Sustainable Ethanol Production from Common Reed (Phragmites australis) through Simultaneuos Saccharification and Fermentation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Feedstock
2.2. Biomass Pretreatment
2.3. SSF (Simultaneous Saccharification and Fermentation)
2.4. Experimental Setup
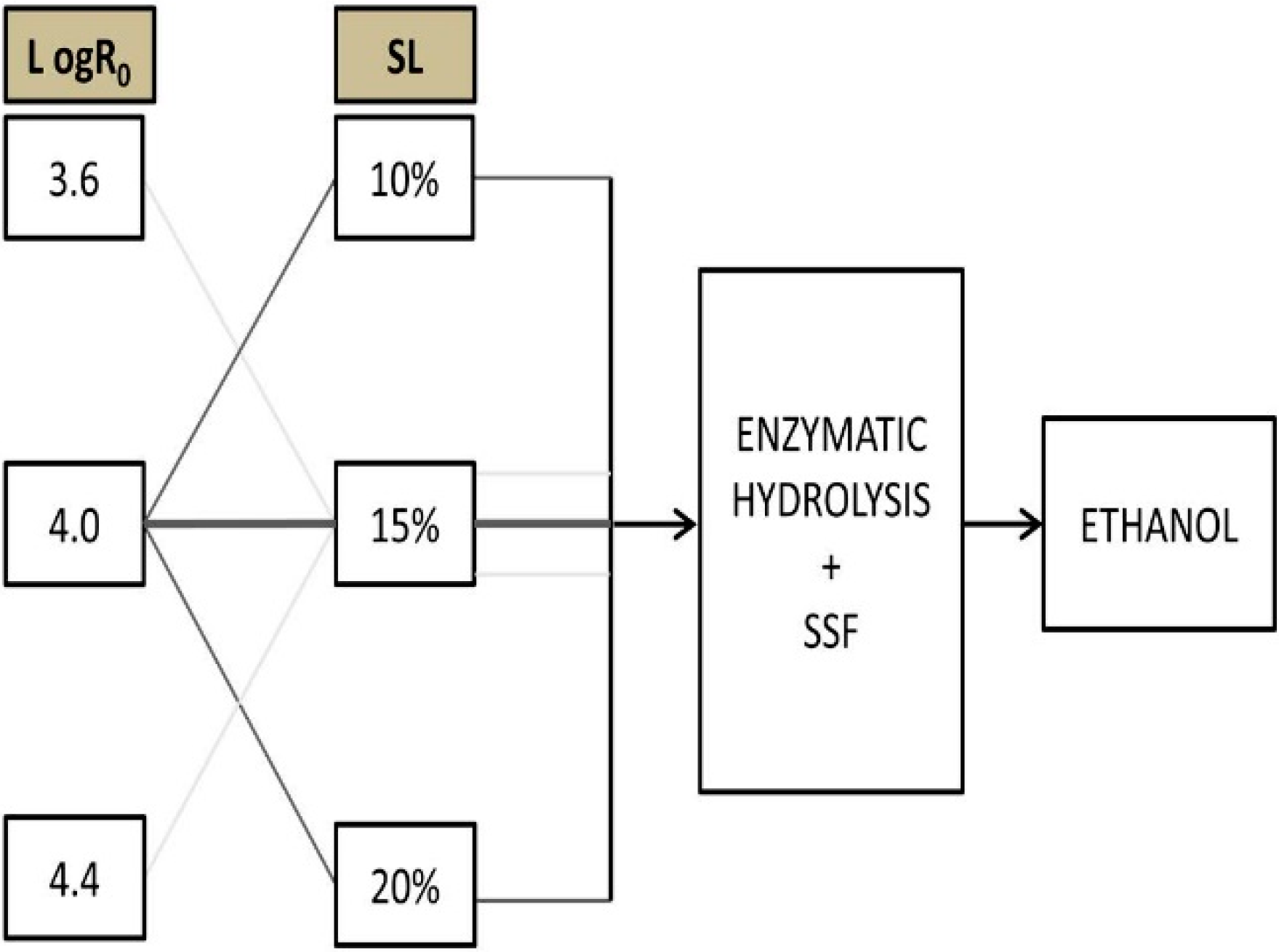
3. Results and Discussion
3.1. Phragmites australis Characterization
| H% | C% | Ac% | L% | E% | A% | Total% | Other% | |
|---|---|---|---|---|---|---|---|---|
| Phragmites australis | 20.51 | 38.13 | 3.92 | 23.02 | 6.90 | 4.25 | 96.72 | 3.28 |
| Standard deviation | 0.62 | 0.36 | 0.16 | 0.92 | 0.23 | 0.04 | 1.20 | 1.20 |
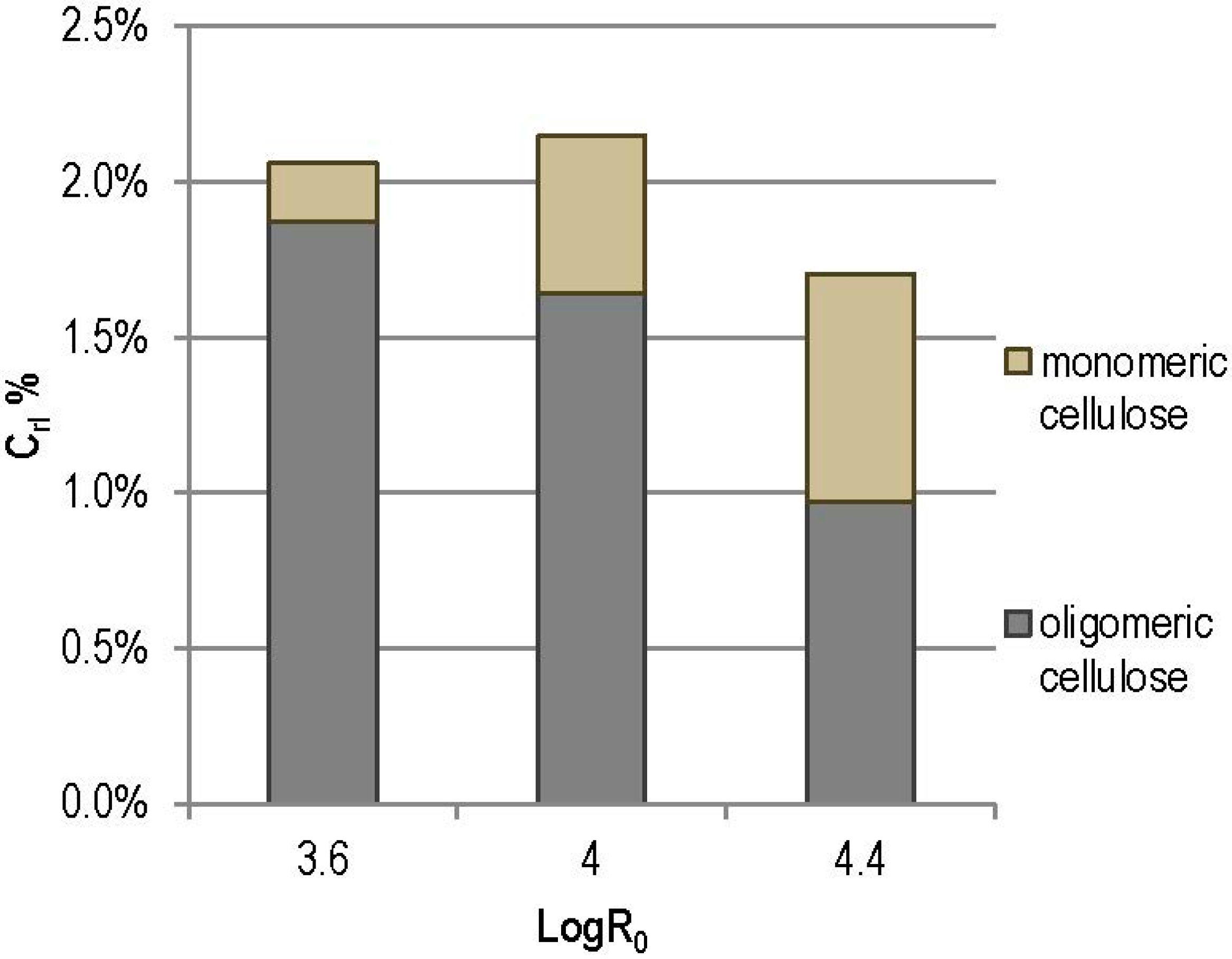
3.2. Steam Explosion
| Log R0 | H% | C% | Ac% | L% | A% | Total% | Other |
|---|---|---|---|---|---|---|---|
| 3.6 | 12.50 ± 0.05 | 48.96 ± 0.27 | 2.19 ± 0.06 | 28.87 ± 0.80 | 3.34 ± 0.15 | 95.86 ± 0.82 | 4.14 ± 0.82 |
| 4.0 | 4.01 ± 0.9 | 54.61 ± 0.28 | 0.42 ± 0.42 | 33.99 ± 0.51 | 3.19 ± 0.21 | 96.22 ± 0.67 | 3.78 ± 0.67 |
| 4.4 | 1.20 ± 0.10 | 52.07 ± 0.53 | 0.39 ± 0.01 | 38.63 ± 0.29 | 4.21 ± 0.15 | 96.49 ± 0.79 | 3.51 ± 0.79 |


3.3. SSF
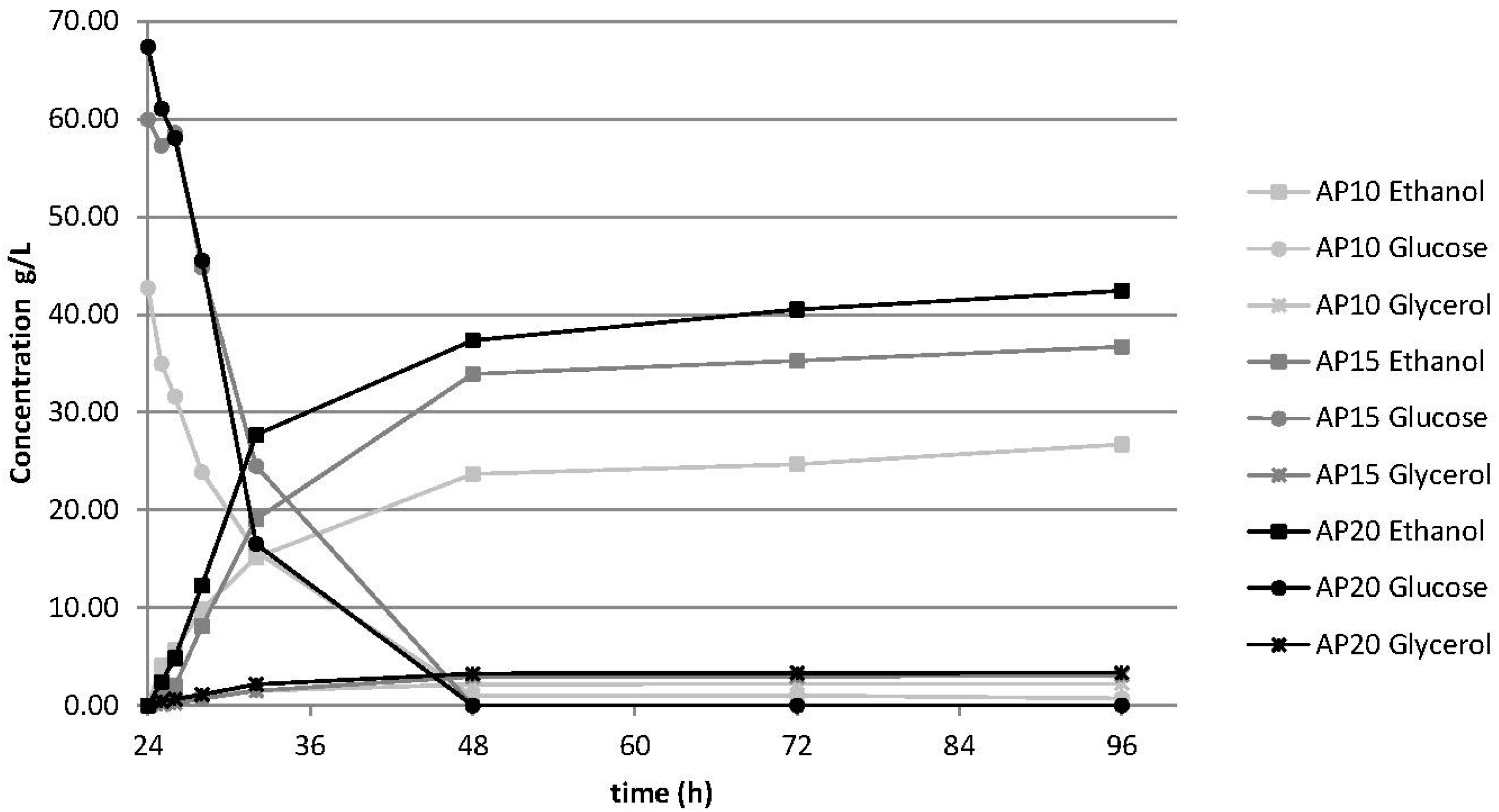
3.3.1. Effect of Solid Loading
| WIS | LogR0 | SL% (w/w) | Hy24% | Hy96% | ∆Hy% a | Fy% | OY |
|---|---|---|---|---|---|---|---|
| AP10 | 4 | 10 | 60.27 | 84.22 | 21.84 | 87.70 | 16.35 |
| AP15 | 4 | 15 | 49.63 | 70.80 | 21.17 | 88.24 | 12.92 |
| AP20 | 4 | 20 | 42.25 | 58.33 | 16.08 | 89.41 | 11.54 |
3.3.2. Effect of LogR0
| WIS | LogR0 | SL% (w/w) | Cr% | Hy24% | Hy96% | ∆Hy% a | Fy% | OY |
|---|---|---|---|---|---|---|---|---|
| AP3.6 | 3.6 | 15 | 98.17 | 38.88 | 38.88 | - | 98.39 | 8.64 |
| AP4.0 | 4.0 | 15 | 96.08 | 49.63 | 70.80 | 21.17 | 88.41 | 12.92 |
| AP4.4 | 4.4 | 15 | 90.77 | 62.38 | 91.78 | 29.40 | 82.55 | 15.80 |

3.3.3. Relative Overall Ethanol Yields and Ethanol Concentrations

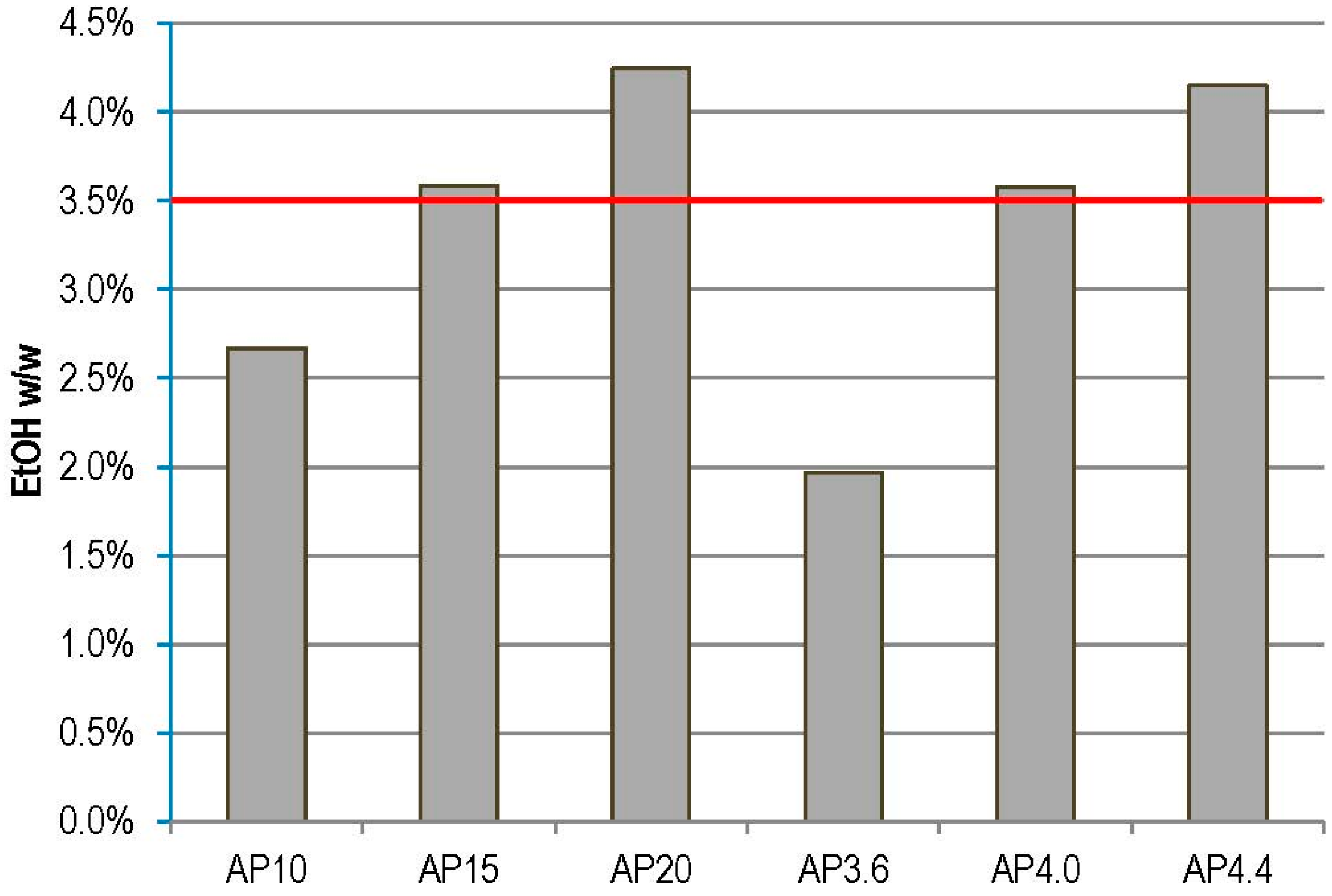
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Salata, F.; de Lieto Vollaro, A.; de Lieto Vollaro, R.; Mancieri, L. Method for energy optimization with reliability analysis of a trigeneration and teleheating system on urban scale: A case study. Energy Build. 2015, 86, 118–136. [Google Scholar] [CrossRef]
- De Lieto Vollaro, R.; Guattari, C.; Evangelisti, L.; Battista, G.; Carnielo, E.; Gori, P. Building energy performance analysis: A case study. Energy Build. 2015, 87, 87–94. [Google Scholar] [CrossRef]
- De Lieto Vollaro, R.; Evangelisti, L.; Carnielo, E.; Battista, G.; Gori, P.; Guattari, C.; Fanchiotti, A. An integrated approach for an historical buildings energy analysis in a smart cities perspective. Energy Procedia 2014, 45, 372–378. [Google Scholar] [CrossRef]
- Salata, F.; de Lieto Vollaro, A.; de Lieto Vollaro, R. A model for the evaluation of heat loss from underground cables in non-uniform soil to optimize the system design. Therm. Sci. 2015, 19, 461–474. [Google Scholar] [CrossRef]
- Naik, S.N.; Goud, V.V.; Rout, P.K.; Dalai, A.K. Production of first and second generation biofuels: A comprehensive review. Renew. Sustain. Energy Rev. 2010, 14, 578–597. [Google Scholar] [CrossRef]
- Sathitsuksanoh, N.; Zhu, Z.; Templeton, N.; Rollin, J.A.; Harvey, S.P.; Zhang, Y.-H.P. Saccharification of a Potential Bioenergy Crop., Phragmites australis (Common Reed), by Lignocellulose Fractionation Followed by Enzymatic Hydrolysis at Decreased Cellulase Loadings. Ind. Eng. Chem. Res. 2009, 48, 6441–6447. [Google Scholar] [CrossRef]
- Szijarto, N.; Kádár, Z.; Varga, E.; Thomsen, A.B.; Costa-Ferreira, M.; Réczey, K. Pretreatment of Reed by Wet Oxidation and Subsequent Utilization of the Pretreated Fibers for Ethanol Production. Appl. Biochem. Biotechnol. 2009, 155, 386–396. [Google Scholar] [PubMed]
- Sun, Y.; Cheng, J. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef]
- Kumar, P.; Barrett, D.M.; Delwiche, M.J.; Stroeve, P. Methods for Pretreatment of Lignocellulosic Biomass for Efficient Hydrolysis and Biofuel Production. Ind. Eng. Chem. Res. 2009, 48, 3713–3729. [Google Scholar] [CrossRef]
- Cotana, F.; Cavalaglio, G.; Gelosia, M.; Coccia, V.; Petrozzi, A.; Ingles, D.; Pompili, E. A comparison between SHF and SSSF processes from cardoon for ethanol production. Ind. Crop. Prod. 2015, 69, 424–432. [Google Scholar] [CrossRef]
- Rana, V.; Eckard, A.D.; Ahring, B.K. Comparison of SHF and SSF of wet exploded corn stover and loblolly pine using in-house enzymes produced from T. reesei RUT C30 and A. saccharolyticus. SpringerPlus 2014. [Google Scholar] [CrossRef] [PubMed]
- Philippidis, G.P.; Smith, T.K.; Wyman, C.E. Study of the enzymatic hydrolysis of cellulose for production of fuel ethanol by the simultaneous saccharification and fermentation process. Biotechnol. Bioeng. 1993, 41, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Kádár, Z.; Szengyel, Z.; Réczey, K. Simultaneous saccharification and fermentation (SSF) of industrial wastes for the production of ethanol. Ind. Crop. Prod. 2004, 20, 103–110. [Google Scholar] [CrossRef]
- Yoon, K.Y.; Woodams, E.E.; Hang, Y.D. Enzymatic production of pentoses from the hemicellulose fraction of corn residues. LWT Food Sci. Technol. 2006, 39, 388–392. [Google Scholar] [CrossRef]
- Mamman, A.S.; Lee, J.-M.; Kim, Y.-C.; Hwang, I.T.; Park, N.-J.; Hwang, Y.K.; Chang, J.-S.; Hwang, J.-S. Furfural: Hemicellulose/xylosederived biochemical. Biofuels Bioprod. Biorefin. 2008, 2, 438–454. [Google Scholar] [CrossRef]
- Sluiter, J.; Sluiter, A. Summative Mass Closure. Technical Report NREL/TP-510-48087-revised July 2011. Available online: http://www.nrel.gov/docs/gen/fy11/48087.pdf (accessed on 25 August 2015).
- Cotana, F.; Cavalaglio, G.; Gelosia, M.; Nicolini, A.; Coccia, V.; Petrozzi, A. Production of Bioethanol in a Second Generation Prototype from Pine Wood Chips. Energy Procedia 2014, 45, 42–51. [Google Scholar] [CrossRef]
- Overend, R.P.; Chornet, E.; Gascoigne, J. Fractionation of lignocellulosics by steam-aqueous pretreatments [and discussion]. Philos. Trans. Royal Soc. Lond. 1987, 321, 523–536. [Google Scholar] [CrossRef]
- Sluiter, A. Determination of Sugars, Byproducts, and Degradation Products in Liquid Fraction Process Samples; National Renewable Energy Laboratory: Golden, CO, USA, 2006. [Google Scholar]
- Ruiz, H.A.; Rodríguez-Jasso, R.M.; Fernandes, B.D.; Vicente, A.A.; Teixeira, J.A. Hydrothermal processing, as an alternative for upgrading agriculture residues and marine biomass according to the biorefinery concept: A review. Renew. Sustain. Energy Rev. 2013, 21, 35–51. [Google Scholar] [CrossRef] [Green Version]
- Palmqvist, E.; Hahn-Hägerdal, B. Fermentation of lignocellulosic hydrolysates. II: Inhibitors and mechanisms of inhibition. Bioresour. Technol. 2000, 74, 25–33. [Google Scholar] [CrossRef]
- Almeida, J.R.M.; Modig, T.; Petersson, A.; Hähn-Hägerdal, B.; Lidén, G.; Gorwa-Grauslund, M.F. Increased tolerance and conversion of inhibitors in lignocellulosic hydrolysates by Saccharomyces cerevisiae. J. Chem. Technol. Biotechnol. 2007, 82, 340–349. [Google Scholar] [CrossRef]
- Cotana, F.; Cavalaglio, G.; Gelosia, M.; Coccia, V.; Petrozzi, A.; Nicolini, A. Effect of Double-Step Steam Explosion Pretreatment in Bioethanol Production from Softwood. Appl. Biochem. Biotechnol. 2014, 174, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Horn, S.J.; Nguyen, Q.D.; Westereng, B.; Nilsen, P.J.; Eijsink, V.G.H. Screening of steam explosion conditions for glucose production from non-impregnated wheat straw. Biomass Bioenergy 2011, 35, 4879–4886. [Google Scholar] [CrossRef]
- Kim, S.-K.; Park, D.H.; Song, S.H.; Wee, Y.J.; Jeong, G.T. Effect of fermentation inhibitors in the presence and absence of activated charcoal on the growth of Saccharomyces cerevisiae. Bioprocess Biosyst. Eng. 2013, 36, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Demeke, M.M.; Dietz, H.; Li, Y.; Foulquié-Moreno, M.R.; Mutturi, S.; Deprez, S.; Den Abt, T.; Bonini, B.M.; Liden, G.; Dumortier, F.; et al. Development of a D-xylose fermenting and inhibitor tolerant industrial Saccharomyces cerevisiae strain with high performance in lignocellulose hydrolysates using metabolic and evolutionary engineering. Biotechnol. Biofuels 2013. [Google Scholar] [CrossRef] [PubMed]
- Spindler, D.; Wyman, C.E.; Mohagheghi, A.; Grohmann, K. Thermotolerant yeast for simultaneous saccharification and fermentation of cellulose to ethanol. Appl. Biochem. Biotechnol. 1988, 17, 279–293. [Google Scholar] [CrossRef]
- Andrić, P.; Meyer, A.S.; Jensen, P.A.; Dam-Johansen, K. Reactor design for minimizing product inhibition during enzymatic lignocellulose hydrolysis: I. Significance and mechanism of cellobiose and glucose inhibition on cellulolytic enzymes. Biotechnol. Adv. 2010, 28, 308–324. [Google Scholar] [CrossRef] [PubMed]
- Várnai, A.; Siika-aho, M.; Viikari, L. Restriction of the enzymatic hydrolysis of steam-pretreated spruce by lignin and hemicellulose. Enzym. Microb. Technol. 2010, 46, 185–193. [Google Scholar] [CrossRef]
- Grethlein, H.E. The Effect of Pore Size Distribution on the Rate of Enzymatic Hydrolysis of Cellulosic Substrates. Nat. Biotechnol. 1985, 3, 155–160. [Google Scholar] [CrossRef]
- Fan, L.T.; Gharpuray, M.M.; Lee, Y.H. Cellulose Hydrolysis; Springer-Verlag: Berlin, Germany, 1987. [Google Scholar]
- Hendriks, A.; Zeeman, G. Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour. Technol. 2009, 100, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Ando, S.; Arai, I.; Kiyoto, K.; Hanai, S. Identification of aromatic monomers in steam-exploded poplar and their influences on ethanol fermentation by Saccharomyces cerevisiae. J. Fermentation Technol. 1986, 64, 567–570. [Google Scholar] [CrossRef]
- Vane, L.M. Separation technologies for the recovery and dehydration of alcohols from fermentation broths. Biofuels Bioprod. Biorefin. 2008, 2, 553–588. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cotana, F.; Cavalaglio, G.; Pisello, A.L.; Gelosia, M.; Ingles, D.; Pompili, E. Sustainable Ethanol Production from Common Reed (Phragmites australis) through Simultaneuos Saccharification and Fermentation. Sustainability 2015, 7, 12149-12163. https://doi.org/10.3390/su70912149
Cotana F, Cavalaglio G, Pisello AL, Gelosia M, Ingles D, Pompili E. Sustainable Ethanol Production from Common Reed (Phragmites australis) through Simultaneuos Saccharification and Fermentation. Sustainability. 2015; 7(9):12149-12163. https://doi.org/10.3390/su70912149
Chicago/Turabian StyleCotana, Franco, Gianluca Cavalaglio, Anna Laura Pisello, Mattia Gelosia, David Ingles, and Enrico Pompili. 2015. "Sustainable Ethanol Production from Common Reed (Phragmites australis) through Simultaneuos Saccharification and Fermentation" Sustainability 7, no. 9: 12149-12163. https://doi.org/10.3390/su70912149







