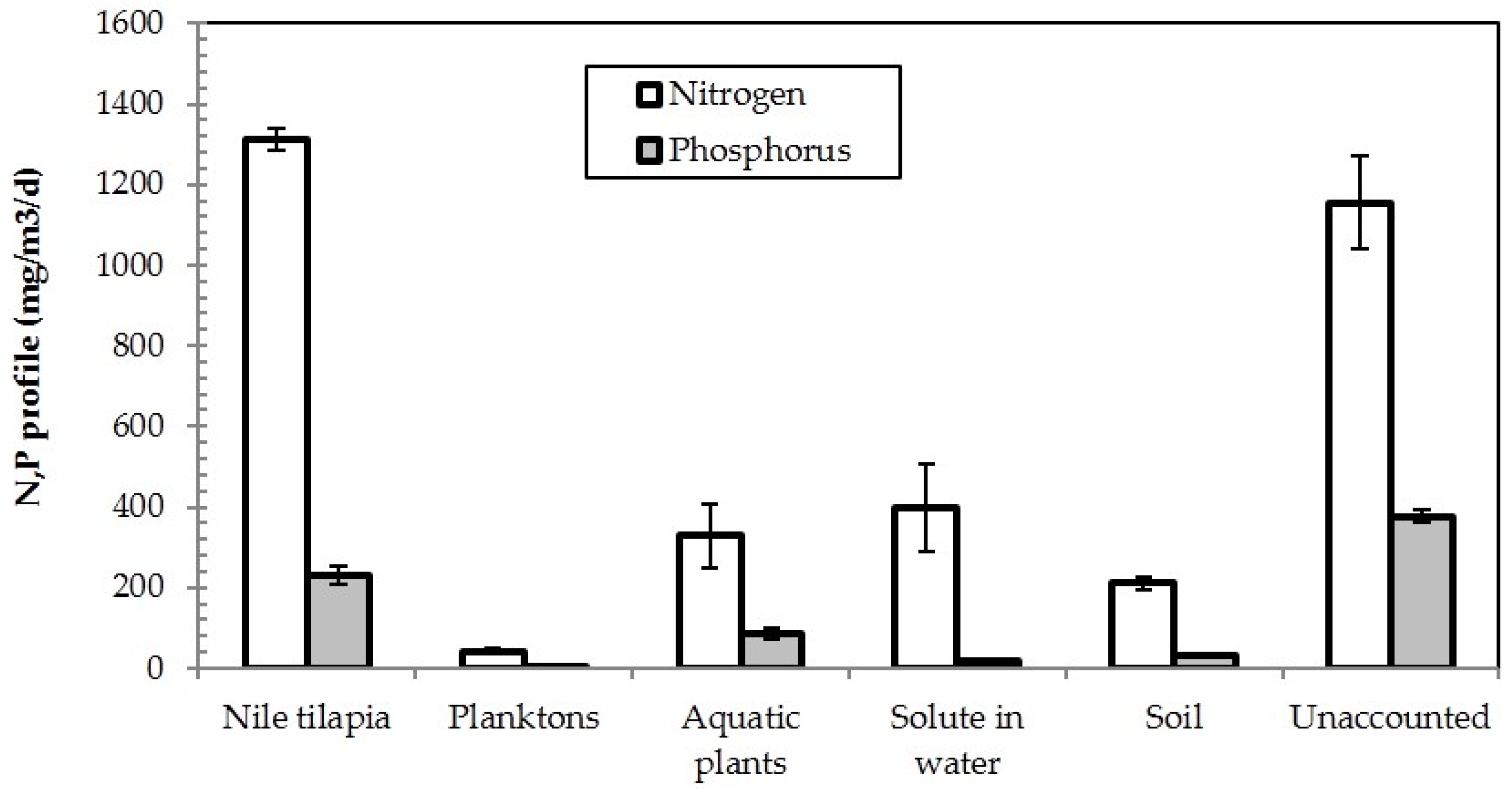
3.5. Nitrogen and Phosphorus Mass Balances
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bahnasawy, M.H.; El-Ghobashy, A.E.; Abdel-Hakim, N.F. Culture of the Nile tilapia (Oreochromis niloticus) in a recirculating water system using different protein levels. Egypt. J. Aquat. Biol. Fish. 2009, 12, 1–15. [Google Scholar]
- Menasveta, P.; Panritdam, T.; Sihanonth, P.; Powtongsook, S.; Chuntapa, B.; Lee, P. Design and function of a closed, recirculating seawater system with denitrification for the culture of black tiger shrimp broodstock. Aquac. Eng. 2001, 25, 35–49. [Google Scholar] [CrossRef]
- Silapakul, S.; Powtongsook, S.; Pavasant, P. Nitrogen compounds removal in a packed bed external loop airlift bioreactor. Korean J. Chem. Eng. 2005, 22, 393–398. [Google Scholar] [CrossRef]
- Uemoto, H.; Morita, M. Nitrogen removal with a dual bag system capable of simultaneous nitrification and denitrification. Biochem. Eng. J. 2010, 52, 104–109. [Google Scholar] [CrossRef]
- Wei, H.; Zhang, Y.; Jiang, Y.; Zhang, C. Influence of carbon source on simultaneous nitrification and denitrification of biomembrane reactor. J. Beijing Univ. Technol. 2010, 36, 506. [Google Scholar]
- Zhang, Y.; Guo, Y.; Bai, Y.; Tan, L.; Wang, Y.; Koyama, T. Simultaneous nitrification and denitrification by catching bed biofilm reactor. Huan Jing Ke Xue 2010, 31, 134–139. [Google Scholar] [PubMed]
- Brito, L.O.; Chagas, A.M.; Silva, E.P.D.; Soares, R.B.; Severi, W.; Gálvez, A.O. Water quality, vibrio density and growth of pacific white shrimp Litopenaeus vannamei (Boone) in an integrated biofloc system with red seaweed Gracilaria birdiae (Greville). Aquac. Res. 2014, 47, 940–970. [Google Scholar] [CrossRef]
- Correia, E.S.; Wilkenfeld, J.S.; Morris, T.C.; Wei, L.; Prangnell, D.I.; Samocha, T.M. Intensive nursery production of the Pacific white shrimp Litopenaeus vannamei using two commercial feeds with high and low protein content in a biofloc-dominated system. Aquac. Eng. 2014, 59, 48–54. [Google Scholar] [CrossRef]
- De Souza, D.M.; Martins, Á.C.; Jensen, L.; Wasielesky, W., Jr.; Monserrat, J.M.; Garcia, L.d.O. Effect of temperature on antioxidant enzymatic activity in the pacific white shrimp Litopenaeus vannamei in a BFT (Biofloc technology) system. Mar. Freshwat. Behav. Physiol. 2014, 47, 1–10. [Google Scholar] [CrossRef]
- Emerenciano, M.; Ballester, E.L.; Cavalli, R.O.; Wasielesky, W. Biofloc technology application as a food source in a limited water exchange nursery system for pink shrimp Farfantepenaeus brasiliensis (Latreille, 1817). Aquac. Res. 2012, 43, 447–457. [Google Scholar] [CrossRef]
- Emerenciano, M.; Cuzon, G.; Arevalo, M.; Gaxiola, G. Biofloc technology in intensive broodstock farming of the pink shrimp Farfantepenaeus duorarum: Spawning performance, biochemical composition and fatty acid profile of eggs. Aquac. Res. 2014, 45, 1713–1726. [Google Scholar] [CrossRef]
- Lewis, W.M.; Yopp, J.H.; Schramm, H.L., Jr.; Brandenburg, A.M. Use of hydroponics to maintain quality of recirculated water in a fish culture system. Trans. Am. Fish. Soc. 1978, 107, 92–99. [Google Scholar] [CrossRef]
- Naegel, L.C. Combined production of fish and plants in recirculating water. Aquaculture 1977, 10, 17–24. [Google Scholar] [CrossRef]
- Seawright, D.E.; Stickney, R.R.; Walker, R.B. Nutrient dynamics in integrated aquaculture–hydroponics systems. Aquaculture 1998, 160, 215–237. [Google Scholar] [CrossRef]
- Thanakitpairin, A.; Pungrasmi, W.; Powtongsook, S. Nitrogen and phosphorus removal in the recirculating aquaculture system with water treatment tank containing baked clay beads and Chinese cabbage. EnvironmentAsia 2014, 7, 81–88. [Google Scholar]
- Trang, N.T.D.; Brix, H. Use of planted biofilters in integrated recirculating aquaculture-hydroponics systems in the Mekong Delta, Vietnam. Aquac. Res. 2014, 45, 460–469. [Google Scholar] [CrossRef]
- Ardjosoediro, I.; Ramnarine, I.W. The influence of turbidity on growth, feed conversion and survivorship of the Jamaica red tilapia strain. Aquaculture 2002, 212, 159–165. [Google Scholar] [CrossRef]
- Coward, K.; Bromage, N. Reproductive physiology of female tilapia broodstock. Rev. Fish Biol. Fish. 2000, 10, 1–25. [Google Scholar] [CrossRef]
- El-Sayed, A.-F.M. Tilapia Culture; CABI: Wallingford, UK, 2006. [Google Scholar]
- Hassanien, H.A.; Elnady, M.; Obeida, A.; Itriby, H. Genetic diversity of Nile tilapia populations revealed by randomly amplified polymorphic DNA (RAPD). Aquac. Res. 2004, 35, 587–593. [Google Scholar] [CrossRef]
- Toguyeni, A.; Fauconneau, B.t.; Fostier, A.; Abucay, J.; Mair, G.; Baroiller, J.-F. Influence of sexual phenotype and genotype, and sex ratio on growth performances in tilapia, Oreochromis niloticus. Aquaculture 2002, 207, 249–261. [Google Scholar] [CrossRef]
- El-Sayed, A.-F.M.; Kawanna, M. Effects of photoperiod on the performance of farmed Nile tilapia Oreochromis niloticus: I. Growth, feed utilization efficiency and survival of fry and fingerlings. Aquaculture 2004, 231, 393–402. [Google Scholar] [CrossRef]
- Ross, L. Environmental physiology and energetics. In Tilapias: Biology and Exploitation; Springer: Heidelberg, Germany, 2000; pp. 89–128. [Google Scholar]
- Siddiqui, A.; Howlader, M.; Adam, A. Culture of Nile tilapia, Oreochromis niloticus (l.), at three stocking densities in outdoor concrete tanks using drainage water. Aquac. Res. 1989, 20, 49–58. [Google Scholar] [CrossRef]
- Burut-Archanai, S.; Eaton-Rye, J.J.; Incharoensakdi, A.; Powtongsook, S. Phosphorus removal in a closed recirculating aquaculture system using the cyanobacterium Synechocystis sp. PCC 6803 strain lacking the SphU regulator of the Pho regulon. Biochem. Eng. J. 2013, 74, 69–75. [Google Scholar] [CrossRef]
- Körner, S.; Das, S.K.; Veenstra, S.; Vermaat, J.E. The effect of pH variation at the ammonium/ammonia equilibrium in wastewater and its toxicity to Lemna gibba. Aquat. Bot. 2001, 71, 71–78. [Google Scholar] [CrossRef]
- Lin, C.K.; Shrestha, M.K.; Yi, Y.; Diana, J.S. Management to minimize the environmental impacts of pond effluent: Harvest draining techniques and effluent quality. Aquac. Eng. 2001, 25, 125–135. [Google Scholar] [CrossRef]
- Thompson, F.L.; Abreu, P.C.; Wasielesky, W. Importance of biofilm for water quality and nourishment in intensive shrimp culture. Aquaculture 2002, 203, 263–278. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater; APHA, AWWA, WPCF: Washington DC, USA, 2005. [Google Scholar]
- Riche, M.; Garling, D. Feeding tilapia in intensive recirculating systems. North Cent. Reg. Aquac. Center Fact Sheet Ser. 2003, 114, 1–4. [Google Scholar]
- Ribeiro, F.B.; Lanna, E.A.T.; Bomfim, M.A.D.; Donzele, J.L.; Freitas, A.S.d.; Sousa, M.P.D.; Quadros, M. Dietary total phosphorus levels for Nile tilapia fingerlings. Rev. Bras. Zootec. 2006, 35, 1588–1593. [Google Scholar] [CrossRef]
- Lee, C.; Kim, M.; Sanjay, K.; Kwag, J.; Ra, C. Biomass production potential of Chlorella vulgaris under different CO2 concentrations and light intensities. J. Anim. Sci. Technol. 2011, 53, 261–268. [Google Scholar] [CrossRef]
- Sung, K.-D.; Lee, J.-S.; Shin, C.-S.; Park, S.-C. Effect of iron and EDTA on the growth of Chlorella sp. KR-1. J. Microbiol. Biotechnol. 1998, 8, 409–411. [Google Scholar]
- Al-Hafedh, Y.S.; Alam, A. Operation of a water recirculating greenwater system for the semi-intensive culture of mixed-sex and all-male Nile tilapia, Oreochromis niloticus. J. Appl. Aquac. 2005, 17, 47–59. [Google Scholar] [CrossRef]
- Gibtan, A.; Getahun, A.; Mengistou, S. Effect of stocking density on the growth performance and yield of Nile tilapia [Oreochromis niloticus (l., 1758)] in a cage culture system in lake Kuriftu, Ethiopia. Aquac. Res. 2008, 39, 1450–1460. [Google Scholar] [CrossRef]
- Sri-uam, P.; Linthong, C.; Powtongsook, S.; Kungvansaichol, K.; Pavasant, P. Manipulation of biochemical compositions of chlorella sp. Eng. J. 2015, 19, 13–24. [Google Scholar] [CrossRef]
- Martínez-Jerónimo, F.; Gutierrez-Valdivia, A. Fecundity, reproduction, and growth of Moina macrocopa fed different algae. Hydrobiologia 1991, 222, 49–55. [Google Scholar] [CrossRef]
- Cossu, R.; Haarstad, K.; Lavagnolo, M.C.; Littarru, P. Removal of municipal solid waste COD and NH 4–N by phyto-reduction: A laboratory–scale comparison of terrestrial and aquatic species at different organic loads. Ecol. Eng. 2001, 16, 459–470. [Google Scholar] [CrossRef]
- Zhao, Y.; Fang, Y.; Jin, Y.; Huang, J.; Bao, S.; Fu, T.; He, Z.; Wang, F.; Zhao, H. Potential of duckweed in the conversion of wastewater nutrients to valuable biomass: A pilot-scale comparison with water hyacinth. Bioresour. Technol. 2014, 163, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Ostroumov, S. Biological filtering and ecological machinery for self-purification and bioremediation in aquatic ecosystems: Towards a holistic view. Riv. Biol. 1998, 91, 221–232. [Google Scholar] [PubMed]
- Cheng, J.J.; Stomp, A.-M. Growing duckweed to recover nutrients from wastewaters and for production of fuel ethanol and animal feed. CLEAN—Soil Air Water 2009, 37, 17–26. [Google Scholar] [CrossRef]
- Kapuscinski, K.L.; Farrell, J.M.; Stehman, S.V.; Boyer, G.L.; Fernando, D.D.; Teece, M.A.; Tschaplinski, T.J. Selective herbivory by an invasive cyprinid, the rudd Scardinius erythrophthalmus. Freshw. Biol. 2014, 59, 2315–2327. [Google Scholar] [CrossRef]
- Hart, P.; O’Sullivan, D.; (University of Tasmania. National Key Centre for Teaching and Research in Aquaculture). Recirculation Systems: Design, Construction and Management; Turtle Press: Tasmania, Australia, 1993. [Google Scholar]
- Liao, P.B.; Mayo, R.D. Salmonid hatchery water reuse systems. Aquaculture 1972, 1, 317–335. [Google Scholar] [CrossRef]
- Masser, M.P.; Rakocy, J.; Losordo, T.M. Recirculating aquaculture tank production systems. In Management of Recirculating Systems; SRAC Publication: Stoneville, MA, USA, 1999. [Google Scholar]
- Van Dam, A.A.; Pauly, D. Simulation of the effects of oxygen on food consumption and growth of Nile tilapia, Oreochromis niloticus (l.). Aquac. Res. 1995, 26, 427–440. [Google Scholar] [CrossRef]
- Balarin, J.D.; Haller, R.D. The intensive culture of tilapia in tanks, raceways and cages. In Recent Advances in Aquaculture; Muir, J.F., Roberts, R.J., Eds.; Croom Helm: London, UK, 1982; pp. 266–355. [Google Scholar]
- Chervinski, J. Environmental physiology of tilapias. In The Biology and Culture of Tilapias: Proceedings of the International Conference on the Biology and Culture of Tilapias, Issue 7 of Iclarm Conference Proceedings; Pullin, R.S.V., Lowe-McConnell, R.H., Eds.; WorldFish: Manila, Philipphines, 1982; pp. 119–128. [Google Scholar]
- Philippart, J.-C.; Ruwet, J.-C. Ecology and distribution of tilapias. In The Biology and Culture of Tilapias: Proceedings of the International Conference on the Biology and Culture of Tilapias, Issue 7 of Iclarm Conference Proceedings; Pullin, R.S.V., Lowe-McConnell, R.H., Eds.; WorldFish: Manila, Philipphines, 1982; pp. 15–60. [Google Scholar]
- Rafiee, G.; Saad, C.R. Nutrient cycle and sludge production during different stages of red tilapia (Oreochromis sp.) growth in a recirculating aquaculture system. Aquaculture 2005, 244, 109–118. [Google Scholar] [CrossRef]


| Growth Parameter | Fish Stocking (Fish/m3) | ||
|---|---|---|---|
| 20 | 25 | 50 | |
| Initial mean weight (g) | 2.6 ± 0.5 | 2.6 ± 0.6 | 2.4 ± 0.6 |
| Final mean weight (g) | 344 ± 69 | 252 ± 37 | 240 ± 16 |
| Weight gain (g) | 342 ± 15 | 249 ± 3 | 237 ± 16 |
| Daily weight gain (g/d) | 3.1 ± 0.1 | 2.23 ± 0.03 | 2.1 ± 0.1 |
| Feed Conversion Ratio; FCR | 1.36 ± 0.06 | 1.2 ± 0.1 | 1.5 ± 0.2 |
| Survival rate (%) | 95 | 86 ± 3 | 91 ± 1 |
| Productivity (kg/m3) | 6.8 ± 0.3 | 5.3 ± 0.5 | 11 ± 1 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sri-uam, P.; Donnuea, S.; Powtongsook, S.; Pavasant, P. Integrated Multi-Trophic Recirculating Aquaculture System for Nile Tilapia (Oreochlomis niloticus). Sustainability 2016, 8, 592. https://doi.org/10.3390/su8070592
Sri-uam P, Donnuea S, Powtongsook S, Pavasant P. Integrated Multi-Trophic Recirculating Aquaculture System for Nile Tilapia (Oreochlomis niloticus). Sustainability. 2016; 8(7):592. https://doi.org/10.3390/su8070592
Chicago/Turabian StyleSri-uam, Puchong, Seri Donnuea, Sorawit Powtongsook, and Prasert Pavasant. 2016. "Integrated Multi-Trophic Recirculating Aquaculture System for Nile Tilapia (Oreochlomis niloticus)" Sustainability 8, no. 7: 592. https://doi.org/10.3390/su8070592






