Soil Warming Elevates the Abundance of Collembola in the Songnen Plain of China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Experimental Design
- (1)
- Warming (W): The open top chamber (OTC) which was hexagon-based pyramid and made of polymethyl methacrylate sheet was used to warm the plot. The dimensions of the OTC are 50 cm height, 1.5 m open-top hexagon, 60° inclination and 2.08 m basal diameter. The detailed design was described in the Temperature Enhancement Experiments of ITEX [19]. The warming device was fixed on the ground, and the bottom margin of OTC was 1cm above the ground.
- (2)
- Warming with low precipitation (WLP): The OTC was used to warm the plot. A 40-cm-wide polymethyl methacrylate sheet covered 30% of the OTC hexagon to reduce 30% of the precipitation.
- (3)
- Warming with high precipitation (WHP): The OTC was used to warm the plot. Thirty percent of the precipitation was sprayed into the plot after the rain fall each time. The data of precipitation was collected from the automatic weather station of the research station which was about 500 m from the plot.
- (4)
- Control (CK): Only the framework of the OTC was installed on the field.
2.3. Sampling and Species Identification
2.4. Data Analyses
3. Results and Discussion
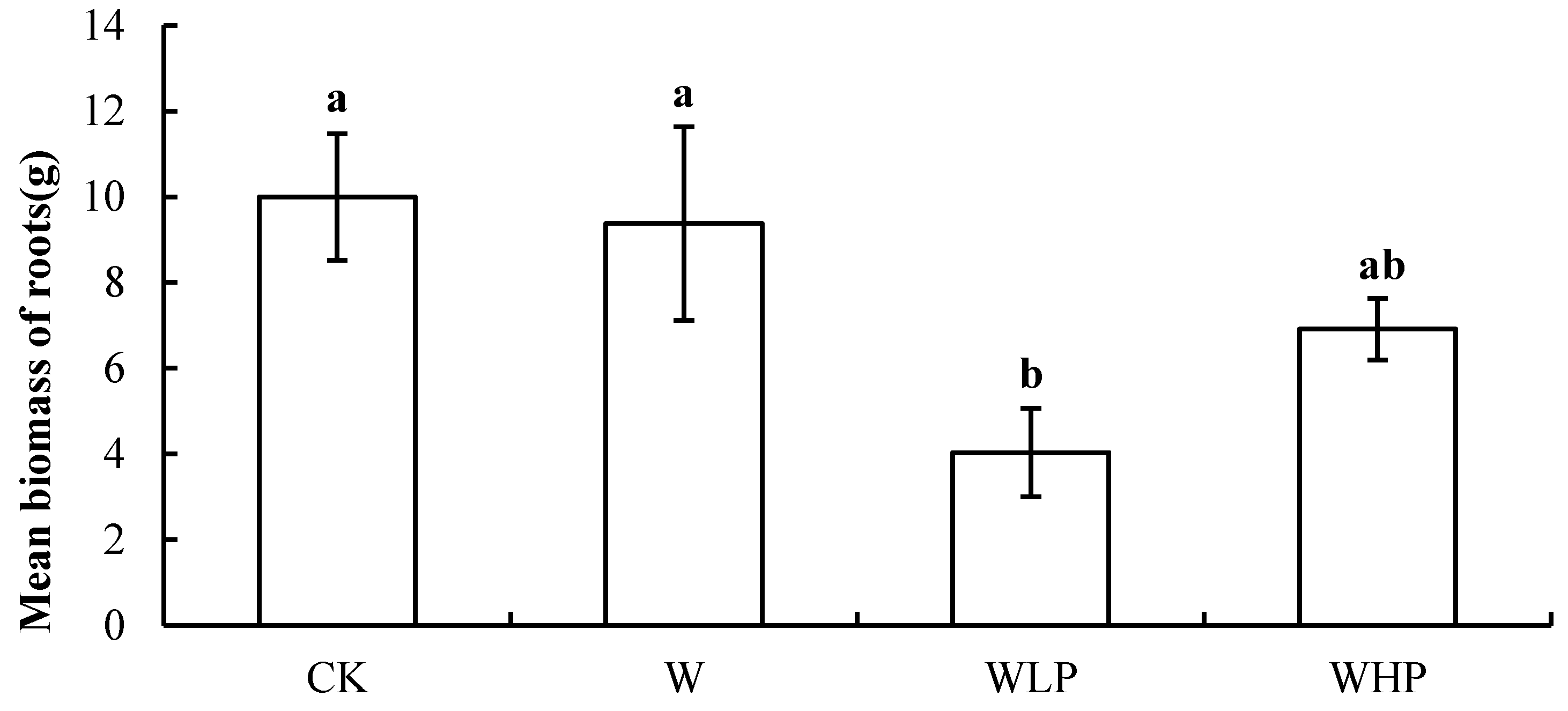
3.1. Environmental Factors and Plant Community
| Treatment | CK | W | WLP | WHP |
|---|---|---|---|---|
| Species richness | 2.00 ± 0.45 | 2.17 ± 0.40 | 2.00 ± 0.26 | 1.50 ± 0.34 |
| Diversity(H) | 0.82 | 1.38 | 1.04 | 1.06 |
| Evenness | 0.62 | 0.96 | 0.93 | 0.80 |
| Dominant species | Medicago falcata | Sonchus arvensis | Medicago falcata | Sonchus arvensis |
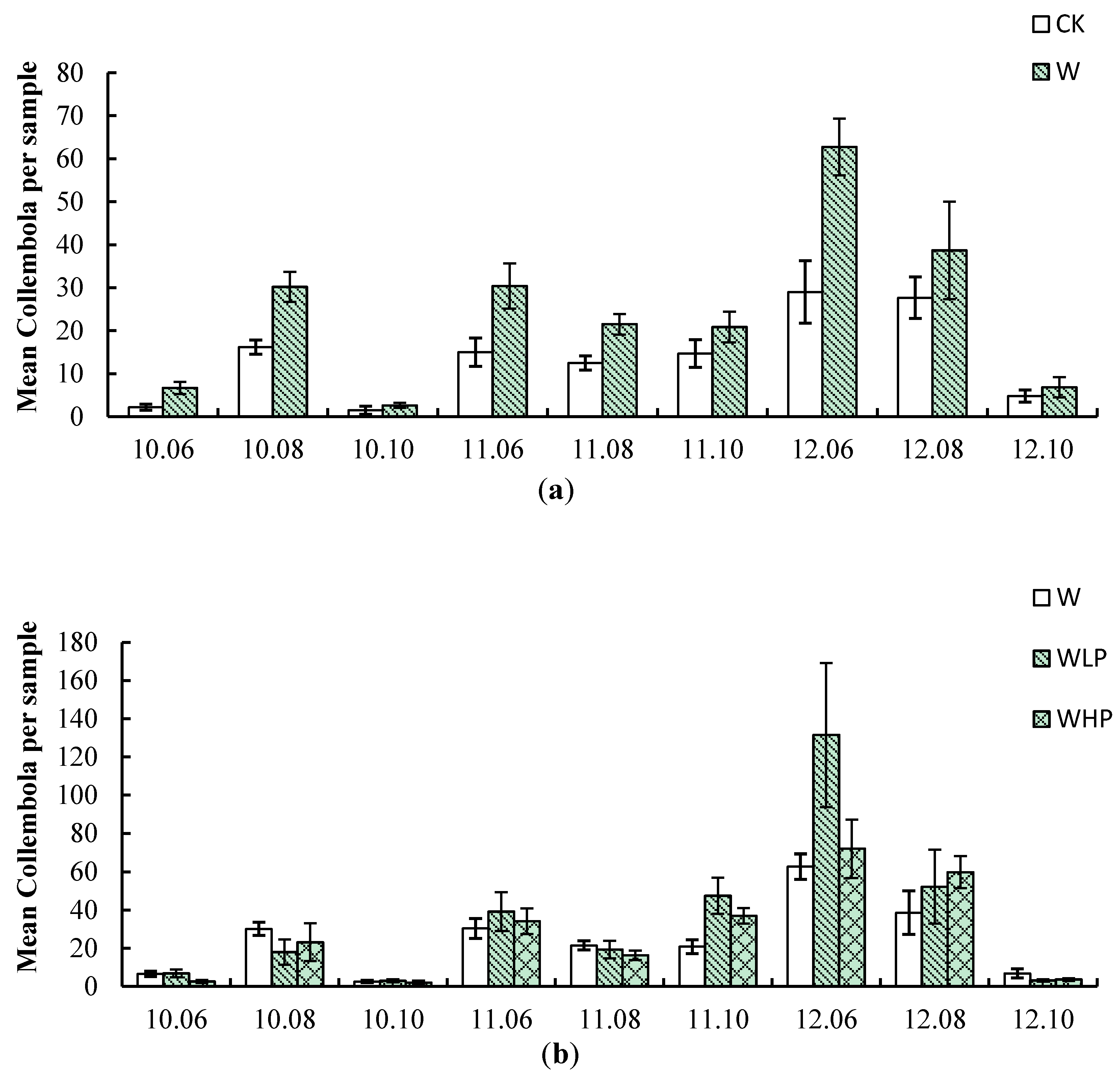
3.2. Composition, Abundance and Species Richness of Collembola
| Collembola | CK (N = 53) | W (N = 52) | WLP (N = 54) | WHP (N = 54) |
|---|---|---|---|---|
| Orchesellides sp.1 | 532 a | 867 b | 1190 b | 1024 b |
| Entomobrya sp.1 | 17 - | 2 - | 61 - | 5 - |
| Mesaphorura sp.1 | 54 a | 97 ab | 279 b | 128 ab |
| Heterosminthurus sp.1 | 38 | 33 | 16 | 35 |
| Bourletiella sp.1 | 41 | 33 | 49 | 51 |
| Ptenothrix sp.1 | 12 - | 3 - | 4 - | 2 - |
| Ptenothrix sp.2 | 78 - | 41 - | 42 - | 76 - |
| Friesea sp.1 | 25 | 42 | 24 | 35 |
| Arrhopalites sp.1 | 0 - | 0 - | 1 - | 0 - |
| Arrhopalites sp.2 | 2 - | 1 - | 2 - | 3 - |
| Isotomiella sp.1 | 83 | 104 | 194 | 102 |
| Proisotoma sp.1 | 16 | 19 | 30 | 33 |
| Lepidocyrtus felipei | 1 - | 11 - | 3 - | 0 - |
| Entomobrya sp.2 | 12 | 19 | 29 | 11 |
| Total | 911 | 1272 | 1924 | 1505 |
| Treatment | CK | W | WLP | WHP | |
|---|---|---|---|---|---|
| 2010 | 1.53 ± 0.17 | 1.82 ± 0.18 | 1.47 ± 0.17 | 2.2 ± 0.28 | |
| Species richness | 2011 | 4.18 ± 0.32 | 3.71 ± 0.33 | 3.83 ± 0.28 | 4.11 ± 0.28 |
| 2012 | 2.31 ± 0.25 | 2.53 ± 0.36 | 2.89 ± 0.32 | 2.67 ± 0.29 | |
| 2010 | 0.78 | 0.79 | 0.95 | 1.06 | |
| Diversity (H) | 2011 | 1.67 | 1.60 | 1.35 | 1.43 |
| 2012 | 0.93 | 0.95 | 1.08 | 0.89 | |
| 2010 | 0.72 | 0.66 | 0.77 | 0.76 | |
| Evenness | 2011 | 0.80 | 0.84 | 0.70 | 0.68 |
| 2012 | 0.66 | 0.68 | 0.70 | 0.62 | |
| Dominant Species and Dominant percent | Orchesellides sp.1 | 58.4 | 68.2 | 61.9 | 68.0 |
| Mesaphorura sp.1 | 5.9 | 7.6 | 14.5 | 8.5 | |
| Ptenothrix sp.2 | 8.6 | - | - | - | |
| Isotomiella sp.1 | 9.1 | 8.2 | 10.1 | 6.8 |
4. Conclusions
Acknowledgments
Author Contributions
Appendix
| Sampling time | CK | W | WLP | WHP |
|---|---|---|---|---|
| June 2010 | 15.38 | 20.56 | 13.94 | 22.94 |
| August 2010 | 8.27 | 15.40 | 6.35 | 11.50 |
| October 2010 | 20.43 | 25.44 | 18.54 | 28.47 |
| June 2011 | 14.07 | 16.12 | 16.99 | 16.62 |
| August 2011 | 17.19 | 12.74 | 10.31 | 17.90 |
| October 2011 | ns | ns | ns | ns |
| June 2012 | 23.86 | 24.35 | 21.14 | 28.81 |
| August 2012 | 27.46 | 25.39 | 21.39 | 30.57 |
| October 2012 | 22.60 | 20.43 | 15.59 | 24.14 |
Conflicts of Interest
References
- Kappelle, M.; van Vuuren, M.M.; Baas, P. Effects of climate change on biodiversity: A review and identification of key research issues. Biodivers. Conserv. 1999, 8, 1383–1397. [Google Scholar] [CrossRef]
- Tylianakis, J.M.; Didham, R.K.; Bascompte, J.; Wardle, D.A. Global change and species interactions in terrestrial ecosystems. Ecol. Lett. 2008, 11, 1351–1363. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.I.; Moorhead, D.L.; Neher, D.A.; Ryoo, M.I. A modeling study of soil temperature and moisture effects on population dynamics of Paronychiurus. kimi (Collembola: Onychiuridae). Biol. Fertil. Soils 2006, 43, 69–75. [Google Scholar] [CrossRef]
- Haimi, J.; Laamanen, J.; Penttinen, R.; Räty, M.; Koponen, S.; Kellomäki, S.; Niemelä, P. Impacts of elevated CO2 and temperature on the soil fauna of boreal forests. Appl. Soil Ecol. 2005, 30, 104–112. [Google Scholar] [CrossRef]
- Zhu, T.C. Yang-Cao Biological Ecology; Jilin Science and Technology Press: Jilin, China, 2004. [Google Scholar]
- Li, X.; Li, Q.; Wang, Z.; Liu, X. A research on characteristics and rational exploitation of soda saline land in the Western Songnen Plain. Res. Agric. Mod. 2002, 5, 361–364. [Google Scholar]
- Luan, Z.; Zhang, G.; Deng, W.; Hu, J.; Zhou, D. Studies on Changes of Air Temperature and Precipitation for Last 50 Years in Songnen Plain. Chin. J. Agrometeorol. 2007, 28, 355–358. [Google Scholar]
- Sun, F.; Yang, S.; Chen, P. Climatic warming-drying trend in Northeastern China during the last 44 years and its effects. Chin. J. Ecol. 2005, 24, 751–755. [Google Scholar]
- Zhou, G.; Wang, Y.; Jiang, Y.; Xu, Z. Carbon balance along the Northeast China Transect (NECT-IGBP). Sci. China (Series C) 2002, 45, 18–29. [Google Scholar] [CrossRef]
- Wu, D.; Yin, W.; Yan, R. Influence of vegetation reclamation type on the characteristics of soil Collembola community in seriously alkalinized and degraded grasslands of Songnen Plain. China Environ. Sci. 2008, 5, 466–470. [Google Scholar]
- Hopkin, S.P. Biology of the Springtails: (Insecta: Collembola); Oxford University Press: Oxford, UK, 1997. [Google Scholar]
- Christiansen, K. Bionomics of Collembola. Annu. Rev. Entomol. 1964, 9, 147–178. [Google Scholar] [CrossRef]
- Siepel, H. Life-history tactics of soil microarthropods. Biol. Fertil. Soils 1994, 18, 263–278. [Google Scholar] [CrossRef]
- Bandow, C.; Karau, N.; Römbke, J. Interactive effects of pyrimethanil, soil moisture and temperature on Folsomia candida and Sinella curviseta (Collembola). Appl. Soil Ecol. 2014, 81, 22–29. [Google Scholar] [CrossRef]
- Huhta, V.; Hänninen, S. Effects of temperature and moisture fluctuations on an experimental soil microarthropod community. Pedobiologia 2001, 45, 279–286. [Google Scholar] [CrossRef]
- Bokhorst, S.; Huiskes, A.; Convey, P.; van Bodegom, P.M.; Aerts, R. Climate change effects on soil arthropod communities from the Falkland Islands and the Maritime Antarctic. Soil Biol. Biochem. 2008, 40, 1547–1556. [Google Scholar] [CrossRef]
- Kardol, P.; Nicholas Reynolds, W.; Norby, R.J.; Classen, A.T. Climate change effects on soil microarthropod abundance and community structure. Appl. Soil Ecol. 2011, 47, 37–44. [Google Scholar] [CrossRef]
- Petersen, H. Collembolan communities in shrublands along climatic gradients in Europe and the effect of experimental warming and drought on population density, biomass and diversity. Soil Org. 2011, 83, 463–488. [Google Scholar]
- Marion, M.G. Temperature Enhancement Experiments. In Proceedings of the the ITEX Manual Danish Polar Center, Copenhagen, Denmark; 1996; pp. 14–19. [Google Scholar]
- Bellinger, P.F.; Christiansen, K.A.; Janssens, F. Checklist of the Collembola of the World. 2012. Available online: http://www.collembola.org (accessed on 15 February 2012).
- Yin, W.Y. Pictorial Keys to Soil Animals of China; Science Press: Beijing, China, 1998. [Google Scholar]
- Potapov, M. Synopses on Palaearctic Collembola: Isotomidae; Dunger, W., Ed.; Staatliches Museum für Naturkunde: Görlitz, Germany, 2001. [Google Scholar]
- Pomorski, R.J. Onychiurinae of Poland (Collembola: Onychiuridae); Polish Taxonomical Society: Wrocklaw, Poland, 1998; pp. 1–201. [Google Scholar]
- Christiansen, K.; Bellinger, P. The Collembola of North America North of the Rio Grand; Grinnell College: Grinnell, IA, USA, 1980. [Google Scholar]
- Lindberg, N.; Bengtsson, J. Population responses of oribatid mites and collembolans after drought. Appl. Soil Ecol. 2005, 28, 163–174. [Google Scholar] [CrossRef]
- Coulson, S.; Hodkinson, I.; Wooley, C.; Webb, N.; Block, W.; Worland, M.; Bale, J.; Strathdee, A. Effects of experimental temperature elevation on high-arctic soil microarthropod populations. Polar Biol. 1996, 16, 147–153. [Google Scholar] [CrossRef]
- Sinclair, B.J. Effects of increased temperatures simulating climate change on terrestrial invertebrates on Ross Island, Antarctica. Pedobiologia 2002, 46, 150–160. [Google Scholar] [CrossRef]
- Butcher, J.W.; Snider, R.; Snider, R.J. Bioecology of edaphic Collembola and Acarina. Annu. Rev. Entomol. 1971, 16, 249–288. [Google Scholar] [CrossRef]
- Wiles, J.A.; Krogh, P.A. Tests with the collembolans Isotoma viridis, Folsomia candida and Folsomia fimetaria. In Handbook of Soil Invertebrate Toxicity Tests; Løkke, H., Van Gestel, C.A.M., Eds.; John Wiley: Chichester, UK, 1998. [Google Scholar]
- Babenko, A.B. Temperature preferendum of Collembola species from arctic tundra of Taimyr. Entomol. Rev. 1993, 72, 89–101. [Google Scholar]
- Wang, R.; Gao, Q.; Chen, Q. Effects of climatic change on biomass and biomass allocation in Leymus. chinensis (Poaceae) along the North-east China Transect (NECT). J. Arid Environ. 2003, 54, 653–665. [Google Scholar] [CrossRef]
- Yang, L.; Han, M.; Zhou, G. The changes in water-use efficiency and stoma density of Leymus. chinensis along Northeast China Transect. Acta Ecol. Sin. 2007, 27, 16–23. [Google Scholar] [CrossRef]
- Jian, N. Plant functional types and climate along a precipitation gradient in temperate grasslands, north-east China and south-east Mongolia. J. Arid Environ. 2003, 53, 501–516. [Google Scholar] [CrossRef]
- Greenslade, P. Survival of Collembola in arid environments: Observations in South Australia and the Sudan. J. Arid Environ. 1981, 4, 219–228. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, X.; Ni, Z.; Chang, L.; Wang, K.; Wu, D. Soil Warming Elevates the Abundance of Collembola in the Songnen Plain of China. Sustainability 2015, 7, 1161-1171. https://doi.org/10.3390/su7021161
Yan X, Ni Z, Chang L, Wang K, Wu D. Soil Warming Elevates the Abundance of Collembola in the Songnen Plain of China. Sustainability. 2015; 7(2):1161-1171. https://doi.org/10.3390/su7021161
Chicago/Turabian StyleYan, Xiumin, Zhen Ni, Liang Chang, Kehong Wang, and Donghui Wu. 2015. "Soil Warming Elevates the Abundance of Collembola in the Songnen Plain of China" Sustainability 7, no. 2: 1161-1171. https://doi.org/10.3390/su7021161





