Variations of Heavy Metals from Geothermal Spring to Surrounding Soil and Mangifera Indica–Siloam Village, Limpopo Province
Abstract
:1. Introduction
2. Experimental Section
2.1. Study Area
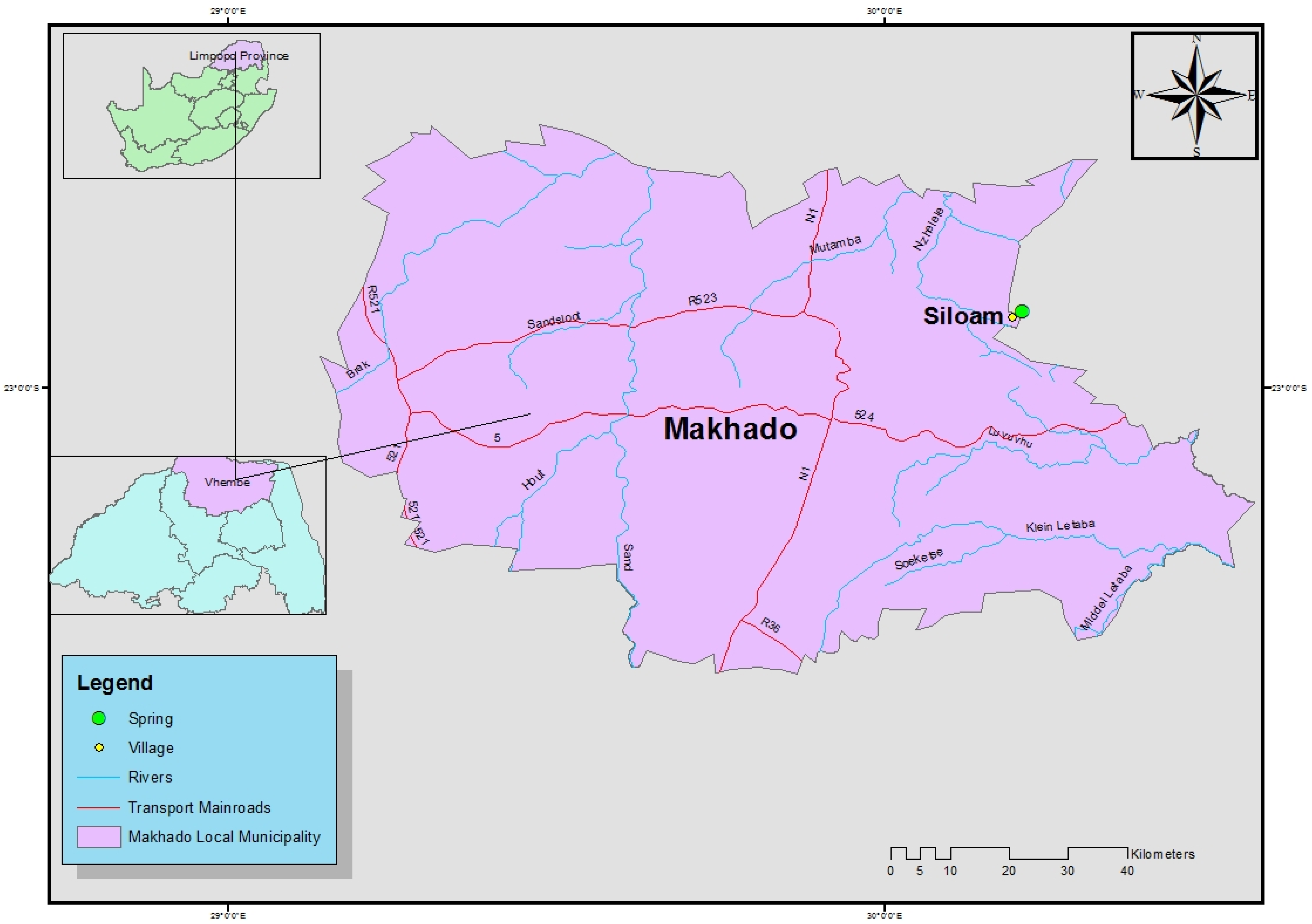
2.2. Sampling
2.3. Experimental Procedure
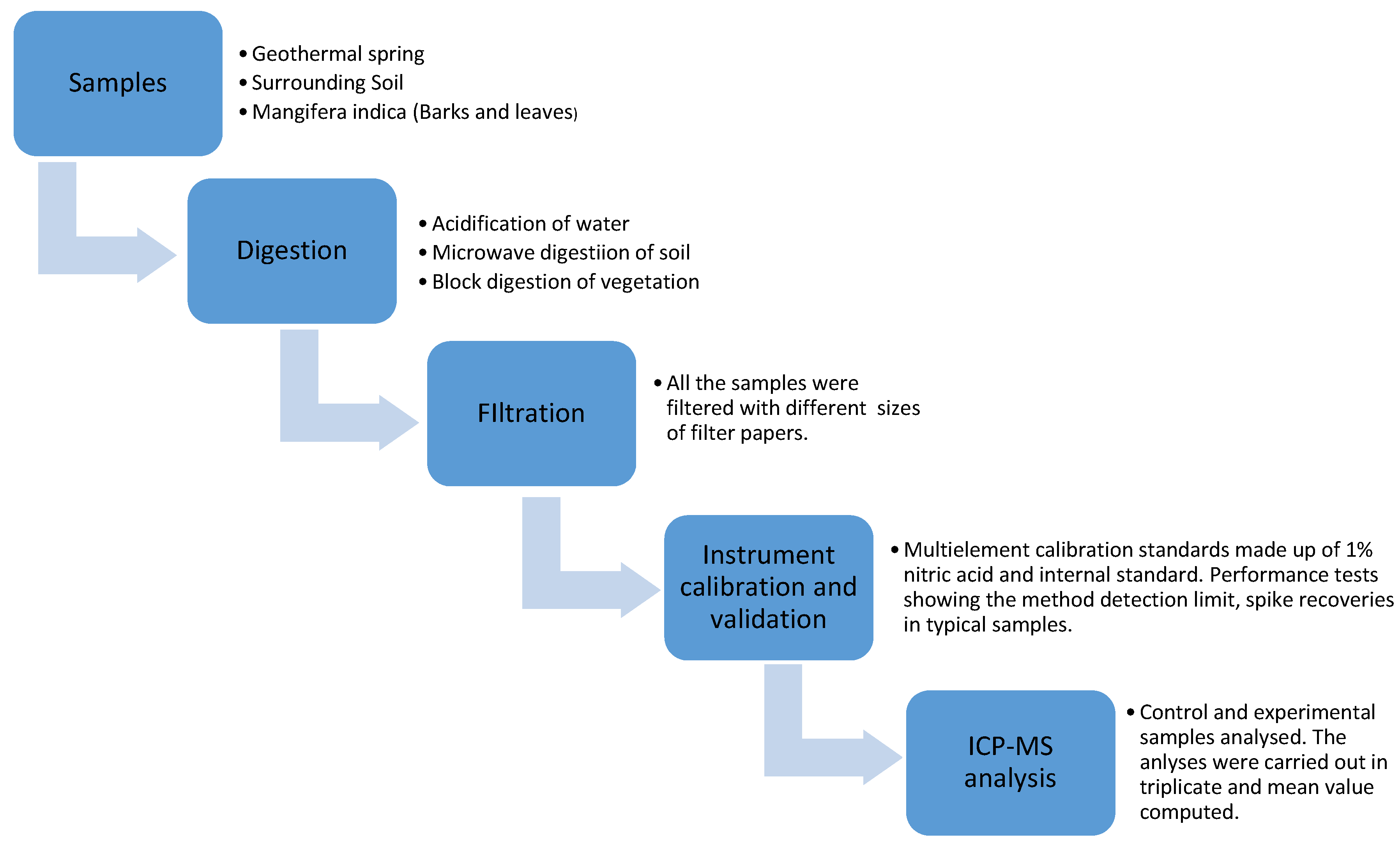
2.4. Quality Assurance/Quality Control
2.5. Statistical Analysis
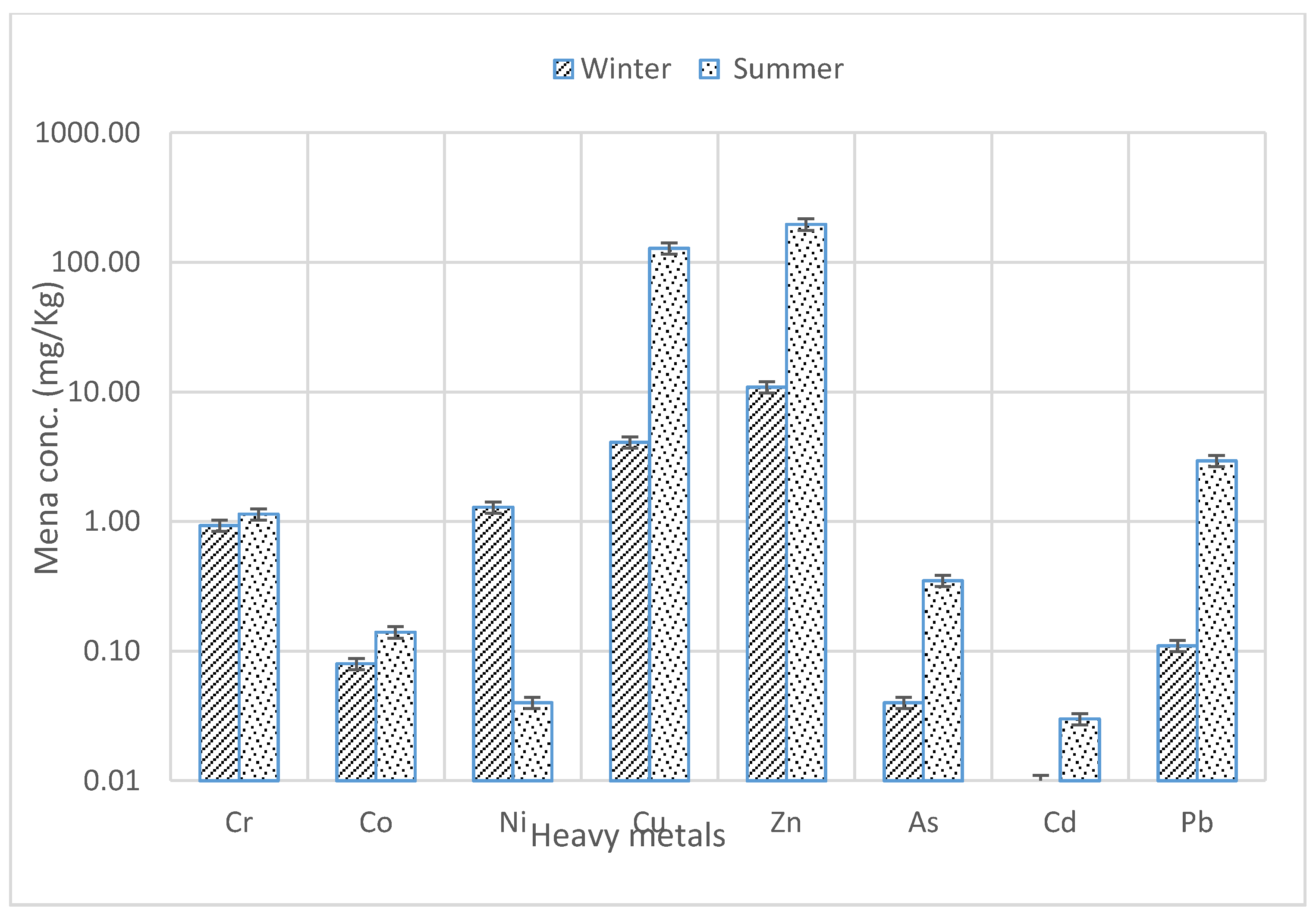
3. Results and Discussion
| Elements | Cr | Co | Ni | Cu | Zn | As | Cd | Pb |
|---|---|---|---|---|---|---|---|---|
| Water analysis (µg/L) | ||||||||
| WHO;SABS | 50;100 | 20;150 | 2000;1000 | 3000;5000 | 10;10 | 10;20 | ||
| Control W | 0.03 ± 0.01 | 0.16 ± 0.06 | 2.62 ± 0.54 | 0.21 ± 0.06 | 50.7 ± 6.22 | 0.09 ± 0.06 | 0.06 ± 0.03 | 0.09 ± 0.03 |
| Control S | 0.03 ± 0.03 | 0.16 ± 0.04 | 2.64 ± 0.51 | 0.21 ± 0.01 | 58.7 ± 1.84 | 0.09 ± 0.03 | 0.1 ± 0.01 | 0.13 ± 0.03 |
| Water W | 0.11 ± 0.03 | 0.02 ± 0.00 | 0.01 ± 0.00 | 0.06 ± 0.03 | 0.24 ± 0.06 | 0.63 ± 0.10 | 0.01 ± 0.00 | 0.12 ± 0.03 |
| Water S | 0.33 ± 0.10 | 0.24 ± 0.08 | 0.18 ± 0.03 | 0.03 ± 0.03 | 0.13 ± 0.04 | 462.11 ± 11.16 | 211 ± 12.73 | 342 ± 14.14 |
| Soil analysis (mg/Kg) | ||||||||
| DNHPD, 1991 | 80 | 20 | 50 | 100 | 185 | 2 | 2 | 56 |
| Control W | 54.00 ± 5.66 | 19.10 ± 2.12 | 25.03 ± 2.08 | 70.46 ± 2.83 | 50.19 ± 4.24 | 1.39 ± 0.28 | 0.23 ± 0.04 | 21.40 ± 1.41 |
| Control S | 58.46 ± 1.66 | 19.14 ± 2.83 | 25.08 ± 6.96 | 70.56 ± 6.28 | 50.23 ± 6.75 | 1.39 ± 0.14 | 0.25 ± 0.01 | 21.42 ± 2.23 |
| Soil W | 65.12 ± 2.83 | 39.04 ± 1.41 | 59.33 ± 5.19 | 95.22 ± 1.95 | 174.51 ± 7.06 | 0.83 ± 0.28 | 0.17 ± 0.04 | 74.60 ± 4.81 |
| Soil S | 92.06 ± 1.41 | 43.11 ± 2.83 | 58.19 ± 4.24 | 266.97 ± 4.29 | 236.05 ± 5.59 | 0.655 ± 0.03 | 0.11 ± 0.03 | 45.74 ± 1.78 |
| Mangifera indica (mg/Kg) | ||||||||
| Control W | 8.09 ± 1.21 | 1.41 ± 0.30 | 33.06 ± 2.06 | 35.42 ± 2.23 | 120.73 ± 6.04 | 1.65 ± 0.28 | 0.02 ± 0.01 | 1.07 ± 0.03 |
| Control S | 15.32 ± 1.24 | 3.26 ± 0.91 | 33.45 ± 0.78 | 46.12 ± 2.66 | 129.12 ± 8.32 | 2.14 ± 0.23 | 0.02 ± 0.01 | 2.01 ± 0.08 |
| Bark W | 0.87 ± 0.01 | 0.34 ± 0.03 | 1.78 ± 0.03 | 2.60 ± 0.28 | 10.40 ± 0.42 | 0.03 ± 0.00 | 0.01 ± 0.00 | 0.14 ± 0.03 |
| Bark S | 2.06 ± 0.01 | 0.48 ± 0.03 | 0.04 ± 0.00 | 7.32 ± 0.03 | 16.22 ± 0.03 | 0.37 ± 0.03 | 0.02 ± 0.01 | 0.01 ± 0.00 |
| Mangifera indica (mg/Kg) | ||||||||
| Control W | 7.67 ± 0.14 | 3.01 ± 0.28 | 31.74 ± 2.06 | 24.04 ± 1.41 | 24.04 ± 0.04 | 0.34 ± 0.06 | 0.84 ± 0.03 | 1.34 ± 0.03 |
| Control S | 10.28 ± 1.94 | 7.98 ± 0.35 | 32.10 ± 2.69 | 32.12 ± 7.95 | 46.87 ± 3.30 | 0.79 ± 0.28 | 1.23 ± 0.10 | 2.31 ± 0.27 |
| Leaf W | 0.93 ± 0.03 | 0.08 ± 0.14 | 1.29 ± 0.14 | 4.09 ± 1.41 | 10.90 ± 1.41 | 0.04 ± 0.01 | 0.01 ± 0.00 | 0.11 ± 0.02 |
| Leaf S | 1.14 ± 0.14 | 0.14 ± 0.03 | 0.04 ± 0.01 | 128.11 ± 0.61 | 196.58 ± 1.41 | 0.35 ± 0.07 | 0.03 ± 0.00 | 2.94 ± 0.06 |

| Elements | Cr | Co | Ni | Cu | Zn | As | Cd | Pb |
|---|---|---|---|---|---|---|---|---|
| Cr | 1.00 | |||||||
| Co | 0.99 ** | 1.00 | ||||||
| Ni | 0.98 ** | 0.99 ** | 1.00 | |||||
| Cu | 0.84 ** | 0.78 * | 0.75 * | 1.00 | ||||
| Zn | 0.77 * | 0.76 * | 0.75 * | 0.93 ** | 1.00 | |||
| As | −0.22 | −0.22 | −0.23 | −0.26 | −0.34 | 1.00 | ||
| Cd | −0.22 | −0.22 | −0.22 | −0.26 | −0.32 | 1.00 ** | 1.00 | |
| Pb | −0.09 | −0.01 | 0.05 | −0.12 | −0.16 | 0.97 ** | 0.97 ** | 1.00 |
| Elements | Factor | |
|---|---|---|
| 1 | 2 | |
| Cr | 0.97 | −0.26 |
| Co | 0.95 | −0.28 |
| Ni | 0.93 | −0.28 |
| Cu | 0.91 | −0.21 |
| Zn | 0.96 | −0.27 |
| As | −0.32 | 0.95 |
| Cd | −0.32 | 0.95 |
| Pb | −0.17 | 0.98 |
| Total variance | 58.64 | 38.67 |


4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Durowoju, O.; Odiyo, J.; Ekosse, G. Hydrogeochemical setting of geothermal springs in Limpopo Province, South Africa- A Review. Res. J. Chem. Environ. 2015, 19, 77–88. [Google Scholar]
- Olivier, J.; Venter, J.S.; Jonker, C.Z. Thermal and Chemical Characteristics of Thermal Springs in the Northern Part of the Limpopo Province, South Africa. Water SA 2011, 34, 163–174. [Google Scholar]
- Taylor, J.; Phillips, F. Balneology or Taking Waters. 2007. Available online: http://www.selfgrowth.com (accessed on 21 October 2014).
- Kent, L.E. Thermal waters of the Union of South Africa and South West Africa. Trans. Geol. Soc. WS. Afr. 1949, 52, 231–264. [Google Scholar]
- Witcher, J.C. Masson Radium Springs Farm; GHC BULLETIN; Southwest Technology Institute, New Mexico State University: Las Cruces, NM, USA, 2002. [Google Scholar]
- Odiyo, J.O.; Makungo, R. Fluoride concentrations in groundwater and impact on human health in Siolam Village, Limpopo Province, South Africa. Water SA 2012, 38, 731–736. [Google Scholar] [CrossRef]
- Manda, L.; Suzuki, K.T. Arsenic around the world: A review. Talanta 2012, 58, 201–235. [Google Scholar] [CrossRef]
- Romero, L.; Alomso, H.; Campano, P.; Fanfani, L.; Cidu, R.; Dadea, C.; Keegan, T.; Thornton, I.; Farago, M. Arsenic enrichment in waters and sediments of the Rio Loa (Second Region, Chile). Appl. Geochem. 2003, 18, 1399–1416. [Google Scholar] [CrossRef]
- Churchill, R.K.; Clinkenbeard, J.P. Perspectives on mercury contributions to watersheds from historic mercury mines and non-mine mercury sources: Examples from the sulphur creek mining district. In Proceedings of the Cordilleran Section–101st Annual Meeting, Piedmont, Italy, 29 April–1 May 2005.
- Ifegwu, C.; Anyakora, C. Screen for eight heavy metals in groundwater samples from highly industrialized area of Lagos, Nigeria. Afri. J. Pharm. Sci. Pharm. 2012, 3, 1–16. [Google Scholar]
- Hutton, M.; Symon, C. The Quantities of Cadmium, Lead, Mercury and Arsenic Entering the U.K. Environment from Human Activities. Sci. Total Environ. 1986, 57, 129–150. [Google Scholar] [CrossRef]
- Battarbee, R.; Anderson, N.; Appleby, P.; Flower, R.G.; Fritz, S.; Haworth, E.; Higgit, S.; Jones, V.; Kreiser, A.; Munro, M.A.; et al. Lake Acidification in the United Kingdom; ENSIS: London, UK, 1988. [Google Scholar]
- Nriagu, J.O.; Pacyna, J. Quantitative Assessment of Worldwide Contamination of Air, Water and Soil by Trace Metals. Nature 1988, 333, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Garbarino, J.R.; Hayes, H.; Roth, D.; Antweider, R.; Brinton, T.I.; Taylor, H. Contaminants in the Mississipi River; U.S. Geological Survey Circular: Rapid City, SD, USA, 1995.
- Hawkes, J.S. Heavy Metals. J. Chem. Educ. 1997, 74, 1374. [Google Scholar] [CrossRef]
- Yahaya, M.I.; Mohammad, S.; Abdullahi, B.K. Seasonal variations of heavy metal concentration in Abattoir dumping site soil in Nigeria. J. Appl. Sci. Environ. Manag. 2009, 13, 9–13. [Google Scholar] [CrossRef]
- Gune, M.S.; Alpaslan, M.; Inal, A. Plant Growth and Fertilizer; Ankara university of Agriculture: Ankara, Turkey, 2004. [Google Scholar]
- Trueby, P. Impact of Heavy Metals on Forest Trees from Mining Areas. In Proceedings of the International Conference on Mining and the Environment, Sudbury, ON, Canada, 25–28 May 2003.
- Durowoju, O.; Odiyo, J.; Ekosse, G. Horizontal status of trace elements from Siloam and Tshipise geothermal springs to their surface soil. Water SA 2015. under review. [Google Scholar]
- Aggett, P.J. Trace element deficiencies in man. In Role of Trace Elements for Health Promotion and Disease Prevention; Sandström, B., Walter, P., Eds.; Karger: Basel, Switzerland, 1998; Volume 54, pp. 18–28. [Google Scholar]
- McLaughlin, M.J.; Hamon, R.E.; McLaren, R.G.; Speir, T.W.; Rogers, S.L. Review: A bioavailability-based rationale for controlling metal and metalloid contamination of agricultural land in Australia and New Zealand. Aust. J. Soil Res. 2000, 38, 1037–1086. [Google Scholar] [CrossRef]
- Brandl, G. The geology of the Pietersburg area. In Explanation Sheet, Geological Survey of South Africa; Geological Maps: Pietersburg, South Africa, 1986; Volume 2328, p. 43. [Google Scholar]
- Mundalamo, H.R. Investigation of Water Quality in Nzhelele Valley, Limpopo Province, South Africa. Honours Mini-Dissertation, University of Venda, Thohoyandou, South Africa, 2003. [Google Scholar]
- United State Environmental Protection Agency (USEPA). Guideline for Water Reuse; EPA: Washington, DC, USA, 2004.
- Pleysier, L.J. Soil sampling and sample preparation. Available online: http://www.cglrc.cgiar.org/iita/soilSampling/ (accessed on 3 January 2016).
- SR ISO 11466. Soil Quality-Extraction of Trace Elements Soluble in Aqua Regia; International Organization for Standardization: Geneva, Switzerland, 1999. [Google Scholar]
- Pyle, S.M.; Nocerino, J.M.; Deming, S.N.; Palasota, J.A.; Palasota, J.M.; Miller, E.L.; Hillman, D.C.; Kuharic, C.A.; Cole, W.H.; Fitzpatrick, P.M.; et al. Comparison of AAS, ICP-AES, PSA, and XRF in determining Lead and Cadmium in Soil. Environ. Sci. Tech. 1996, 30, 204–213. [Google Scholar] [CrossRef]
- United State Environmental Protection Agency (USEPA). (1994) Method 200.8. Determination of Trace Elements in Waters and Wastes by ICP, Revision 5.4. Available online: http://www.epa.gov/sam/pdfs/EPA-200.8.pdf (accessed on 14 December 2014).
- Todd, D.K. Groundwater Hydrology, 2nd ed.; Wiley: New York, NY, USA, 1980; pp. 535–552. [Google Scholar]
- World Health Organisation (WHO). Bottled Drinking Water. 2000. Available online: http://www.who.int/mediacentre/factsheets/fs256/en/print.html (accessed on 19 August 2014).
- South African Bureau of Standards (SABS). Class 1 Potable Water Standards; SABS 241:1999; South African Bureau of Standards: Pretoria, South Africa, 1999.
- Department of National Health and Population Development (DNHPD). Guide: Permissible Utilisation and Disposal of Sewage Sludge; Ref: A11/2/5/4; DNHPD: Pretoria, South Africa, 1991.
- Chouba, L.; Kraiem, M.; Njimi, W.; Tissaoui, C.H.; Thompson, J.R.; Flower, R.J. Seasonal variation of heavy metals (Cd, Pb and Hg) in sediments and in mullet, Mugil cephalus (Mugilidae), from the Ghar El Melh Lagoon (Tunisia). Waters Bull. 2007, 4, 45–52. [Google Scholar]
- McGrath, S.P.; Chang, A.C.; Page, A.L.; Wilter, E. Land application of sewage sludge: Scientific perspective of heavy metal loading limits in Europe and United States. Environ. Rev. 1994, 2, 108–118. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durowoju, O.S.; Odiyo, J.O.; Ekosse, G.-I.E. Variations of Heavy Metals from Geothermal Spring to Surrounding Soil and Mangifera Indica–Siloam Village, Limpopo Province. Sustainability 2016, 8, 60. https://doi.org/10.3390/su8010060
Durowoju OS, Odiyo JO, Ekosse G-IE. Variations of Heavy Metals from Geothermal Spring to Surrounding Soil and Mangifera Indica–Siloam Village, Limpopo Province. Sustainability. 2016; 8(1):60. https://doi.org/10.3390/su8010060
Chicago/Turabian StyleDurowoju, Olatunde S., John O. Odiyo, and Georges-Ivo E. Ekosse. 2016. "Variations of Heavy Metals from Geothermal Spring to Surrounding Soil and Mangifera Indica–Siloam Village, Limpopo Province" Sustainability 8, no. 1: 60. https://doi.org/10.3390/su8010060







